Abstract
Rodlike ZSM-5 zeolite microspheres (R-Z5) were synthesized via a seed induced hydrothermal method with S-1 seeds in the presence of β-cyclodextrin (CD). The synthesized ZSM-5 zeolites were characterized with XRD, SEM, N2 adsorption and desorption, NH3-TPD, the catalytic performance of R-Z5 catalysts was studied for the methanol to propylene (MTP) reaction. The effects of the ratio of CD/SiO2 on the morphologies and textural properties of the products were discussed in detail. A possible formation process of R-Z5 was proposed. The results showed that CD played a key role in the preparation of R-Z5, which could be ascribed to the amphipathy of CD for the formation of rodlike ZSM-5 crystals. Compared with the conventional seed induced method, R-Z5 zeolites possessed the larger BET surface area and mesopore volumes and the more moderate acidic sites. Moreover, the synthesized R-Z5 catalysts exhibited excellent conversion activity (64 h) and propylene selectivity (41.35%) in the primary catalytic performance of MTP reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Propylene is one of the most essential organic chemical materials in petrochemical industries, mainly produced via naphtha steam cracking and fluid catalytic cracking [1,2,3,4]. In recent years, with the increasing demand for petrochemical materials, traditional routes seem unable to fill the projected propylene gap in the markets. The methanol to propylene (MTP) process developed by Lurgi, as an alternative route to produce propylene from non-petroleum sources such as coal, biomass and natural gas, has attracted significant attention [5,6,7,8]. ZSM-5 zeolite catalyst has been proven to be one of the most promising catalysts for the MTP reaction, which has unique topological structure, adjustable acidity and high hydrothermal stability due to its MFI structure with a three-dimensional 10-membered ring micropore [9,10,11,12,13,14]. However, deposition of coke and catalyst deactivation will occur in the catalytic reaction process, which tends to suffer from an intracrystalline diffusion limitation and a rapid deactivation, it is still a huge challenge to further improve catalytic lifetime and propylene selectivity of MTP reaction over ZSM-5 catalysts [15].
To solve the above problems, the composited ZSM-5 zeolites containing mesopores as well as micropores have been prepared in recent years. Some secondary mesoscale templates have been added in the preparation process, such as sucrose [16], rectorite [17], gemini-type triquaternary ammonium surfactant [18]. As a result, the topography, nature, number and strength of acid sites are changed for mesopore-containing ZSM-5 zeolites. Therefore, molecule diffusion and coke resistance are improved, enhanced catalytic performance are shown in methanol conversion reactions [19,20,21]. We also have made a great effort to the preparation of hierarchical ZSM-5 in our previous work, aggregates of nano-sized ZSM-5 crystals have been synthesized by adding cetyltrimethyl ammonium bromide (CTAB) with a seed-induced method, the synthesized ZSM-5 catalysts with abundant mesopores have revealed higher catalytic activity compared with conventional ZSM-5 [22, 23]. Although there are many ways to prepare hierarchical ZSM-5 zeolites up to now, the catalytic lifetime and propylene selectivity are needed to improve for MTP reaction to satisfy the practical production [10].
In this work, we have prepared mesopore-containing rodlike ZSM-5 zeolite microspheres (R-Z5) by a seed-induced method in the presence of β-cyclodextrin (CD), this process is relatively simple, low-cost and facile. The effect of CD on textural properties and catalytic performance of R-Z5 catalysts has been systematically characterized by XRD, SEM, N2 adsorption and desorption, NH3-TPD. The catalytic activity of R-Z5 has been evaluated on MTP reaction. The formation process of R-Z5 has also been proposed. The products have unique morphology, which are microsphere aggregates of rodlike ZSM-5, thus resulting in abundant mesopores and adjustable surface acidity. As a result, R-Z5 catalysts exhibited an excellent catalytic performance in MTP reaction.
2 Experimental
2.1 Preparation of R-Z5 samples
Silicate-1 (S-1) zeolites were synthesized following our previous work [3, 22]. The obtained S-1 zeolites were used directly as seeds for the following synthesis without purification.
R-Z5 samples were synthesized from the starting gels with the molar composition of 10 Na2O: 100 SiO2: 0.6 Al2O3: 2000 H2O: x CD, where x is the concentration of CD. A typical synthesis procedure is presented as followed: 0.1217 g NaAlO2 and 0.9298 g NaOH were added into 30 g H2O, and stirred for 10 min at room temperature. Then, 8.74 g S-1 seeds with respect to 8% of total SiO2 and 1.42 g CD were introduced into the clear solution, and 17.06 g silica sol was dropwise added. Then, the homogeneous aluminosilicate gel was formed after stirring for 1 h. The hydrogel was placed into a Teflon-lined steel autoclave and heated at 120 °C for 24 h and further at 170 °C for 12 h. The products were separated by centrifugation, repetitively washed with distilled water until the pH reached 8, dried at 120 °C for 12 h and calcined at 550 °C for 6 h. To obtain the H-type ZSM-5, the samples were ion exchanged three times in 1 M NH4Cl solution at 80 ºC for 2 h, and then calcined at 550 °C again. The obtained samples are denoted as R-Z5-x (x = 0, 0.5, 1, 1.5 and 2).
2.2 Characterization methods
Scanning electron microscopy (SEM) images of samples were obtained on an S-4800 field emission scanning electron microscope. X-ray diffraction (XRD) patterns were acquired on a Rigaku D/max2500 diffractometer. Fourier transform infrared (FT-IR) spectra were recorded on a Bruker Vertex 7.0 spectrometer. Nitrogen adsorption and desorption isotherms of the samples were measured at 77 K using a Micromeritics TriStar 3000 automated physisorption instrument. Temperature-programmed desorption of ammonia (NH3-TPD) measurements were recorded using a TP-5080D chemical adsorption instrument equipped with a thermal conductivity detector (Xianquan Industrial and Trading Co., Ltd). The elemental composition of ZSM-5 zeolites was measured by an inductively coupled plasma optical emission spectroscope (ICP-OES) using a Varian Vista-MPX emission spectrometer, the SiO2/Al2O3 ratio was calculated according to the elemental composition.
2.3 Catalytic performance
The MTP reaction was performed in a fixed bed microreactor at 480 °C under atmospheric pressure. The catalyst (0.5 g) with 20–40 mesh was loaded in a stainless steel reactor and activated before the test at 480 °C for 1 h in a N2 flow of 60 ml min− 1. The feed with aqueous solution of methanol (50 mol%) was injected into the reactor under N2 at a flow rate of 60 mL/min, and the weight hourly space velocity (WHSV) was kept constant at 6 h− 1. The products were analyzed using an on-line gas chromatograph (GC-SP-6800 A) equipped with a flame ionization detector and a capillary column (Agilent HP-PLOT-Q: 30 m × 0.32 mm × 20 μm). The reaction performance in terms of methanol conversion and product selectivity was subsequently evaluated.
3 Results and discussion
3.1 Physicochemical properties of R-Z5 samples
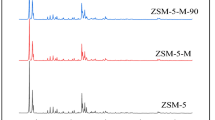
The XRD patterns for all R-Z5 samples synthesized with different CD/SiO2 ratios are presented in Fig. 1. The typical diffraction characteristic peaks observed at 2θ of 7.9°, 8.7°, 23.1°, 23.9° and 24.4° [24], corresponding to (101), (200), (332), (051) and (303), respectively [25, 26], reveal that the MFI structure is formed in all the samples [27]. Moreover, no other crystalline phase is observed. FT-IR analysis was carried out to further confirm the formation of MFI structure (shown in Fig. S1). The absorption bands at 450, 550, 1098 and 1220 cm− 1 are observed, which are due to the Si-O-Si bending, external symmetric stretching, internal and external asymmetric stretching vibrations of the five-membered rings [28]. As absorbance band around 550 cm− 1 does not appear in amorphous silica, therefore the optical density ratio of the absorbance bands at 550 cm− 1 and 450 cm− 1 (I550/I450) can be used to determine the crystallinity [29]. The I550/I450 ratio for R-Z5-1.5 sample is around 0.7, demonstrating well ZSM-5 phase.
SEM analysis was performed for all ZSM-5 samples, to investigate the effect of CD on the sizes and morphologies. The obtained SEM images are shown in Fig. 2. Although all the ZSM-5 samples have spherical shape structure, it exhibits that the sizes and morphologies have a large difference with varying the additive amount of CD. The average sizes of particles are 400 nm, 500 nm, 1 μm, 5 μm, 10 μm, respectively, which indicates the significant increase of particle sizes by adding a small quantity of CD. Similarly, the morphologies of samples display significant difference in the crystal growth direction. It is interesting to observe that the crystal growth is fast in b axis with the increase of CD. Especially the CD/SiO2 ratio is more than 0.01, as shown in Fig. 2c. Furthermore, Fig. 2d and e reveal that the particles of R-Z5-1.5 are the spherical aggregation of rodlike crystals. However, further increase the amount of CD (CD/SiO2 = 0.02), some impurities are observed, due to overmuch CD resulting in the amorphous phase of R-Z5-2. The result of SEM characterization demonstrates that CD has significant effect on the direction of crystal growth. As a result, the shape and size of ZSM-5 particles are significantly changed.
To date, the growth mechanism of zeolite crystals has not been explained exactly for the seed induced method. Nevertheless, the formation and growth of crystal nuclei are well known in the present. It is supposed that the addition of the amphipathic reagents is an efficient method to control the crystal growth. β-cyclodextrin (CD) as amphipathic reagent has hydrophobic inner cavity and hydrophilic shell, the crystal growth is inhibited by the hydrophobic cavity. As a result, the hydrophilic shell directs the formation of rodlike ZSM-5 crystals, and R-Z5 microspheres are obtained from the aggregation of rodlike ZSM-5 crystals.
3.2 Textural properties of zeolites
The N2 adsorption/desorption isotherms are plotted in Fig. 3. According to the IUPAC classification, the isotherms show that the ZSM-5 samples synthesized with CD exhibit a combination of type-I and IV isotherm with an apparent H-4 hysteresis loop at the P/P0 of 0.45–0.99 except that R-Z5-0 and R-Z5-2 exhibit a type-I isotherm with a micropore structure. The results demonstrate that the prepared samples with CD have abundant intercrystalline mesopores. In addition, the open mesopores connected to the external surface are observed because of largely parallel disposition of the adsorption and desorption branches of the hysteresis loop [30].
The texture properties of all the prepared samples are reported in Table 1. The micropore surface area and micropore volume of zeolites have no significant change after adding CD and increasing the content. More importantly, micropore volume of all the ZSM-5 samples is close to 0.10 cm3 g− 1, which demonstrates the well reservation of micropore structure after adding CD. The BET surface area, external surface area, total volume and mesopore volume all increase after the addition of CD, then decrease when CD/SiO2 ratio is more than 0.02. The larger mesopore volumes of R-Z5-1 (0.31 cm3 g− 1) and R-Z5-1.5 (0.35 cm3 g− 1) comparing to R-Z5-0 (0.15 cm3 g− 1) reveal the formation of abundant mesopores in the crystallization process, due to the aggregation of clubbed ZSM-5 crystals induced by adding CD.
3.3 Acidity of catalysts
The acidity is of great importance for H-ZSM-5 zeolites in MTP reaction, and moderate acidity is significant factor for product distribution, especially for the catalyst stability and propylene selectivity [31]. NH3-TPD profiles of the prepared ZSM-5 zeolites are as shown in Fig. 4. All the catalysts exhibit similar NH3-TPD profiles with the desorption peaks at both low temperature and high temperature, which are generally assigned to the desorbed NH3 from weak acidic sites and strong acidic sites respectively. To produce more propylene, the strength and amount of acidic sites should be adjusted. As seen in Fig. 4, after adding CD in the synthesis, both the peaks of weak and strong acidic sites shift to lower temperature for the R-Z5-0.5, R-Z5-1 and R-Z5-1.5 catalysts in NH3 desorption, the results indicate that the more moderate acidic sites are developed.
The acidic properties of all catalysts are reported in Table 2. The results show that densities of strong acidic sites are essentially the same for all catalysts. On the other hand, the densities of weak acidic sites increase after adding the right amount of CD resulting in the increase of total acid sites, and the R-Z5-1.5 has the most amounts of weak acidic sites with 0.195 mmol g− 1. In general, weak acidic sites contribute to side-chain methylation, which lead to olefinic products in the MTP catalysis, according to the hydrocarbon pool mechanism [32]. Therefore, the hydrogen transfer reactions are hindered, and the formation of aromatics and heavy hydrocarbons are prevented [15, 33]. The results demonstrate that the distribution of weak acidic sites is changed due to more Si-OH on the external surface by adding CD. The increased weak acidic sites and the decreased acid strength are expected to improve catalyst performance in the MTP process.
3.4 Catalytic performance in MTP reaction
The catalysts prepared with different levels of CD were evaluated for MTP reaction under the same condition in a fixed-bed reactor. Reaction would be suspended, when the methanol conversion decreased to below 90% or showed an observably downward trend. Accordingly, the lifetime of catalysts is obtained by the beginning time to the stopping time.
The catalytic lifetime and propylene selectivity of all catalysts in MTP reaction are presented in Fig. 5. The methanol conversion of all the catalysts shows a long stable stage with 100%. However, different catalysts display significant difference in the lifetime. The lifetime is only 37 h for R-Z5-0 catalyst. After the addition of CD, the lifetime is prominently enhanced. In other words, the lifetime of R-Z5-0.5 catalyst is noticeably raised to 52 h. And that, increasing the amounts of CD within certain limits leads to sustaining increase in the lifetime, 57 and 64 h for R-Z5-1 and R-Z5-1.5, respectively. However, excess addition of CD results in a decrease of the catalyst lifetime. As seen by Fig. 3, the lifetime is only 28 h for R-Z5-2. The lifetime of R-Z5 catalyst is much longer than that of Z5-0.1 C samples in the reported paper [3]. The improved catalytic stability is due to the increased mesoporosity and the more moderate acidic sites, which shortens the diffusion path and inhibits coke formation [34]. As have been reported by Ryoo, the improved catalytic performance can be attributed to the generation of mesopores and the tuned acidic properties [35]. On the other side, coke deposition is the main cause of catalyst deactivation in MTP process, and often results in the coverage of acidic sites, the partial acid sites within the micropores of ZSM-5 zeolite cannot be utilized, thus leading to the poor catalytic activity. In most cases, the coke is heavily deposited inside micropores for solely microporous zeolites. On the contrary, the coke formation occurs mainly in mesopore for the mesoporous zeolites, and the coke precursors are easily transferred to the external surface because of the short diffusion path lengths, thus the mesoporous zeolites have more toleration of the carbon deposition to improve the catalytic lifetime. In addition, as discussed earlier about acidic properties, hydrogen transfer reactions are effectively suppressed by the increasing of weak acid sites, and the formation of aromatics compounds is prevented. In a word, the catalysts synthesized by adding CD display a superior catalytic lifetime in MTP reaction.
The overall trend of propylene selectivity is also in agreement with that of the catalyst lifetime. As a result, the average propylene selectivity of the catalysts is increased by 37.11% of R-Z5-0 to 41.35% of R-Z5-1.5. The catalytic performances of MTP reaction for all R-Z5 catalysts are summarized in Table 3. The ethylene selectivity of catalysts shows contrary trend compared with that of propylene selectivity, i.e., the ethylene selectivity of R-Z5-0 (9.23%) is much higher than R-Z5-1.5 (7.59%). It should be noted that the P/E ratios are improved significantly by adding certain amount of CD, due to the increasing propylene selectivity and the decreased ethylene selectivity, the P/E ratio reaches up to 5.2 for R-Z5-1.5 catalyst. As is mentioned before, microporous ZSM-5 zeolite tends to undergo rapid coke deposits for acid-catalyzed MTP reaction, leading to the blockage of micropore channels and the deactivation of catalysts. The mesopore containing ZSM-5 catalyst has outstanding diffusivity, which facilitates the removal of intermediate products from the active center of catalysts, such as propylene molecules [36]. Thus, the more propylene is formed because of the reaction equilibrium shift, and the various secondary reactions are reduced, particularly the formation of higher olefins and aromatics from propylene [37]. On the other hand, the formation of products is based on two possible hydrocarbon pool cycles (olefin-based cycle and aromatic-based cycle) according to the hydrocarbon pool mechanism, the ethylene is formed from the aromatic-based cycle. However, the aromatic-based cycle is greatly weakened over R-Z5 catalysts due to the mesoporosity, leading to the decrease of ethylene selectivity [38]. Therefore, the higher P/E ratio is achieved with mesoporous ZSM-5 catalysts compared with the conventional ZSM-5 catalysts. As a result, the propylene selectivity as well as P/E ratio over R-Z5 catalysts is enhanced. The results of catalyst test indicate that the performance of catalysts is overall improved with the modified seed-induced method by adding CD as a mesoporous reagent, especially compared with the template method [39].
4 Conclusion
In summary, rodlike ZSM-5 zeolite microspheres (R-Z5) were synthesized by seed induced method in the present of β-cyclodextrin (CD). CD has a significant effect on the direction of crystal growth to change the shape and size of ZSM-5 zeolites, the morphology and acidity of R-Z5 catalysts highly depend on the additive amount of CD in synthesis. Interestingly, the obtained samples show the structure of microsphere aggregates assembled by rodlike crystals at CD/SiO2 ratio of 0.015. Abundant intercrystalline mesopores are formed to connect the micropore inside and the external surface. Both weak and strong acidic sites of R-Z5 catalysts are adjusted to the more moderate acidity. The excellent textural and acidic properties of R-Z5 catalysts are attributed to that CD acts as the guide role for crystal growth because of hydrophobic inner cavity and hydrophilic shell.
The R-Z5 catalysts display significantly improved performance as compared to conventional ZSM-5 (R-Z5-0). The catalytic lifetime and propylene selectivity in MTP reaction reach 64 h and 41.35% (vs. 37 h and 37.11%). This may be due to the mesopore structure and suitable acidity, which promotes the diffusion of product molecules and prevents the secondary reaction for coke formation. This work provides a facile and environment friendly strategy for controllable synthesis of hierarchical ZSM-5 zeolites, which are expected to be widely used as catalysts or adsorbents.
Data availability
No datasets were generated or analysed during the current study.
References
T.-L. Cui, L.-B. Lv, W.-B. Zhang, X.-H. Li, J. -S Chen Catal. Sci. Technol. 6, 5262–5266 (2016)
A. Zabihpour, J. Ahmadpour, F Yaripour Chem. Eng. Sci. 273, 118639 (2023)
H. Chen, Y. Wang, C. Sun, X. Wang, C Wang Catal. Commun. 112, 10–14 (2018)
M.A. Ali, S. Ahmed, N. Al-Baghli, Z. Malaibari, A. Abutaleb, Yousef Catal. Lett. 149, 3395–3424 (2019)
U. Olsbye, S. Svelle, M. Bjorgen, P. Beato, T.V. Janssens, F. Joensen, S. Bordiga, K P Lillerud Angew Chem. Int. Ed. Engl. 51, 5810–5831 (2012)
X. Wang, M. Wen, C. Wang, J. Ding, Y. Sun, Y. Liu, Y Lu Chem. Commun. 50, 6343–6345 (2014)
Y. Jiao, X. Yang, C. Jiang, C. Tian, Z. Yang, J. Zhang J. Catal. 332, 70–76 (2015)
L. Bu, Y. Wang, C. Sun, T. Zhao, J. Zhao, Z. Wang, W. Liu, J. Lu, S. Wu, M. Shi, J. Porous Mat. 29, 1165–1175 (2022)
S. Ilias, A. Bhan, ACS Catal. 3, 18–31 (2012)
W. Dai, L. Zhang, R. Liu, Z. Huo, W. Dai, N Guan Mater. Today Sustain. 22, 100364 (2023)
M. Firoozi, M. Baghalha, M Asadi Catal. Commun. 10, 1582–1585 (2009)
C. Sun, Y. Yang, J. Du, F. Qin, Z. Liu, W. Shen, H. Xu, Y Tang Chem. Commun. 48, 5787–5789 (2012)
M. Li, Y. Zhou, C. Ju, Y. Fang, Appl. Catal. A-Gen. 512, 1–8 (2016)
R. Feng, X. Yan, X. Hu, J. Wu, Z. Yan Micropor Mesopor Mater. 302, 110246 (2020)
H. Roohollahi, M. Hamidzadeh, F. Yaripour, S Shifteh Catal. Commun. 183, 106782 (2023)
L. Jin, T. Xie, S. Liu, Y. Li, H Hu Catal. Commun. 75, 32–36 (2016)
J. Ding, H. Liu, P. Yuan, G. Shi, X Bao ChemCatChem. 5, 2258–2269 (2013)
B. Liu, C. Li, Y. Ren, Y. Tan, H. Xi, Y Qian Chem. Eng. J. 210, 96–102 (2012)
C. Sun, J. Du, J. Liu, Y. Yang, N. Ren, W. Shen, H. Xu, Y Tang Chem. Commun. 46, 2671 (2010)
S. Fan, J. Zhou, J. Lv, M. Liu, H. Huang, J. Zhang, T -S Zhao Chem. Lett. 44, 1697–1699 (2015)
L. Bu, Y. Wang, W. Liu, K. Chu, N. Guo, Y. Huang, L. Qu, X. Su, X. Zhang, Y. Li, Appl. Catal. A-Gen. 665, 119393 (2023)
H. Chen, Y. Wang, F. Meng, C. Sun, H. Li, Z. Wang, F. Gao, X. Wang, S. Wang, Micropor Mesopor Mater. 244, 301–309 (2017)
H. Chen, Y. Wang, C. Sun, F. Gao, L. Sun, C. Wang, Z. Wang, X Wang Catal. Commun. 100, 107–111 (2017)
Y. Khani, S. Pyo, K.-E. Jeong, C.-U. Kim, M.A. Khan, B.-H. Jeon, K.-Y.A. Lin, S.Q. Choi, Y -K Park Catal. Today 425, (2024)
R. Feng, X. Yan, X. Hu, Y. Zhang, J. Wu, Z. Yan Appl. Catal. A-Gen. 594, 117464 (2020)
H. Huang, C. Yuan, P. Zhong, W. Fan, S. He, D. Xu, J. Gao, Q. Zhang, C. Li, Catal. Lett. 152, 2178–2185 (2021)
T. Fu, Y. Han, C. Li, M. Guo, G. Zhan, Z. Li Chem. Eng. Sci. 284, 119457 (2024)
S. Narayanan, J.J. Vijaya, S. Sivasanker, L.J. Kennedy, S K Jesudoss Powder Technol. 274, 338–348 (2015)
J. Yu, D. He, D. Chen, J. Liu, J. Lu, F. Liu, P. Liu, Y. Zhao, Z. Xu, Y. Luo, Appl. Surf. Sci. 420, 21–27 (2017)
D. Ren, T. Xu, H. Yan, X. Zhang, Z. Li, Y. Li, J. Leng, Q. Zhang, J. Li, D. Zhao, H. Shi, H. Jiang, Y. Liu, X. Chen, C Yang Micropor Mesopor Mater. 364, 112867 (2024)
M. Rostamizadeh, A. Taeb, J. Ind. Eng. Chem. 27, 297–306 (2015)
A. Sassi, M.A. Wildman, H.J. Ahn, P. Prasad, J.B. Nicholas, J.F. Haw, J. Phys. Chem. B 106, 2294–2303 (2002)
Y. Gao, G. Wu, F. Ma, C. Liu, F. Jiang, Y. Wang, A. Wang, Micropor Mesopor Mater. 226, 251–259 (2016)
P. Peng, Y. Wang, Z. Zhang, K. Qiao, X. Liu, Z. Yan, F. Subhan, S. Komarneni, Chem. Eng. J. 302, 323–333 (2016)
J. Kim, M. Choi, R. Ryoo, J. Catal. 269, 219–228 (2010)
J. Ahmadpour, M Taghizadeh C R Chim. 18, 834–847 (2015)
M. Milina, S. Mitchell, P. Crivelli, D. Cooke, J. Pérez-Ramírez, Nat. Commun. 5, 3922 (2014)
M. Bjørgen, F. Joensen, M. Spangsberg Holm, U. Olsbye, K.-P. Lillerud, S Svelle Appl. Catal. A-Gen. 345, 43–50 (2008)
S. Eslamdoost Jami, A. Nakhaei Pour, A. Mohammadi, J. Iran. Chem. Soc. 19, 401–411 (2021)
Author information
Authors and Affiliations
Contributions
HC carried out most of the experiment, data analysis and wrote the main manuscript. TL prepared the zeolites and edited the manuscript. ZL and BL assisted the centrifugation of zeolites suspension and contributed to the discussion of results, MZ supervised and conceptualized the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, H., Li, T., Li, Z. et al. Facile synthesis of rodlike ZSM-5 zeolite microspheres and catalytic performance in methanol to propylene reaction. J Porous Mater 31, 1175–1182 (2024). https://doi.org/10.1007/s10934-024-01590-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-024-01590-z