Abstract
A systematic study on the effect of monovalent cation doping on structural, magnetic, and magnetocaloric properties of Pr0.85 A 0.15MnO3 (A = Ag and K) samples synthesized by a sol-gel method has been carried out. The crystal structure and morphology have been worked by X-ray diffraction (XRD) and scanning electron microscopy (SEM) imaging measurements. The XRD results indicate that both samples have orthorhombic structure. Magnetization versus temperature measurements show that our samples display a ferromagnetic-to-paramagnetic phase transition with increasing temperature. The ferromagnetic-to-paramagnetic phase transition temperature (T C) values were found as 74 and 116 K for Pr0.85Ag0.15MnO3 and Pr0.85 K 0.15MnO3, respectively. The magnetic entropy changes were evaluated from isothermal magnetization curves measured at various temperatures near T C by steps of 4 K. The values of the magnetic entropy change were determined as 0.99 and 1.39 J kg −1 K −1 for Pr0.85Ag0.15MnO3 and Pr0.85 K 0.15MnO3 under external field changes of 10 kOe, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The magnetocaloric effect (MCE), described as cooling or heating of a magnetic material under magnetic field change, offers an alternative technology for refrigeration [1]. The magnetic refrigeration (MR) technology has basic advantages over a classical system such as not emitting environmentally harmful gases and being more energy efficient [2–4]. It would be possible to achieve mentioned advantages only if desired magnetic materials can be produced. For this reason, MCE has been investigated widely to find a proper refrigerant material that can produce large entropy change when it goes through magnetization-demagnetization process [5–7]. So far, it has been reported that the large spontaneous magnetization and a sharp change in the magnetization with changing temperature are important requirements for magnetic material to possess large magnetic entropy change [8, 9]. The perovskite manganites having the general formula RE1−x A x MnO3 (RE and A are a trivalent rare-earth cation and a divalent or monovalent cation, respectively) have attracted much attention due to their interesting physical and magnetic properties such as colossal magnetoresistance (CMR) and MCE [10–12]. It is known that REMnO3 is an antiferromagnetic insulator [13]. The magnetic and transport properties of these base samples are strongly affected by the percentage of the divalent or monovalent cation ions, the ionic radii of these ions, and the preparation method of the samples [14, 15]. For RE = Pr case, in the literature, there are a lot of studies about the structural, magnetic, and magnetocaloric properties with divalent substitutions. However, the substitution of monovalent (A = Na, K, Ag, etc.) elements by RE ions has been much less studied [13, 16–21]. Jirák et al. reported structural and physical properties of Pr1−x A x MnO3 manganites 0 ≤x≤ 0.15 for K [16] and 0 ≤x≤ 0.2 for Na [17], respectively. According to their studies, the optimum doping level is determined as 0.15 for the substitution of monovalent cation in PrMnO3 [18]. In the present work, we have explored the effect of the monovalent cation substitution on structural, magnetic, and magnetocaloric properties in Pr0.85 A 0.15MnO3 (A = Ag and K) manganites. The percentage of the monovalent cations has the same ratio for both samples.
2 Experimental Procedure
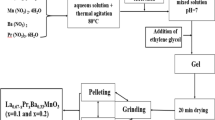
We have prepared polycrystalline Pr0.85 A 0.15MnO3 for A = Ag and K samples, labeled as PAM and PKM, respectively. The sol-gel method was used to obtain high-purity and homogenous powder samples. An appropriate amount of Pr(NO3)3⋅6H2O, MnO2, AgNO3, and KNO3 powders was liquefied in suitable diluents to obtain desired stoichiometry for PAM and PKM samples. We used 87.91 g (five times of the total mole number of main compound) citric acid monohydrate (CAS number: 5949-29-1) with ≥99.95 % purity and 23.38 cm 3 (five times of the total mole number of main compound) mono-ethylene glycol with 99.8 % purity (CAS number: 107-21-1) as reagents and solvents, respectively. Then, the solution was stirred at 300 ∘C until dry gel was formed. The dry gel was first burned at 500 ∘C for an hour and then calcined at 550 ∘C for 5 h so that organic substances will be removed from the materials. To get fine powders, the materials were ground by using an agate mortar. After that, the microcrystalline powder was pressed into disks and sintered at 970 and 1150 ∘C during 24 h for PAM and PKM, respectively. The crystal structure of the powder samples was identified by X-ray diffraction (XRD) using Cu K α radiation. The microstructural properties and elemental analysis of the samples were investigated by scanning electron microscopy (SEM) combined with energy-dispersive X-ray spectroscopy (EDS). Magnetic measurements were performed by using a vibrating sample magnetometer (VSM). The magnetic entropy changes (ΔS M) were obtained from isothermal magnetization curves which were taken up to 1 T at various temperatures near T C by intervals of 4 K.
3 Results and Discussions
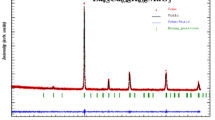
Figure 1 shows the X-ray diffraction patterns recorded at room temperature using Cu K α radiation for Pr0.85 A 0.15MnO3 (A = Ag and K) samples. It can be seen from the figure that both samples have been crystallized as perovskite structure. The peaks become larger and narrower which indicate better crystallization in the PKM than in the PAM sample. The XRD patterns of the PAM and PKM samples are indexed in the orthorhombic structure with a Pbnm space group by using PANalytical X’Pert HighScore Plus software. From Fig. 1, the PAM sample has a small amount of impurity phases (4.90 % of the compound) related to the presence of PrMn2 O 5 symbolized by a triangle. We believed that the presence of this impurity phase does not affect the observed magnetocaloric behavior of the sample since it is non-ferromagnetic impurity [22]. The lattice parameters of samples are obtained as a= 5.435 Å, b= 5.490 Å, and c= 7.670 Å for PAM and a= 5.439 Å, b= 5.485 Å, and c= 7.724 Å for PKM from the XRD data. The unit cell volumes of the samples are found as 228.858 and 230.429 Å 3 for PAM and PKM samples, respectively. It is seen that there is a slight difference in the value of lattice parameters and the unit volume of samples. The average crystallite sizes, calculated from the Scherrer relation [23], are found as 38 and 56 nm for PAM and PKM, respectively. The average crystallite size of PKM is obviously bigger than that of PAM. The reason of differences of lattice parameters and crystallite sizes between PAM and PKM might be related to having larger ionic radii of K (1.51 Å) than Ag (1.28 Å) [24] The lattice distortion can be estimated by a tolerance factor formulated as \(t_{\mathrm {f}} ={\left ({r_{\mathrm {A}} +r_{0} } \right )} \left /{\sqrt {2} \left ({r_{\text {Mn}} +r_{0} } \right )}\right .\) where r A, r Mn, and r O are the ionic radii of A site, Mn site, and oxygen ion, respectively [25]. The A site average ionic size (〈r A〉) and the tolerance factor (t f) of samples were calculated using values of ionic radii by Shannon for coordination number 8 for the A site cations and coordination number 12 for oxygen [24]. The values of the 〈r A〉 and the t f are given in Table 1. The value of the 〈r A〉 for the PKM sample is bigger than that of the PAM due to having a bigger ionic radius of K than Ag. The t f value of both samples is in the constant range of perovskite structure [26]. Figure 2a, b shows the SEM images and EDS spectra for PAM and PKM samples, respectively. It is seen from the SEM images that each sample is formed in different shape grains. The average grain sizes of the samples, calculated from 100 arbitrary grains, are found as 0.71 and 0.94 μm for PAM and PKM samples, respectively. The average grain sizes are larger than those calculated from the XRD data. It is evident that the grains are formed from several crystallites [27]. It is seen from the EDS spectra that the samples contain all of the expected elements and any impurity element has not been identified.
In order to determine the magnetic behavior of the samples, we have performed the temperature M(T) and applied magnetic field M(H) dependence of the magnetization measurements. M(T) curves of both samples measured in zero-field-cooled (ZFC) procedure at a magnetic field of 250 Oe are shown in Fig. 3a, b. It can be seen that magnetization increases by increasing the temperature from 10 K to the magnetic phase transition point, which depends on doping of K or Ag elements. This behavior may have arisen due to the suppression of magnetization caused by the coexistence of ferromagnetic/antiferromagnetic couplings and/or spin glass that yields to an anisotropic field inside the samples [28–31]. When the temperature is even increased, the magnetization starts to decrease which indicate a ferromagnetic-to-paramagnetic phase transition at temperature (T C). From the minimum point of the dM/d T−T curve given as an inset graph in Fig. 3a, b, the T C values of samples were determined as 74 and 116 K for PAM and PKM samples, respectively (Table 2). The small ionic radius of Ag with respect to the K causes the decrease of the 〈r A〉. This decrease leads to a change in Mn–O–Mn bond angle and Mn–O length. Therefore, a double-exchange interaction might be weaker because of the changing of bandwidth and mobility of e g electrons [32, 33] and, consequently, the Curie temperature value of the samples decreases from 116 to 74 K by changing K with Ag [28]. It can be seen from Fig. 3a, b that the phase transition becomes sharper by changing Ag with K and this situation supports the former explanations given above as well. Figure 3a, b also displays the temperature dependence of the inverse magnetic susceptibility, 1/ χ−T. At high temperature region, they show a linear behavior corresponding with paramagnetic state. By fitting the inverse susceptibility data with the Curie-Weiss law given by χ = C/(T−𝜃), where C is the Curie constant and 𝜃 is the paramagnetic Curie temperature, we have determined the 𝜃 value as 81 and 117 K for Ag and K, respectively. The 𝜃 value is nearly in accordance with T C for the PKM sample. However, for the PAM sample, the 𝜃 value is higher than T C, which might be related to the magnetic inhomogeneity and impurity [34]. The Curie constant is given by \(C=\frac {N\mu _{\text {eff}}^{2} \mu _{\mathrm {B}}^{2} }{3k_{\mathrm {B}} }\) where N is Avogadro’s number, μ B is the Bohr magneton, μ eff is the effective magnetic moment, and k B is the Boltzmann constant. The Curie constant values of the samples are calculated from the slopes of 1/ χ−T curves. By using these values, μ eff values were determined as 3.43 μ B and 3.99 μ B, for PAM and PKM, respectively. These values are smaller than theoretically estimated effective magnetic moment value (\(\mu _{\text {eff}}^{\text {cal}}\), 4.61 μ B), which indicates the canted situation of the spin ordering [35–37]. Figure 4a, b represents the magnetic field dependence of magnetization curves, M(H), measured in the vicinity of transition temperature for each sample. The M(H) curves of the samples display a nonlinear behavior as a typical ferromagnetic state at T<T C [38], and then they become linear that shows a paramagnetic behavior above T C. In the vicinity of T C, we crossed out H/M versus M 2 (the so-called Arrott plots) to define the type of the magnetic phase transition. The Arrott plots of each sample are shown in Fig. 5a, b. According to the Banerjee criterion, the slope of the H/M versus M 2 curves indicates the order of magnetic phase transition. Since it is well known that the positive (negative) slope of the curve indicates the second (first)-order magnetic phase transition [39], our both PAM and PKM samples show a second-order magnetic phase transition due to the positive slope of their Arrott plots.
From isothermal M(H) measurements, the magnetic entropy change (ΔS M) has been determined using the classical thermodynamic theory based on Maxwell’s relation [40]
where M i and M i+1 are the experimental values of the magnetization at temperatures T i and T i+1, respectively, under a magnetic field H i . Figure 6a, b shows the magnetic entropy change curves as a function of temperature in a magnetic field change of 1 T for PAM and PKM samples. In a magnetocaloric system, the biggest magnetic entropy change occurs around the transition temperature where the magnetization has an abrupt variation. Thus, we have performed isothermal magnetization measurements around T C. The values of \({\Delta } S_{\mathrm {M}}^{\max } \) are found as 0.99 and 1.39 J kg −1 K −1 for PAM and PKM at ΔH= 1 T, respectively. These results are comparable with the values of monovalent or divalent perovskite manganites [15, 41, 42]. The \({\Delta } S_{\mathrm {M}}^{\max } \) value of PKM is obviously larger than that of PAM. The lower \({\Delta } S_{\mathrm {M}}^{\max } \) value of the PAM sample might be arisen from (i) the presence of impurity phases that increases the volume fraction of non-magnetocaloric part of PAM and (ii) the broader magnetic phase transition of the PAM sample.
Amaral et al. [43] and Amaral and Amaral [44] focused on more accurate theoretical modeling of MCE that was based on the Landau theory of phase transitions. According to the theory, the magnitude and the temperature dependence of the ΔS M are closely related with magnetoelastic coupling and electron condensation energy. In order to analyze the ΔS M properties of the sample in accordance with this theory, we have used the Gibbs free energy expressed in the following equation [43, 44]:
where A, B, and C are the Landau coefficients. The Landau theory of ferromagnetism assumes that equilibrium condition around T C so that the magnetic free energy can be minimized. From the minimization, \(\frac {\partial G(M,T)}{\partial M}=0\), a magnetic equation of state is obtained as
The corresponding magnetic entropy change is obtained from differentiation of the magnetic part of the free energy with respect to the temperature
where A ′(T), B ′(T), and C ′(T) are the temperature derivatives of the expansion coefficients. Using these values, we have calculated the temperature dependence of magnetic entropy change in an applied external magnetic field of 1 T of the samples. Figure 7 shows the temperature dependence of magnetic entropy change curves for both samples. It is seen from Fig. 7 that the magnetic entropy change curve calculated by the Landau theory is consistent with experimental curve. From this result and the theory explained above, it can be concluded that both the magnetoelastic coupling and the electron condensation energy play an important role on the MCE properties in our samples [45, 46].
The relative cooling power (RCP), which is related to the amount of heat transfer between cold and hot parts of refrigerator in an ideal thermodynamic cycle [47], is an important parameter in determining the efficiency of cooling. The RCP is defined as [48]
where \(\left | {-{\Delta } S_{\mathrm {M}}^{\max } } \right |\) is the absolute value of maximum magnetic entropy change and δ T FWHM is the full width at half maximum of the magnetic entropy change curve. The RCP values of the samples were determined from Fig. 6a, b. Although the \(-{\Delta } S_{\mathrm {M}}^{\max } \) value of the PKM is higher than that of the PAM, its RCP value (33.7 J kg −1) is lower than that of PAM (35.6 J kg −1) at the same magnetic field change. This situation results from the broadening of the \({\Delta } S_{\mathrm {M}}^{\max } \) curve which yields to increase the applicability of the magnetic cooling materials [49].
4 Conclusions
We have investigated the influence of monovalent element doping on structural, magnetic, and magnetocaloric properties of Pr0.85 A 0.15MnO3 (A = Ag and K) polycrystalline samples. The X-ray powder diffraction analysis reveals that the PAM and PKM samples have orthorhombic structure. The unit cell volumes and the lattice parameters vary monotonically with the 〈r A〉. The ferromagnetic-to-paramagnetic phase transition temperature increases with increasing the 〈r A〉. It is assumed that increasing 〈r A〉 leads to an increase of double-exchange interaction. Both samples show second-order magnetic phase transition nature. The maximum magnetic entropy changes were determined as 0.99 and 1.39 J kg −1 K −1 for PAM and PKM samples for a field change of 1 T, respectively. Under the same magnetic field change, the RCP value of the PAM sample is bigger than that of the PKM due to having a broader magnetic phase transition of the PAM sample. As explained above, the obtained values are comparable to magnetocaloric Pr-based manganites.
References
Tozri, A., Khelifi, J., Dhahri, E., Hlil, E. K.: Influence of Pr-doping on magnetic phase transition and magnetocaloric effect of La0.7−x Pr x Ba0.3MnO3 manganite. Mater. Chem. Phys. 149-150, 728–733 (2015)
Mahaton, R. N., Sethupathi, K., Sankaranarayanan, V., Nirmala, R.: Co-existence of giant magnetoresistance and large magnetocaloric effect near room temperature in nanocrystalline La0.7Te0.3MnO3. J. Magn. Magn. Mater. 322, 2537–2540 (2010)
Xu, Y., Meier, M., Das, P., Koblischka, M. R., Hartmann, U.: Perovskite manganites: Potential materials for magnetic cooling at or near room temperature. Cryst. Eng. 5, 383–389 (2002)
Zimm, C. B., Jastrab, A., Sternberg, A., Pecharsky, V. K., Gschneidner Jr., K. A., Osborne, M., Anderson, I.: Description and Performance of a Near-Room Temperature Magnetic Refrigerator. Adv. Cryog. Eng. 43, 1759–1766 (1998)
Das, S., Dey, T. K.: Magnetic entropy change in polycrystalline La1−x K x MnO3 perovskites. J. Alloys Compd. 440, 30–35 (2007)
Das, S., Dey, T. K.: Above room temperature magnetocaloric properties of La0.7Ba0.3−z Na z MnO3 compounds. Mater. Chem. Phys. 108, 220–226 (2008)
Cherif, R., Hlil, E. K., Ellouze, M., Elhalouani, F., Obbade, S.: Magnetic and magnetocaloric properties of La0.6Pr0.1Sr0.3Mn1−x Fe x O 3 (0≤x≤0.3) manganites. J. Solid Stat. Chem. 215, 271–276 (2014)
Dan’kov, S. Y., Tishin, A. M., Pecharsky, V. K., Gschneidner, K. A.: Magnetic phase transitions and the magnetothermal properties of gadolinium. Phys. Rev. B 57, 3478–3490 (1998)
Pan, M. H., Yu, S. C., Hur, N. H.: Excellent magnetocaloric properties of La0.7Ca0.3−x Na x MnO3 0.05≤x≤0.25 single crystals. Appl. Phys. Lett. 86, 072504–3 (2005)
Anwar, M. S., Ahmed, F., Koo, B. H.: Structural distortion effect on the magnetization and magnetocaloric effect in Pr modified La0.65Sr0.35MnO3 manganite. J. Alloys Compd. 617, 893–898 (2014)
Cheikh-Rouhou Koubaa, W., Koubaa, M., Cheikhrouhou, A.: Effect of potassium doping on the structural, magnetic and magnetocaloric properties of La0.7Sr0.3−x K x MnO3 perovskite manganites. J. Alloys Compd. 470, 42–46 (2009)
Çetin, S. K., Acet, M., Ekicibil, A., Sarıkürkçü, C., Kıymaç, K.: Reversibility in the adiabatic temperature change of Pr0.73Pb0.27MnO3. J. Alloys Compd. 565, 139–143 (2013)
Ur. Rehman, Z., Anwar, M. S., Koo, B. H.: Influence of Barium Doping on the Magnetic and Magnetocaloric Properties of Pr1−x Ba x MnO3. J. Supercond. Nov. Magn. 28, 1629–1634 (2015)
Righi, L., Gorria, P., Insausti, M., Gutiérrez, J., Barandiaran, J. M.: Influence of Fe in giant magnetoresistance ratio and magnetic properties of La0.7Ca0.3Mn1−x Fe x O 3 perovskite type compounds. J. Appl. Phys. 81, 5767–5769 (1997)
Maignan, A., Damay, F., Martin, C., Raveau, B.: Nickel-induced metal-insulator transition in the small a cation manganites Ln0.5Ca0.5MnO3. Mater. Res. Bull. 32, 965–972 (1997)
Jirák, Z., Hejtmánek, J., Knízek, K., Sonntag, R.: Structure and properties of the Pr1−x K x MnO3 perovskites (x=0−0.15). J. Solid Stat. Chem. 132, 98–106 (1997)
Jirák, Z., Hejtmánek, J., Knízek, K., Maryško, M., Pollert, E., Dlouhá, M., Vrastislav, S., Kužel, R., Hervieu, M.: Structure and magnetism in the Pr1−x Na x MnO3 perovskites (0≤x≤0.2). J. Magn. Magn. Mater. 250, 275–287 (2002)
Zouari, S., Cheikh-Rouhou, A., Strobel, P., Pernet, M., Pierre, J.: Structural and magnetic properties of alkali-substituted praseodymium manganites Pr1−x A x MnO3 (A = Na, K). J. Alloys Compd. 333, 21–27 (2002)
Thaljaoui, R., Boujelben, W., Pȩkała, M., Pȩkała, K., Fagnard, J.-F., Vanderbemden, P., Donten, M., Cheikhrouhou, A.: Magnetocaloric effect of monovalent K doped manganites Pr0.6Sr0.4−x K x MnO3 (x = 0 to 0.2). J. Magn. Magn. Mater. 352, 6–12 (2014)
Reis, M. S., Amaral, V. S., Araújo, J. P., Tavares, P. B., Gomes, A. M., Oliveira, I. S: Magnetic entropy change of Pr1−x Ca x MnO3 manganites (0.2≤x≤0.95). Phys. Rev. B 71, 144413–144418 (2005)
Chen, P., Du, Y. W., Ni, G.: Low-field magnetocaloric effect in Pr0.5Sr0.5MnO3. Europhys. Lett. 52, 589–593 (2000)
Alonso, J. A., Casais, M. T., Martinez-Lope, M. J., Martinez, J. L., Fernandez-Diaz, M. T.: A structural study from neutron diffraction data and magnetic properties of RMn2O5 (R = La, rare earth). J. Phys. Condens. Matter 9, 8515–8526 (1997)
Shindea, K. P., Deshpande, N. G., Eom, T., Lee, Y. P., Pawara, S. H.: Solution-combustion synthesis of La0.65Sr0.35MnO3 and the magnetocaloric properties. Mater. Sci. Eng. B 167, 202–205 (2010)
Shannon, R. D.: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976)
Ahmed, A. M., Papavassiliou, G., Mohamed, H. F., Ibrahim, E. M. M.: Structural, magnetic and electronic properties on the Li-doped manganites. J. Magn. Magn. Mater. 392, 27–41 (2015)
Thaljaoui, R., Boujelben, W., Pȩkała, M., Pociecha, D., Syzdłowska, J., Cheikhrouhou, A.: Room temperature magnetocaloric and magneto-transport properties of monovalent doped Pr0.6Sr0.35Na0.05MnO3 manganite. J. Alloys Compd. 530, 138–143 (2012)
Das, S., Dey, T. K.: Structural and magnetocaloric properties of La1−y Na y MnO3 compounds prepared by microwave processing. J. Phys. D: Appl. Phys. 40, 1855–1863 (2007)
Bohigas, X., Tejada, J., Del Barco, E., Zhang, X. X., Sales, M.: Tunable magnetocaloric effect in ceramic perovskites. Appl. Phys. Lett. 73, 390–392 (1998)
Ho, T. A., Dang, N. T., Phan, T. L., Yang, D. S., Lee, B. W., Yu, S.C.: Magnetic and magnetocaloric properties in La0.7Ca0.3−x Na x MnO3 exhibiting first-order and secondorder magnetic phase transitions. J. Alloys Compd. (2016) doi:10.1016/j.jallcom.2016.03.156
Nam, D. N. H., Mathieu, R., Nordblad, P., Khiem, N. V., Phuc, N. X.: Ferromagnetism and frustration in Nd0.7Sr0.3MnO3. Phys. Rev. B 62, 1027 (2000)
Freitas, R.S., Ghivelder, L., Damay, F., Dias, F., Cohen, L.F.: Magnetic relaxation phenomena and cluster glass properties of La0.7−x Y x Ca0.3MnO3 manganites. Phys. Rev. B 64, 144404 (2001)
Radaelli, P. G., Iannone, G., Marezio, M., Hwang, H. Y., Cheong, S. -W., Jorgensen, J. D., Argyriou, D. N.: Structural effects on the magnetic and transport properties of perovskite A\(_{1-x}\textit {A}_{x}^{\prime }\)MnO3 (x=0.25,0.30). Phys. Rev. B 56, 8265–8276 (1997)
Anwar, M. S., Kumar, S., Ahmed, F., Heo, S. N., Kim, G. W., Koo, B. H.: Study of magnetic entropy change in La0.65Sr0.35Cu0.1Mn0.9 O 3 complex perovskite. J. Electroceramics 30, 46–50 (2013)
Mtiraoui, N., Dhahri, J., Oumezine, M., Dhahri, E.: Magnetic and Magnetocaloric Properties of La0.67Pb0.33−x Ag x MnO3 Compounds. Supercond. Nov. Magn. 25, 1937–1945 (2012)
Raju, K., Pavan Kumar, N. P., Venugopal Reddy, P. V., Yoon, D. H.: Influence of Eu doping on magnetocaloric behavior of La0.67Sr0.33MnO3. Phys. Lett. A. 379, 1178–1182 (2015)
Ayas, A. O., Akyol, M., Cetin, S. K., Akca, G., Ekicibil, A., Ozcelik, B.: Magnetocaloric Properties of La0.85Ag0.15MnO3 and (La0.80Pr0.20)0.85Ag0.15MnO3 Compounds. J. Supercond. Nov. Magn. 28, 1649–1658 (2015)
Ayas, A. O., Akyol, M., Ekicibil, A.: Structural and magnetic properties with large reversible magnetocaloric effect in (La1−x Pr x )0.85Ag0.15MnO3 (0.0≤x≤0.5) compounds. Philos. Mag. 96, 922–937 (2016)
Bebenin, N. G., Zainullina, R. I., Ustinov, V. V., Mukovskii, Y. M.: Magnetic properties of La0.7−x Pr x Ca0.3MnO3 single crystals: When is Banerjee criterion applicable?. J. Magn. Magn. Mater. 354, 76–80 (2014)
Banerjee, S. K.: On a generalised approach to first and second order magnetic transitions. Phys. Lett. 12, 16–17 (1964)
Foldeaki, M., Chahine, R., Bose, T. K.: Magnetic measurements: A powerful tool in magnetic refrigerator design. J. Appl. Phys. 77, 3528–3537 (1995)
Zhang, P., Yang, H., Zhang, S., Ge, H., Pan, M.: Effect of Li doping on the magnetic and magnetocaloric properties of Pr0.5Sr0.5−x Li x MnO3 (0≤x≤0.3). J. Magn. Magn. Mater. 334, 16–20 (2013)
Suemitsu, M., Nakagawa, T., Hirayama, Y., Seino, S., Yamamoto, T. A.: Magnetocaloric effect of La0.7−x Pr x Ca0.3MnO3 perovskites. J. Alloys Compd. 551, 195–199 (2013)
Amaral, J. S., Reis, M. S., Amaral, V. S., Mendonça, T. M., Araújo, J. P., Tavares, P. B., Vieira, J. M.: Magnetocaloric effect in Er- and Eu-substituted ferromagnetic La-Sr manganites. J. Magn. Magn. Mater. 290–291, 686–689 (2005)
Amaral, V S, Amaral, J S: Magnetoelastic coupling influence on the magnetocaloric effect in ferromagnetic materials. J. Magn. Magn. Mater. 272, 2104–2105 (2004)
Anwar, M. S., Kumar, S., Ahmed, F., Arshi, N., Kim, G. W., Koo, B. H.: Above room temperature magnetic transition and magnetocaloric effect in La0.66Sr0.34MnO3. J. Korean Physical Society 60, 1587–1592 (2012)
Varvescu, A., Deac, I. G.: Critical magnetic behavior and large magnetocaloric effect in Pr0.67Ba0.33MnO3 perovskite manganite. Physica B 470-471, 96–101 (2015)
Tishin, A. M., Spichkin, Y. I.: The magnetocaloric effect and its applications. Institute of Physics Publishing, Bristol, UK (2003)
Gschneidner, K. A., Pecharsky, V. K.: Magnetocaloric Materials. Annu. Rev. Mater. Sci. 30, 387–429 (2000)
Debnath, J. C., Zeng, R., Kim, J. H., Dou, S. X.: Improvement of refrigerant capacity of La0.7Ca0.3MnO3 material with a few percent Co doping. J. Magn. Magn. Mater. 323, 138–143 (2011)
Acknowledgments
This work is supported by the Research Fund of Adıyaman University, Adıyaman, Turkey, under grant contract no. FEFBAP/2014-0008 and the Research Fund of Çukurova University, Adana, Turkey, under grant contract nos. FBA-2015-5028 and FEF2012D12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akça, G., Ayaş, A.O., Çetin, S.K. et al. Effect of Monovalent Cation Doping on Structural, Magnetic, and Magnetocaloric Properties of Pr0.85 A 0.15MnO3 (A = Ag and K) Manganites. J Supercond Nov Magn 30, 1515–1525 (2017). https://doi.org/10.1007/s10948-016-3946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3946-8