Abstract
A type of mesoporous SBA-15 supported imidazolium functionalized ionic liquids were prepared, characterized and tested as effective and practical catalysts for the synthesis of 2-oxazolidinones by the cycloaddition of CO2 and aziridines. The effects of reaction parameters such as type of catalysts, catalyst amount, CO2 pressure, reaction temperature and catalyst stability have also been investigated in detail, the catalyst SBA-15@DMIL-HOCH2COO exhibited high activity with good yields and excellent regioselectivities. In addition, the catalyst can be easily and effectively recycled for five times with no significant loss in its catalytic activity and selectivity. This work introduces a new, convenient, highly efficient and environmentally friendly pathway to explore the supported ionic liquids for the chemical fixation of carbon dioxide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chemical fixation of carbon dioxide (CO2) into valuable chemicals has attracted considerable attention due to its prominent features of abundance, nontoxicity, inexpensive, and ubiquitous C1 source [1, 2]. In the past decade, many synthetic processes have been developed to convert CO2 into value-added products, such as cyclic carbonates, carboxylic acids, 2-oxazolidinones, etc. [3,4,5,6,7,8,9,10,11,12]. Among those processes, the selective cycloaddition of CO2 with aziridines is one of the most promising 100% atom economic approaches as the obtained products 2-oxazolidinones are widely used as high active building blocks for the formation of various pharmaceuticals, auxiliaries and other fine chemicals. Up to now, a number of catalysts have been developed for the cycloaddition of CO2 with aziridines to produce 2-oxazolidinones, such as DBN [13], metal complexes [14,15,16], MCM-41-IPr-CO2 [17], amine functionalized MCM-41 [18], [NH2Et2]I [19], and others [20,21,22]. Nevertheless, most of these protocols suffered from disadvantages such as harsh reaction conditions, tedious process, need of co-catalyst or co-solvent, and catalyst recycle problems. Thus, the search for a novel, sustainable and efficient catalytic systems for the conversion of CO2 to oxazolidinones under mild reaction conditions remains a great challenge.
Ionic liquids (ILs) have found various industrial applications in synthesis and catalysis due to their distinctive properties of negligible vapour pressure, thermal and chemical stability, non-flammable, nonvolatile and strong structural design prospects [23,24,25,26,27]. Through the functional design of anions and cations of ionic liquids, the use of ILs as catalysts in the efficient synthesis of 2-oxazolidinones have been developed [28,29,30]. However, most of the catalytic systems have the disadvantages of isolation of pure ILs and reusability. Hence, the immobilization of functionalized ILs over solid supports to explore solid base heterogeneous catalytic systems could make the process easier through their easy handling, simplifying isolation and recycling of the catalyst [31,32,33,34,35,36]. Among these soild supports, mesoporous silica materials offer significant advantages of large surface area, highly ordered structure, thermal and chemical stabilities. These materials can be used as a platform for the loading and dispersion of ILs active components, thereby increasing their catalytic activities and have been widely utilized as supports in the case of heterogeneous catalysis [37,38,39,40,41,42,43,44]. We herein report the preparation of a type of mesoporous SBA-15 supported imidazolium functionalized ionic liquids, which served as single-component catalysts for the product of 2-oxazolidinones from the cycloaddition of CO2 with aziridines under mild conditions (Scheme 1). The present work offers a novel and high-efficient strategy for CO2 fixation and conversion to value-added 2-oxazolidinones. Moreover, the recycling performance of catalyst and possible catalytic mechanism were explored.
2 Experimental
2.1 Materials and apparatus
All chemicals and reagents were purchased from firms and used without further purification. Pluronic 123 (EO20PO70EO20) was purchased from Sigma-Aldrich. Scanning electron microscopy (SEM) experiments, including energy dispersive X-ray spectroscopy (EDX) for mapping of elements, were performed on a JSM-7500F electron microscope. Each sample was well dispersed in ethanol, drop casted on a silicon wafer, dried and coated with gold using a working distance of 5–12 mm and a voltage of 12–20 kV. FT-IR spectra of all catalysts (KBr pellets) were obtained in the range of 4000–400 cm−1 using PerkinElmer Spectrum I FT-IR equipment. Powder X-ray diffraction (XRD) data were obtained using a Rigaku Ultima IV diffractometer equipped with Cu-Kα radiation (1.5418 Å) at 40 kV and 40 mA at a scan rate of 2° min−1 in a scan range of 5°–80°. Thermogravimetric analysis (TGA) was carried out on NETZSCH STA 449 F5 (25 − 600 °C, 10 °C min−1, under flowing air gas). UV–Vis spectra were carried out in the range of 200–800 nm using a Shimadzu UV-2450 spectrophotometer, using BaSO4 as the reflectance standard material. N2 adsorption–desorption isotherms were carried out a BELSORP-max instrument and pore size distribution curves were calculated from the analysis of desorption branch of the isotherm by the BJH (Barrett–Joyner–Halenda). Prior to the measurement, all samples were degassed overnight at 130 °C under a dynamic vacuum. The catalytic reaction was monitored by gas chromatography (Agilent 7890) equipped with a flame ionization detector (FID) and a capillary column (HP-5, 30 m × 0.32 mm × 0.25 μm). The temperature program employed was as follows: initial oven temperature at 60 °C for 1 min, ramp at 10 °C/min, to 280 °C and hold isothermally for 5 min. The injection and detector temperatures were 280 °C. 1H NMR spectra was recorded on a Bruker 400 MHz spectrometer with CDCl3 as the solvent and tetramethylsilane as an internal standard. Elemental analysis for C, H, N and O was recorded on a Vario Micro cube Elemental analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany).
2.2 Preparation of supported functionalized ionic liquids
SBA-15 was synthesized as described in literatures [39,40,41]. The supported ionic liquids were synthesized according to literatures (Scheme 2) [34,35,36, 43, 44]. A typical procedure was as follows: Sodium ethoxide (0.5 mol), ethanol (250 mL) and imidazole (0.5 mol) were stirred at 80 °C for 5 h, then 1,3-dichloropropane (0.25 mol) was added, and the mixture was stirred at 70 °C for 3 h. After that, the mixture was filtrated and the filtrate was evaporated, washed with ethanol and dried under vacuum at 50 °C to give 1. Next, 4-chloro-1-butano (0.3 mol), 1 (0.3 mol) and ethanol (150 mL) was refluxed for 24 h. After evaporating the solvent, the mixture was washed with ethyl acetate and dried under vacuum to give 2. Subsequently, (3-chloropropyl) triethoxysilane (0.3 mol), and 2 (0.3 mol), and toluene (200 mL) were stirred at 95 °C under nitrogen for 24 h, thereafter, the solvent was isolated and dried to give 3. Then, 3 (0.2 mol), HOCH2COONa or KPF6 or NaBF4 (0.4 mol), acetonitrile (100 mL) were stirred at 60 °C for 24 h, then the solvent was evaporated, the obtained residue was washed with water and dried under vacuum to give ionic liquid 4 DMIL-HOCH2COO or DMIL-PF6 or DMIL-BF4. Finally, SBA-15 (1.0 g) and 4 (0.2 g) were added into a solution of dry toluene (100 mL), and the mixture was stirred and refluxed for 24 h under nitrogen. Then the resulting solid was filtered, cleansed twice with diethyl ether and dried under vacuum to give the supported ionic liquids.
2.3 Catalytic synthesis of 2-oxazolidinones from CO2 and aziridines
In a typical reaction process, aziridine (10 mmol), SBA-15@DMIL-HOCH2COO (0.3 g) were added into a 50 mL stainless-steel autoclave equipped with a magnetic stirrer. Then CO2 was introduced into the autoclave and kept for 0.4 MPa pressure after the air evacuated, and the mixture was stirred at 80 °C for mentioned time period. The reaction mixture was quantitatively analyzed by GC. After the reaction, the remaining CO2 was released slowly. Meanwhile, the reactor was cooled to room temperature and the product was obtained by filtration and separation. Fresh substrates were then recharged to the recovered catalyst and then recycled under identical reaction conditions. All target products are commercial, thus they were recognized by comparison with those of standard compounds or by 1H NMR and Elemental analysis.
2.4 Spectroscopic data for products
5-Methyloxazolidin-2-one (Table 2, entry 1): 1H NMR (400 MHz, CDCl3): δ 1.42 (d, CH3, 3H), 3.12 (dd, CH2, 2H), 4.78 (m, CH, 1H), 6.24 (s, NH, 1H) ppm; Elemental analysis for C4H7NO2: C, 47.49; H, 6.95; N, 13.83; O, 31.62. Found: C, 47.52; H, 6.98; N, 13.85; O, 31.65.
Oxazolidin-2-one (Table 2, entry 2): 1H NMR (400 MHz, CDCl3): δ 3.18 (t, CH2, 2H), 4.71 (t, CH2, 2H), 6.27 (s, NH, 1H) ppm; Anal. Calcd. for C3H5NO2: C, 41.35; H, 5.76; N, 16.09; O, 36.71. Found: C, 41.38; H, 5.79; N, 16.09; O, 36.75.
4,5-Dimethyloxazolidin-2-one (Table 2, entry 3): 1H NMR (400 MHz, CDCl3): δ 1.28–1.39 (m, 2CH3, 6H), 4.74 (m, CH, 1H), 5.19 (m, CH, 1H), 6.28 (s, NH, 1H) ppm; Anal. Calcd. for C5H9NO2: C, 52.12; H, 7.83; N, 12.15; O, 27.76. Found: C, 52.16; H, 7.88; N, 12.17; O, 27.79.
5-Phenyloxazolidin-2-one (Table 2, entry 4): 1H NMR (400 MHz, CDCl3): δ 3.57 (d, CH2, 2H), 5.63 (t, CH, 1H), 6.43 (s, NH, 1H), 7.32–7.43 (m, Ar–H, 5H) ppm; Elemental analysis for C9H9NO2: C, 66.23; H, 5.52; N, 8.55; O, 19.57. Found C, 66.25; H, 5.56; N, 8.58; O, 19.61.
3-Methyloxazolidin-2-one (Table 2, entry 5): 1H NMR (400 MHz, CDCl3): δ 3.21 (s, CH3, 3H), 3.59 (t, CH2, 2H), 4.34 (t, CH2, 2H) ppm; Elemental analysis for C4H7NO2: C, 47.48; H, 6.94; N, 13.82; O, 31.61. Found C, 47.52; H, 6.98; N, 13.85; O, 31.65.
5-Methyl-3-phenyloxazolidin-2-one (Table 2, entry 6): 1H NMR (400 MHz, CDCl3): δ 1.54 (d, CH3, 3H), 3.68 (d, CH2, 2H), 4.76 (m, CH, 1H), 7.11–7.15 (m, Ar–H, 1H), 7.35–7.51 (m, Ar–H, 4H); Elemental analysis for C10H11NO2: C, 67.75; H, 6.23; N, 7.86; O, 18.02. Found C, 67.78; H, 6.26; N, 7.90; O, 18.06.
3 Results and discussion
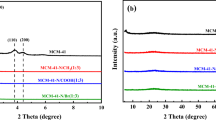
Figure 1 presented the XRD patterns of our synthesized supported ionic liquids. Compared to the XRD pattern of pure SBA-15, the spectra of pure SBA-15 and supported ILs are almost the same, only one broad peak appears at about 2θ = 22.7°, which is the characteristic structure of SBA-15 [40, 41]. No typical peaks corresponding to ionic liquids were observed, suggested that the ionic liquid species were well‐dispersed on the parent support framework. The surface morphology and elemental composition of the supported ILs catalysts are characterized by SEM and EDX. As can be seen from the SEM image (Fig. 2), the SBA-15 exhibited characteristic rope-like morphology. After the immobilization of ionic liquid on the SBA-15 support, the typical aggregated small spherical particles can be seen in the support framework. The SEM images of SBA-15 and SBA-15 supported ionic liquids indicated that both of them have irregular particle morphologies, which are almost identical to the literature [40,41,42]. The EDX images of the supported ILs were exhibited in Fig. 3, in which the corresponding elemental signals were distributed homogeneously and consistently, indicating the successful formation of the supported ILs species.
The UV–Vis spectras for the supported ILs were presented in Fig. 4, the intensity of diffraction peak at around 305 nm was attributed to Si–O species of SBA-15. The supported ILs exhibited characteristic absorption peaks around 200–240 nm and since pure SBA-15 shows no absorption in this area, which are assigned to the presence of ligand ionic liquid charge transfer and is usually used as the direct proof that organic ILs have been incorporated into the framework of mesoporous SBA-15 [38,39,40,41]. Obviously, after immobilization of ionic liquids, no significant peaks attributed to the ionic liquids particles were found in the UV–Vis spectras, which may be owing to the well-dispersed ionic liquids particles encapsulated in mesoporous channels of the SBA-15 support. FT-IR spectras of the supported ionic liquids were shown in Fig. 5. The peaks in the absorption band from 3580 to 3370 cm−1 suggested the existence of hydroxyl stretching vibration. The peak at around 1096 cm−1 is ascribed to the presence of Si–O–Si. The peak observed at around 728 cm−1 is ascribed to the CH2 chain flexural vibration of lactate. The vibrations of N=N and C–N bonds of imidazole generated the observed peaks at around 1628 cm−1 and 1536 cm−1, respectively [34,35,36,37]. N2 absorption–desorption isotherms and BJH pore size distribution curves of the supported ILs are displayed in Fig. 6. All supported ILs and bare SBA-15 show a type IV isotherms according to the IUPAC nomenclature with an H1 hysteresis loop starting at about 0.6 relative pressure (P/P0) [42,43,44,45,46,47]. This is a characteristics of materials which exhibited mesoporous nature, indicating the presence of mesoporous structure in the supported ILs. On the other hand, pore size distributions curves of the supported ILs presents the probable pore size centered at 6.8026 nm, 6.8640 nm, and 6.7462 nm for SBA-15@DMIL-PF6, SBA-15@DMIL-BF4, and SBA-15@DMIL-HOCH2COO, respectively, which also indicated mesoporous structure in the supported ILs. The bare SBA-15 exhibits 546.52 m2g−1 of BET surface area and 0.7628 cm3g−1 of pore volume. The BET surface area of SBA-15@DMIL-PF6, SBA-15@DMIL-BF4, and SBA-15@DMIL-HOCH2COO composites are 319.65 m2g−1, 289.86 m2g−1, and 302.57 m2g−1 respectively, which were smaller than SBA-15 due to the anchoring of ionic liquid species, which had nearly a small surface area (Table 1). At the same time, after the immobilization of ionic liquids on the SBA-15 support, the pore volume of the supported ILs was also gradually decreased, which was due to the incorporation of ionic liquid species in the pore walls. These results are in good agreement with the fact that the surface of SBA-15 has been successfully immobilized by functional ionic liquid.
After the characterization of supported ionic liquids, they were applied in the catalytic synthesis of 5-methyloxazolidin-2-one by the model cycloaddition of 2-methylaziridine and CO2 for condition optimization (Table 2). Firstly, all resulting the supported ionic liquids catalysts SBA-15@DMIL-PF6, SBA-15@DMIL-BF4 and SBA-15@DMIL-HOCH2COO were evaluated for the catalytic synthesis of 5-methyloxazolidin-2-one (Table 1, entries 1–3). The catalytic activity of SBA-15@DMIL-PF6 or SBA-15@DMIL-BF4 is low in the reaction conditions studied whereas SBA-15@DMIL-HOCH2COO showed the excellent performance in the cycloaddition with 95% yield and 99.1% selectivity (Table 2, entry 3). The catalytic performance of bulk ionic liquids DMIL-BF4, DMIL-PF6, DMIL-HOCH2COO and the support SBA-15 as the catalysts in the cycloaddition were compared (Table 2, entries 4–7). Results showed that both bulk ionic liquids and support SBA-15 exhibited very low catalytic activities in the reaction. The amount of catalysts had strong effect on the reaction, and the effect of amount of best catalyst SBA-15@DMIL-HOCH2COO was studied. The product yield was increased from 58 to 82% when the amount of catalyst was increased from 0.1 to 0.2 g (Table 2, entries 9 and 10). After a steady growth, the product yield reached 95% in the presence of 0.3 g amount of catalyst (Table 2, entry 3). However, the amount of catalyst was further increased to 0.4 g, no obvious enhancement of product yield and selectivity were observed (Table 2, entry 11). A control experiment in the absence of catalyst revealed that SBA-15@DMIL-HOCH2COO was responsible for the efficient reaction transformation (Table 2, entry 8). Therefore, SBA-15@DMIL-HOCH2COO can act as a suitable catalyst for the cycloaddition.
Since SBA-15@DMIL-HOCH2COO shows the better catalytic activity than other catalysts, it is employed as the benchmark to optimize the reaction conditions. The reaction was remarkably influenced by the CO2 pressure. As shown in Fig. 7, the product yield and selectivity was increased with an increase in the CO2 pressure from 0.1 MPa to 0.4 MPa, however, minor decrease in the product yield and selectivity was noticed above this pressure. It was because CO2 was compressed and present in the liquid phase at pressures below 0.4 MPa, thus resulting in improve the product yield and selectivity. At pressures higher than 0.4 MPa, 2-methylaziridine was present in the gaseous form, which decrease the concentration and retard the interaction with SBA-15@DMIL-HOCH2COO, therefore minor decrease in the product yield and selectivity. Figure 8 showed that the reaction temperature exhibited remarkable influence on the reaction. The product yield and selectivity was increased with an increase in the reaction temperature from 50 to 80 °C, however, a decrease in the product yield and selectivity was observed above 80 °C, which was due to the formation of side products at higher temperature (by GC analysis).
In order to craft the greener and economical aspect of the developed catalytic system, the cycling performance of SBA-15@DMIL-HOCH2COO was studied and the corresponding result is plotted in Fig. 9. In each cycle, the catalyst SBA-15@DMIL-HOCH2COO was easily isolated by filtration and can be used directly for consecutive runs. It showed that the catalyst possessed a good reusability and maintained high catalytic performance at least five times without considerable reduction in catalytic activity. The stability of SBA-15@DMIL-HOCH2COO was also confirmed by the thermal gravimetric analysis (Fig. 10). The first step of weight loss (1.05%) below 200 °C was related to the removal of adsorbed water and other volatile solvent residues, whereas the main weight loss (10.47%) from 200 to 600 °C in the second step was corresponded to degradation of the organic moieties of ionic liquid. These observations demonstrated that the catalyst SBA-15@DMIL-HOCH2COO was thermally stable below 200 °C, which was beneficial for the recycling catalytic experiments. The recovered catalyst after five cycles has no obvious change in morphology (Fig. 2e). The FT-IR analysis of SBA-15@DMIL-HOCH2COO after five cycles showed the existence of expected characteristic framework (Fig. 11), which indicated that the characteristic structure of the catalyst remain stable after five cycles.
The above results demonstrated that SBA-15@DMIL-HOCH2COO was an efficient and stable catalyst for the synthesis of 5-methyloxazolidin-2-one from the cycloaddition of 2-methylaziridine with CO2.
Under the optimal reaction conditions, the cycloaddition of CO2 with other aziridines were investigated. As shown in Table 3, the terminal aziridines are transferred into the corresponding 2-oxazolidinones with the satisfied catalytic activities in the presence of SBA-15@DMIL-HOCH2COO. The corresponding 2-oxazolidinones were obtained in good to high yields with excellent selectivities within 4 h. Aziridines with no substituted groups at the nitrogen atom gave the high yields with excellent selectivities (> 99%) whitin 2 h. The aziridines bearing substituted groups at the nitrogen atom, aziridines such as 1-methylaziridine and 2-methyl-1-phenylaziridine with steric hindrance of N-substituted group also afforded the corresponding 2-oxazolidinones in good yields with significant selectivity of 99% (Table 3, entries 5 and 6).
On the basis of experimental results and previous literatures [14,15,16,17,18,19,20,21], a possible catalytic mechanism is proposed for the catalytic synthesis of 2-oxazolidinones from cycloaddition of CO2 with aziridines (Scheme 3). The first step involves the activation of aziridine by the O atom coordination with hydroxyl sites of the catalyst SBA-15@DMIL-HOCH2COO for the formation of intermediate a. At the same time, the catalyst helps to activate CO2 for the formation of the carbonate species. Next, the intermediate a adds to the less sterically hindered C atom of aziridine by nucleophilic attack to give the intermediate b, followed by the nucleophilic interaction to give the intermediate c. Ultimately, cyclization via intramolecular substitution of anion and the desired product was produced along with the regeneration of catalyst for the next cycle.
4 Conclusion
In conclusion, a type of mesoporous SBA-15 supported imidazolium functionalized ionic liquids were prepared and tested as catalysts for the synthesis of 2-oxazolidinones by the cycloaddition of CO2 and aziridines. The experiment demonstrated that the catalyst SBA-15@DMIL-HOCH2COO possessing abundant active sites exhibited excellent activity in high to excellent yields and selectivities. The catalyst having full utilization of active sites can be easily recovered and reused for five times without considerable loss in activity. The protocol was found to be advantageous in terms of high to excellent yields, ease of isolation and reusability of the catalyst. This study provide indication of the application of SBA-15 supported hydroxyacetate anionic functionalized ionic liquid catalyst as an environmentally benign and high efficient alternative in chemical fixation of carbon dioxide.
References
R. Calmanti, M. Selva, A. Perosa, Tandem catalysis: one-pot synthesis of cyclic organic carbonates from olefins and carbon dioxide. Green Chem. 23, 1921–1941 (2021)
W. Kong, B. Shen, H. Lyu, J. Kong, J. Ma, Z. Wang, S. Feng, Review on carbon dioxide fixation coupled with nutrients removal from wastewater by microalgae. J. Clean. Prod. 292, 125975 (2021)
N.A. Tappe, R.M. Reich, V. D’Elia, F.E. Kühn, Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: an update. Dalton Trans. 47, 13281–13313 (2018)
L. Guo, K.J. Lamb, M. North, Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 23, 77–118 (2021)
Y. Hu, R.L. Zhang, D. Fang, Quaternary phosphonium cationic ionic liquid/porous metal-organic framework as an efficient catalytic system for cycloaddition of carbon dioxide into cyclic carbonates. Environ. Chem. Lett. 17, 501–508 (2019)
C.C. Truong, D.K. Mishra, Recent advances in the catalytic fixation of carbon dioxide to value-added chemicals over alkali metal salts. J. CO2 Util. 41, 101252 (2020)
H. Salehizadeh, N. Yan, R. Farnood, Recent advances in microbial CO2 fixation and conversion to value-addedproducts. Chem. Eng. J. 390, 124584 (2020)
T. Zhang, J. Zhong, Z. Wu, Recent advances in catalytic conversion of carbon dioxide to propiolic acids over coinage-metal-based catalysts. J. Energy Chem. 59, 572–580 (2021)
S. Pulla, C.M. Felton, Y. Gartia, P. Ramidi, A. Ghosh, Synthesis of 2-oxazolidinones by direct condensation of 2-aminoalcohols with carbon dioxide using chlorostannoxanes. ACS Sustain. Chem. Eng. 1, 309–312 (2013)
J.M. Chen, L. Qi, L. Zhang, L.J. Li, C.Y. Hou, W. Li, L.J. Wang, Copper/DTBP-promoted oxyselenation of propargylic amines with diselenides and CO2: synthesis of selenyl 2-oxazolidinones. J. Org. Chem. 85, 10924–10933 (2020)
F. Chen, S. Tao, Q.Q. Deng, D. Wei, N. Liu, B. Dai, Binuclear tridentate hemilabile copper(I) catalysts for utilization of CO2 into oxazolidinones from propargylic amines. J. Org. Chem. 85, 15197–15212 (2020)
Y. Yoshida, T. Endo, Synthesis of multifunctional 4-hydroxymethyl 2-oxazolidinones from glycidyl carbamate derivatives catalyzed by bicyclic guanidine. Tetrahedron Lett. 72, 153086 (2021)
Y. Wu, G. Liu, Organocatalyzed cycloaddition of carbon dioxide to aziridines. Tetrahedron Lett. 52, 6450–6452 (2011)
G. Bresciani, S. Zacchini, F. Marchetti, G. Pampaloni, Non-precious metal carbamates as catalysts for the aziridine/CO2 coupling reaction under mild conditions. Dalton Trans. 50, 5351–5359 (2021)
V. Saptal, D.B. Shinde, R. Banerjee, B.M. Bhanage, State-of-the-art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones. Catal. Sci. Technol. 6, 6152–6158 (2016)
C.S. Cao, Y. Shi, H. Xu, B. Zhao, A multifunctional MOF as a recyclable catalyst for the fixation of CO2 with aziridines or epoxides and as a luminescent probe of Cr(VI). Dalton Trans. 47, 4545–4553 (2018)
H. Zhou, Y.M. Wang, W.Z. Zhang, J.P. Qu, X.B. Lu, N-Heterocyclic carbene functionalized MCM-41 as an efficient catalyst for chemical fixation of carbon dioxide. Green Chem. 13, 644–650 (2011)
D.B. Nale, S. Rana, K. Parida, B.M. Bhanage, Amine functionalized MCM-41 as a green, efficient, andheterogeneous catalyst for the regioselective synthesis of5-aryl-2-oxazolidinones, from CO2 and aziridines. Appl. Catal. A: Gen. 469, 340–349 (2014)
G. Bresciani, M. Bortoluzzi, G. Pampaloni, F. Marchetti, Diethylammonium iodide as catalyst for the metal-free synthesis of 5-aryl-2-oxazolidinones from aziridines and carbon dioxide. Org. Biomol. Chem. (2021). https://doi.org/10.1039/D1OB00458A
S. Arayachukiat, P. Yingcharoen, S.V.C. Vummaleti, L. Cavallo, A. Poater, V. D’Elia, Cycloaddition of CO2 to challenging N-tosyl aziridines using a halogen-free niobium complex: catalytic activity and mechanistic insights. Mol. Catal. 443, 280–285 (2017)
A.C. Kathalikkattil, J. Tharun, R. Roshan, H.G. Soek, D.W. Park, Efficient route for oxazolidinone synthesis using heterogeneous biopolymer catalysts from unactivated alkyl aziridine and CO2 under mild conditions. Appl. Catal. A: Gen. 447–448, 107–114 (2012)
S. Ghosh, T.S. Khan, A. Ghosh, A.H. Chowdhury, M.A. Haider, A. Khan, S.M. Islam, Utility of silver nanoparticles embedded covalent organic frameworks as recyclable catalysts for the sustainable synthesis of cyclic carbamates and 2-oxazolidinones via atmospheric cyclizative CO2 capture. ACS Sustain. Chem. Eng. 8, 5495–5513 (2020)
O. Nordness, J.F. Brennecke, Ion dissociation in ionic liquids and ionic liquid solutions. Chem. Rev. 120, 12873–12902 (2020)
P.N. Reddy, P. Padmaja, B.V.S. Reddy, G. Rambabu, Ionic liquid/water mixture promoted organic transformations. RSC Adv. 5, 51035–51054 (2015)
H.M.A. Hassan, M.A. Betiha, R.F.M. Elshaarawy, E.A. Ahmed, Facile tailoring of hierarchical mesoporous Al-SBA-15 by ionic liquid and their applications in heterogeneous catalysis. J. Porous Mater. 25, 63–73 (2018)
A. Yıldırım, An expedient method for kinetically controlled acetonide formation from d-fructose induced by ionic liquid catalyst accompanied with SrCl2·6H2O. Catal. Lett. 150, 2566–2571 (2020)
P. McNeice, P.C. Marr, A.C. Marr, Basic ionic liquids for catalysis: the road to greater stability. Catal. Sci. Technol. 11, 726–741 (2021)
Y. Chen, R. Luo, Z. Yang, X. Zhou, H. Ji, Imidazolium-based ionic liquid decorated zinc porphyrin catalyst for converting CO2 into five-membered heterocyclic molecules. Sustain. Energy Fuels 2, 125–132 (2018)
Y.N. Zhao, Z.Z. Yang, S.H. Luo, L.N. He, Design of task-specific ionic liquids for catalytic conversion of CO2 with aziridines under mild conditions. Catal. Today 200, 2–8 (2013)
Z.Z. Yang, L.N. He, S.Y. Peng, A.H. Liu, Lewis basic ionic liquids-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under solvent-free conditions. Green Chem. 12, 1850–1854 (2010)
P. Virtanen, E. Salminen, J.P. Mikkola, Modeling of supported ionic liquid catalysts systems-from idea to applications. Ind. Eng. Chem. Res. 56, 12852–12862 (2017)
R. Gupta, M. Yadav, R. Gaur, G. Arora, P. Yadav, R.K. Sharma, Magnetically supported ionic liquids: a sustainable catalytic route for organic transformations. Mater. Horiz. 7, 3097–3130 (2020)
S. Askari, M. Jafarzadeh, D.B. Christensen, S. Kegnæs, A synergic activity of urea/butyl imidazolium ionic liquid supported on UiO-66-NH2 metal–organic framework for synthesis of oximes. Catal. Lett. 150, 3159–3173 (2020)
R. Fehrmann, A. Riisager, M. Haumann, Supported Ionic Liquids: Fundamentals and Applications (Wiley-VCH Verlag, Weinheim, 2014)
E. Peris, R. Porcar, M.I. Burguete, E. García-Verdugo, S.V. Luis, Supported ionic liquid-like phases (SILLPs) as immobilised catalysts for the multistep and multicatalytic continuous flow synthesis of chiral cyanohydrins. ChemCatChem 11, 1955–1962 (2019)
B. Sandig, L. Michalek, S. Vlahovic, M. Antonovici, B. Hauer, M.R. Buchmeiser, A monolithic hybrid cellulose-2.5-acetate/polymer bioreactor for biocatalysis under continuous liquid-liquid conditions using a supported ionic liquid phase. Chem. Eur. J. 21, 15835–15842 (2015)
N. Mohamed, Preparation and characterization of silver mesoporous silica nanoshells with promising antibacterial activity. J. Porous Mater. 27, 1277–1285 (2020)
P. Verma, Y. Kuwahara, K. Mori, R. Raja, H. Yamashita, Functionalized mesoporous SBA-15 silica: recent trends and catalytic applications. Nanoscale 12, 11333–11363 (2020)
S. Sadjadi, M.M. Heravi, Current advances in the utility of functionalized SBA mesoporous silica for developing encapsulated nanocatalysts: state of the art. RSC Adv. 7, 30815–30838 (2017)
I. Hierro, S. Gómez-Ruiz, Y. Pérez, P. Cruz, S. Prashar, M. Fajardo, Mesoporous SBA-15 modified with titanocene complexes and ionic liquids: interactions with DNA and other molecules of biological interest studied by solid state electrochemical techniques. Dalton Trans. 47, 12914–12932 (2018)
P. Chawdhury, K.V.S.S. Bhargavi, M. Selvaraj, C. Subrahmanyam, Promising catalytic activity by non-thermal plasma synthesized SBA-15-supported metal catalysts in one-step plasma-catalytic methane conversion to value-added fuels. Catal. Sci. Technol. 10, 5566–5578 (2020)
X. Sheng, Y. Zhou, Y. Yang, Y. Zhang, Z. Zhang, S. Zhou, X. Fu, S. Zhao, Synthesis of immobilized heteropolyanion-based ionic liquids on mesoporous silica SBA-15 as a heterogeneous catalyst for alkylation. RSC Adv. 4, 30697–30703 (2014)
Z. Dokhaee, M. Ghiaci, H. Farrokhpour, G. Buntkowsky, H. Breitzke, SBA-15-supported imidazolium ionic liquid through different linkers as a sustainable catalyst for the synthesis of cyclic carbonates: a kinetic study and theoretical DFT calculations. Ind. Eng. Chem. Res. 59, 12632–12644 (2020)
T. Perumal, V.L. Mangesh, S.K. Perumal, R. Arumugam, N. Subramanian, S. Subramanian, S. Kannan, Isomerization of alkanes over ionic liquids supported on SBA-15. Energy Fuels 34, 14620–14632 (2020)
J. Arfaoui, A. Ghorbel, C. Petitto, G. Delahay, A new V2O5–MoO3–TiO2–SO42− nanostructured aerogel catalyst for diesel DeNOx technology. New J. Chem 44, 16119–16134 (2020)
J. Arfaoui, A. Ghorbel, C. Petitto, G. Delahay, New CeO2–TiO2, WO3–TiO2 and WO3–CeO2–TiO2 mesoporous aerogel catalysts for the low temperature selective catalytic reduction of NO by NH3. J. Porous Mater. (2021). https://doi.org/10.1007/s10934-021-01102-3
J. Arfaoui, A. Ghorbel, C. Petitto, G. Delahay, Promotional effect of ceria on the catalytic behaviour of new V2O5–WO3–TiO2 aerogel solids for the DeNOx process. J. Solid State Chem. 300, 122261 (2021)
Acknowledgements
The authors are grateful to the Research Foundation of Yichang Science and Technology Bureau (A21-3-009), 111 Project (D20015) and analysis and testing center of China Three Gorges University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, C. & Hu, Y.L. Efficient and convenient catalytic regioselective synthesis of 2-oxazolidinones from CO2 and aziridines over reusable SBA-15 supported hydroxyacetate-functionalized ionic liquid. J Porous Mater 29, 131–142 (2022). https://doi.org/10.1007/s10934-021-01153-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01153-6