Abstract
Barium hexagonal ferrites (BaNd x Fe12−x O 19) have been synthesized by initial high-energy milling of the precursors and calcining subsequently. The as-prepared samples are characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and vibrating sample magnetometry (VSM). XRD and SEM examinations reveal that a high-crystallized hexagonal BaNd x Fe12−x O 19 with lamellar morphology is obtained when the precursor is calcined at 1200∘C in air for 3 h. The hexagonal crystalline structure of BaFe12 O 19 is not changed after doping Nd3+ ions in BaFe12 O 19. However, lattice parameters a and b values increase with an increase in Nd content at first, then decrease. Nd substitution may improve the magnetic properties of BaNd x Fe12−x O 19. BaNd0.1Fe11.9 O 19, obtained at 1050∘C, has the highest specific saturation magnetization value (80.81 emu/g) and magnetic moment (16.21 μ B); BaNd0.2Fe11.8 O 19, obtained at 950∘C, has the highest coercivity value, 4075.19 Oe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ferrimagnetic materials have three types, which are spinel, garnet, and hexaferrites. Among them, synthesis and structural characterization of hexaferrites have received a considerable amount of attention due to the diversity of structure types as well as to their potential applications, such as multiple-state memory elements, novel memory media, transducers, and new functional sensors [1–3]. Hexaferrites are classified into five types, including M, W, Y, X, and Z type hexaferrites with general formulae BaFe12 O 19, BaMe2Fe16 O 27, BaMe2Fe12 O 22, Ba2Me2Fe28 O 46, and Ba2Me2Fe24 O 41, respectively, where Me represents any divalent element [4]. BaFe12 O 19 is one of the M type hexaferrites, which the unit cell contains 38 O2− ions, 2 Ba2+ ions, and 24 Fe3+ ions. Sixteen Fe3+ ions with upward spin are located in three octahedral sites (2a, 12k, and 2b), whereas the remaining eight Fe3+ ions with downward spin are at the tetrahedral and trigonal bipyramidal sites (4f1 and 4f2) with a net magnetic moment of 40 μ B per unit cell [5, 6]. The molecular unit of M type hexaferrites is made of one S and one R block, with an overlap of hexagonally and cubically packed layers; the S block consists of two spinel units and the R block consists of three hexagonal units [4]. BaFe12 O 19 is a very important hard magnetic material, which has many unique properties, such as large specific saturation magnetization (Ms) and coercivity (Hc), high values for the magnetic anisotropy field (HA) and Curie temperature, great chemical stability, and excellent high-frequency microwave absorption materials [7, 8]. Substitutions of Fe3+ in BaFe12 O 19 with other metal ions lead to net magnetic moment change per unit and tailor magnetic properties of barium hexaferrites. Therefore, doped barium hexaferrites caused great concern.
Various methods of synthesizing BaFe12 O 19 and doped BaFe12 O 19 with different magnetic properties have been developed, including high-energy milling method [9–11], solid-state reaction method [12–14], co-precipitation method [15, 16], hydrothermal treatment [7], sol–gel synthesis [17, 18], citrate precursor [19, 20], self assembly method [21], etc. The magnetic properties of hexaferrite samples depend strongly on composition and synthesis conditions. For example, Venkateswaran et al. [9] synthesized barium hexagonal ferrite (BaFe12 O 19) by initial high-energy milling of the precursors, followed by sintering at 950∘C in air for 5 h. Specific saturation magnetizations (Ms), remanence (Mr), and coercivity (Hc) of the samples are 45 emu/g, 29 emu/g, and 4500 Oe, respectively. Singh et al. [17] prepared BaLa x Fe12−x O 19 nanohexaferrites via the sol–gel auto-combustion technique. Specific saturation magnetizations of BaLa x Fe12−x O19 increase from 72.00 to 78.72 emu/g when doped La content is increased from x = 0.05 to x = 0.25. Thakur et al. [19] synthesized Sm-doped Ba–Co hexaferrite with composition BaCo0.8Sm x Fe(11.2−x) O 19 (x = 0.2, 0.4, and 0.6) via a citrate precursor method. The BaCo0.8Sm0.2Fe11 O 19 sample, sintered at 900∘C, has the highest specific saturation magnetization (32.55 emu/g) and the highest coercivity (2690.20 Oe) value. However, to the best of our knowledge, the synthesis and magnetic properties of BaNd x Fe12−x O 19 by thermal decomposition of oxalates have not been reported in previous studies.
This study aims to prepare BaNd x Fe12−x O 19 by calcining oxalates in air and study the effect of composition and calcination temperature on magnetic properties of BaNd x Fe12−x O 19. Our results clearly show that the magnetic properties, in particular the specific magnetizations (Ms) and coercivity (Hc) of BaNd x Fe12−x O 19, can be improved after doping Nd3+ ions.
2 Experimental Procedures
All chemicals used are of reagent-grade purity (purity >99.9 %). The BaNd x Fe12−x O 19 was prepared by initial planetary ball milling of the precursors and calcining subsequently. In a typical synthesis (BaFe12 O 19), 1.18 g BaC2 O 4⋅ 2H2O, 9.71 g FeC2O ⋅ 2H2O, and 10 ml ethanol were added to a stainless steel ball milling tank of 100 ml. The mass ratio of the sample to the stainless steel ball is about 1/15. Samples were milled at room temperature for 30 min. The grinding velocity was about 350 circles/min. BaFe12 O 19 precursor was obtained after being dried at 80∘C in air for 5 h. A similar synthesis procedure was used to synthesize other BaNd x Fe12−x O 19 precursor. Finally, the BaNd x Fe12−x O 19 precursor was calcined over 950∘C for 3 h at a heating rate of 2∘C min−1 in air to produce hexagonal BaNd x Fe12−x O 19.
X-ray diffraction (XRD) of the prepared sample was carried out using an X ′pert PRO diffractometer equipped with a graphite monochromator and a Cu target. Radiation applied was Cu K α (λ = 0.15406 nm), operating at 40 kV and 50 mA. XRD scans were conducted from 5 to 75∘ in 2 𝜃, with a step size of 0.01∘. The morphologies of the synthesis products were observed using a S-3400 scanning electron microscope (SEM). The magnetic properties of the samples were studied by vibrating sample magnetometer (VSM, Lake Shore 7410) at room temperature (RT).
3 Results and Discussion
3.1 XRD and SEM Analyses of the Calcined Products
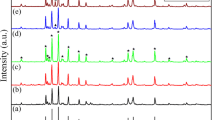
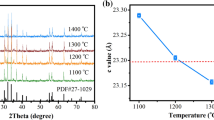
Figure 1 shows XRD patterns of BaNd x Fe12−x O 19 calcined at different temperatures for 3 h. Characteristic diffraction peaks of hexagonal BaFe12 O 19 and an unknown phase appeared when BaNd x Fe12−x O 19 precursors were calcined at 950∘C. Characteristic diffraction peaks of the unknown phase become weak and/or disappeared with the increase in calcination temperature. When BaNd x Fe12−x O 19 precursors were calcined at 1200∘C for 3 h, all diffraction peaks in the pattern were in accord with those of hexagonal BaFe12 O 19 with space group P63/mmc(194) from PDF card 43-0002 except for a weak diffraction peak at 28.39∘ for 2 𝜃. The Nd3+-doped ions do not change the hexagonal crystalline structure of BaFe12 O 19 except that the diffraction peaks slightly shift (Fig. 1e). The lattice parameters of the sample were refined by the Rietveld analysis using MDI Jade (ver. 5.0) software. The refined lattice parameters of BaNd x Fe12−x O 19, obtained at 1200∘C, are shown in Table 1. With increase in Nd doping from x = 0, 0.1, 0.2, to 0.3, lattice parameters (a and b values) increase at first, then decrease; the c value decreases at first, then increases. This could be due to the larger Nd3+ ion (0.099 nm) [22] substituting the Fe3+ ion in a and b axes (with a much smaller ionic radius of 0.067 nm) [23] initially, and then for a higher doping level (x = 0.3), some of the Ba2+ ions (0.161 nm) [24] could enter the c axis.
The crystallite size of BaNd x Fe12−x O 19 is estimated using the following Scherrer formula [25]:
where D is the crystallite size, K = 0.89 (the Scherrer constant), λ = 0.15406 nm (wavelength of the X-ray used), βis the width at half-maximum intensity, and 𝜃 is the corresponding angle. The d (114) interplanar spacing of BaNd x Fe12−x O 19 is determined using the Bragg equation [25]:
The crystallite size (D) of BaNd x Fe12−x O 19, obtained at different temperatures, and d (114) interplanar spacing of BaNd x Fe12−x O 19, obtained at 1200∘C, are shown in Figs. 2 and 3, respectively From Fig. 2, the crystallite size of BaNd x Fe12−x O 19, obtained at 1200∘C, exhibits non-linear variation; the crystallite size of BaNd x Fe12−x O 19 is between 47.8 and 75.7 nm. By contrast, d (114) interplanar spacing of BaNd x Fe12−x O 19 decreases with the increase in Nd content (Fig. 3).
The crystallinity of BaNd x Fe12−x O 19 can be estimated by MDI Jade 5.0 software. The crystallinity of BaNd x Fe12−x O 19 (x = 0, 0.1, 0.2, and 0.3), obtained at different temperatures, is shown in Fig. 4. The crystallinities of BaNd x Fe12−x O 19, obtained at 1200∘C, decrease with increase in Nd content.
Lattice strains of the BaNd x Fe12−x O 19 are determined using the following Williamson–Hall formula [25]:
where β is the full-width at half maximum (in radian) of the peaks, 𝜃 is the peak position, and ε is the lattice strain of the structure. Lattice strains of BaNd x Fe12−x O 19, obtained at 1200∘C, are shown in Fig. 5. The lattice strain of BaNd x Fe12−x O 19 increases with an increase in Nd content (0 ≤ x ≤ 0.2) at first and then decreases (x = 0.3). The lattice strain exists in the BaFe12 O 19, attributed that Ba2+ (0.161 nm) [24] and Fe2+ (0.067 nm) [23] ions have different ionic radii and/or charge, resulting in the distortion of octahedral, tetrahedral, and trigonal bipyramidal. The substitution of Fe3+ ions in a and b axes of the hexagonal by Nd3+ ions with larger ionic radii can increase the distortion of hexagonal in BaNd x Fe12−x O 19, resulting in the increase of lattice strain in BaNd x Fe12−x O 19 with increasing Nd content. However, for a higher doping level (x = 0.3), the replacement of some Fe3+ ions in the c axis by Nd3+ ions decreases the distortion in BaNd x Fe12−x O 19, resulting in the decrease of lattice strain.
The morphologies of the calcined products are shown in Fig. 6. BaNd x Fe12−x O 19 (x = 0, 0.1, 0.2, and 0.3) samples, obtained at 1050 and 1200∘C, are composed of approximately lamellar grains. The particle size increases with an increase of calcination temperature. The particle size of BaNd x Fe12−x O 19, obtained at 1200∘C, is mainly between 500 nm and 3 μ m.
3.2 Magnetic Properties of BaNd x Fe12−x O 19
Figure 7 shows RT magnetic hysteresis loops of the as-prepared BaNd x Fe12−x O 19 samples. Effects of Nd content and calcination temperature on specific magnetization are presented in Fig. 8. Dependence of specific saturation magnetizations of BaNd x Fe12−x O 19 on Nd content exhibits non-linear variation. BaNd0.1Fe11.9 O 19, obtained at 1050∘C, has the highest specific saturation magnetization value (80.81 emu/g); BaNd0.3Fe11.7 O 19, obtained at 1200∘C, has the lowest specific saturation magnetization value (64.22 emu/g). The evolution of specific saturation magnetization of BaNd x Fe12−x O 19, obtained at 1200∘C, can be explained as follows: The magnetic moments per ion for Ba2+, Nd3+, and Fe3+ ions are 0, 3, and 5 μ B, respectively. When Fe3+ ions in hexagonal BaFe12 O 19 are partially substituted by Nd3+ ions, Nd3+ ions preferentially fill the tetrahedral and trigonal bipyramidal sites, resulting in the increase in the net magnetic moment and/or specific saturation magnetization of BaNd x Fe12−x O 19 with the increase of Nd content initially, and then for a higher doping level (x = 0.3), some of the Nd3+ could enter the octahedral sites, resulting in the decrease of the net magnetic moment and/or specific saturation magnetization of BaNd x Fe12−x O 19.
Dependence of remanence (Mr) and coercivity (Hc) on Nd content and calcination temperature is shown in Fig. 9. From Fig. 9a, remanences of BaNd x Fe12−x O 19 decrease with an increase in calcination temperature except for 950∘C; that of BaNd x Fe12−x O 19, obtained at 1200∘C, decreases with an increase in Nd content. By contrast, coercivity of BaNd x Fe12−x O 19 decreases with an increase in calcination temperature (Fig. 9b). Dependence of coercivity of BaNd x Fe12−x O 19 on Nd content exhibits non-linear variation. BaNd0.2Fe11.8 O 19, obtained at 950∘C, has the highest coercivity value (4075.19 Oe); BaNd0.2Fe11.8 O 19, obtained at 1200∘C, has the lowest coercivity value (481.2 Oe). Dependence of squareness (Mr/Ms) on Nd content is shown in Fig. 10a. Squareness (Mr/Ms) of BaNd x Fe12−x O 19 decreases with an increase of calcination temperature; that of BaNd x Fe12−x O 19, obtained at 1200∘C, decreases with an increase in Nd content. BaNd0.3Fe11.7 O 19 has the lowest Mr/Ms, 0.3625.
The magnetic moment of BaNd x Fe12−x O 19 samples obtained at 1200∘C is estimated using the following formula [26]:
where M is the molecular weight of the composition, Ms is the specific saturation magnetization (emu/g), and η B is the magnetic moment (μ B). The results show that dependence of the magnetic moment of BaNd x Fe12−x O 19 on Nd content exhibits non-linear variation (Fig. 10b). BaNd0.1Fe11.9 O 19, obtained at 1050∘C, has the largest magnetic moment value (16.21 μ B); BaNd0.3Fe11.7 O 19, obtained at 1200∘C, has the lowest magnetic moment value (13.09 μ B).
4 Conclusions
Barium hexagonal ferrites (BaNd x Fe12−x O 19) have been successfully synthesized by ball milling a mixture of oxalates at first, followed by calcination in air. XRD and SEM examinations indicate that a high-crystallized hexagonal BaNd x Fe12−x O 19 with lamellar morphology is obtained when the precursor is calcined above 950∘C in air for 3 h. The hexagonal crystalline structure of BaFe12 O 19 is not changed after doping Nd3+ ions in BaFe12 O 19. However, lattice parameters a and b values increase at first, then decrease; the c value decreases at first, then increases. The lattice strain of BaNd x Fe12−x O 19 increases with an increase in Nd content (0 ≤ x ≤ 0.2) at first and then decreases (x = 0.3). Specific saturation magnetization and the magnetic moment of BaNd x Fe12−x O 19 exhibit non-linear variation. Nd substitution may improve magnetic properties of BaNd x Fe12−x O 19. BaNd0.1Fe11.9 O 19, obtained at 1050∘C, has the highest specific saturation magnetization value (80.81 emu/g) and magnetic moment (16.21 μ B); BaNd0.2Fe11.8 O 19, obtained at 950∘C, has the highest coercivity value, 4075.19 Oe.
References
Tawfik, A., Hemeda, O.M., El-Sayed, A.H., Hamad, M.A.: J. Supercond. Nov. Magn. 29, 2085–2088 (2016)
Jaafar, A., Almisaeed, A., Souier, T., Bououdina, M.: J. Supercond. Nov. Magn. (2016). doi:10.1007/s10948-016-3593-0
Torkian, S., Ghasemi, A., ShojaRazavi, R., Tavoosi, M.: J. Supercond. Nov. Magn. 29, 1627–1640 (2016)
Pawar, R.A., Desai, S.S., Tamboli, Q.Y., Shirsath, S.E., Patange, S.M.: J. Magn. Magn. Mater. 378, 59–63 (2015)
Verma, S., Pandey Jr., O.P., A.P., Sharma, P.: Physica B 448, 57–59 (2014)
Wu, C.J., Yu, Z., Yang, Y., Sun, K., Nie, J.L., Liu, Y., Jiang, X.N., Lan, Z.W.: J. Alloy. Compd. 664, 406–410 (2016)
Wang, J., Wu, Y.J., Zhu, Y.J., Wang, P.Q.: Mater. Lett. 61, 1522–1525 (2007)
Mohsen, Q.: J. Alloy. Compd. 500, 125–128 (2010)
Manikandan, M., Venkateswaran, C.: J. Magn. Magn. Mater., 82–86 (2014). 358–359
Shams, M.H., Rozatian, A.S.H., Yousefi, M.H., Valíček, J., Šepelák, V.: J. Magn. Magn. Mater. 399, 10–18 (2016)
Kaynar, M.B., Özcana, Ş., Shah, S.I.: Ceram. Int. 41, 11257–11263 (2015)
Trukhanov, A.V., Turchenko, V.O., Bobrikov, I.A., Trukhanov, S.V., Kazakevich, I.S., Balagurov, A.M.: J. Magn. Magn. Mater. 393, 253–259 (2015)
El-Sayed, S.M., Meaz, T.M., Amer, M.A., El Shersaby, H.A.: Physica B 426, 137–143 (2013)
Yang, H.B., Liu, M., Lin, Y., Dong, G.Q., Hu, L.Y., Zhang, Y., Tan, J.Y.: Mater. Chem. Phys. 160, 5–11 (2015)
Mosleh, Z., Kameli, P., Ranjbar, M., Salamati, H.: Ceram. Int. 40, 7279–7284 (2014)
Amer, M.A., Meaz, T.M., Attalah, S.S., Ghoneim, A.I.: Mater. Sci. Semicond. Process. 40, 374–382 (2015)
Pratap Singh, V., Kumar, G., Kumar, A., Rai, R.S., Valente, M.A., Batoo, K.M., Kotnala, R.K., Singh, M.: Ceram. Int. 42, 5011–5017 (2016)
Chavan, V.C., Shirsath, S.E., Mane, M.L., Kadam, R.H., More, S.S.: J. Magn. Magn. Mater. 398, 32–37 (2016)
Rana, K., Thakur, P., Thakur, A., Tomar, M., Gupta, V., Mattei, J.L., Queffelec, P.: Ceram. Int. 42, 8413–8418 (2016)
Ahmed, M.A., Mansour, S.F., Ismael, H.: J. Magn. Magn. Mater. 378, 376–388 (2015)
Choi, M., Cho, S., Song, Y., Baek, S., Kim, H., Jung, J., Lee, H., Park, C., Park, S., kim, Y.: Curr. Appl. Phys. 14, 1208–1211 (2014)
Yildirim, S., Yurddaskal, M., Dikici, T., Aritman, I., Ertekin, K., Celik, E.: Ceram. Int. 42, 10579–10586 (2016)
Huang, X.S., Zhou, Y., Wu, W.W., Xu, J.W., Liu, S.Q., Liu, D.S., Wu, J.: J. Electron. Mater. 45, 3113–3120 (2016)
Manoun, B., Ezzahi, A., Benmokhtar, S., Ider, A., Lazor, P., Bihd, L., Igartua, J.M.: J. Alloy. Compd. 533, 43–52 (2012)
Huang, X.S., Chen, W., Wu, W.W., Zhou, Y., Wu, J., Wang, Q., Chen, Y.Y.: J. Mater. Sci. - Mater. Electron. 27, 5395–5402 (2016)
Chen, W., Zhou, Y., Lu, J.Y., Huang, X.S., Wu, W.W., Lin, C.W., Wang, Q.: Ceram. Int. 42, 1114–1121 (2016)
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant no. 21161002, 21603040), the Guangxi Natural Science Foundation of China (Grant no. 2016GXNSFDA380034, 2016GXNSFBA380062), and the Guangxi University Student Innovation Foundation of China (Grant no. 30).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Wu, W., Mao, M. et al. Improvement of the Magnetization of Barium Hexaferrites Induced by Substitution of Nd3+ Ions for Fe3+ Ions. J Supercond Nov Magn 30, 707–714 (2017). https://doi.org/10.1007/s10948-016-3886-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3886-3