Abstract
An amine-modified ZSM-5/SBA-16 (ZS) micro-/mesoporous composite was synthesized for CO2 capture using nonionic tri-block copolymer pluronic (F127) as a template, tetraethyl orthosilicate (TEOS) as a silicon source and ZSM-5 zeolite as part of silicon aluminum source by embedding method. The amine-bifunctionalized samples were prepared by first grafting with aminepropyltriethoxysilane (APTES) and then impregnating with different concentrations of tetraethylenepentamine (TEPA). The amine efficiency, adsorption kinetics, thermal stability, regeneration performance and the effects of impregnated amine loadings (30–60%) and temperatures (30–90 °C) on the CO2 adsorption performance were investigated using a thermal gravimetric analyzer (TGA) in the mixed gases (15 vol% CO2 and the balance of N2). The best adsorbent after modification of ZSM-5/SBA-16 composite material was screened to study CO2 adsorption mechanism and adsorption kinetics. With the increase of the TEPA amount, the adsorption capacity of amine-bifunctionalized material first increased and then decreased. At 90 °C, the bifunctionalized ZSM-5/SBA-16 displayed the highest CO2 adsorption performance of 1.630 mmol/g at the TEPA loading of 55%. The CO2 adsorption mechanism of the bifunctionalized material was the synergistic effect of physical adsorption and chemical adsorption. After five adsorption/desorption cycle regenerations, the saturated adsorption capacity of the composite material was 1.496 mmol/g, which was only 8.2% lower than the original adsorption capacity. The composites exhibited good CO2 adsorption performance, indicating the promising industrial applications for CO2 capture form actual flue gas after desulfurization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Accompanied by rapid development of industry, environmental problems are becoming increasingly prominent, especially air pollution. For instance, pulp industry is accompanied by emission of total volatile organic compounds from in chemical pulping process [1] and high CO2 discharge from power industry during coal combustion. In addition, a series of environmental degradation problems such as climate warming, glaciers melting, and sea levels rising caused by excessive CO2 emission related to combustion of fossil for transport or in power plants, metallurgy, cement and chemical production [2] have seriously affected human production activities. Mitigating CO2 emission and preventing the negative effect which have on climate change have received more and more attention. Carbon capture and storage (CCS) is being developed as a promising technology to reduce large scale CO2 emission particularly from coal fired power plants and all efforts are being made to make processes green and environmentally friendly [3]. Several carbon capture technologies have emerged to reduce CO2 emission [4], including post-combustion carbon capture technology, a promising technology with a conventional process that differ from pre-combustion, and oxy-fuel combustion and chemical looping combustion [5], which need redesign. Among the post-combustion technologies, adsorption with amine-modified porous solids has been a focus due to its outstanding advantages of lower energy consumption, low price and little corrosion [6] over absorption with liquid amines [7].There have been various porous solids designed as supports, including metal organic frameworks (MOFs) [8], activated carbon [9], porous carbon [10], amine-functionalized porous silica [11], functioned porous carbons [12], zeolites [13, 14], porous polymers [15, 16] and hydrotalcites [17].
The microporous zeolite molecular sieves have the advantages of strong acidity and high hydrothermal stability, but their pore sizes are small and the gas desorption is slow. Mesoporous molecular sieves have the characteristics of large pore size, high specific surface area, and orderly structure, but their thin pore walls are easy to collapse under high temperature conditions. Fortunately, the advantages of the microporous and mesoporous materials can be combined by means of compounding different materials to overcome the defects of single-stage holes. The compounding means can improve the hydrothermal stability of the composites and obtain large pore size, large specific surface area and high pore order. Xiao et al. [18] proposed a full-atomic mimetic structure of NaX/MCM-41 and the results showed that NaX/MCM-41 has a higher diffusion coefficient of CO2 than NaX due to the mesoporous channels. In addition, Santos et al. [19] also reported that the micro-/mesoporous composite ZSM-12/MCM-41 is significantly better than the single microporous ZSM-12 and mesoporous MCM-41. At the same time, amine-modified zeolite materials exhibited promising candidates for removing carbon dioxide [20, 21].
Regarding the introduction of amines into porous supports, there are four routes to obtain for classes of adsorbents, including impregnation, grafting, in situ polymerization and amine double functionalization [22]. Among them, amine double functionalization technology achieved the improved CO2 adsorption capacity compared with the single amine modified technology due to the increased amine content and the stronger interaction of combined amines with CO2 molecules. The in situ polymerization technology can be regarded as a hybrid method combining physical impregnation and chemical grafting while the molecular design was difficult [23]. Therefore, the novel amine-bifunctionalized composite strategy should receive more attention. Tetraethylenepentamine (TEPA), polyethyleneimine (PEI) and piperazine (PZ) were impregnated into the inner channel of SBA-15 modified by 3-aminepropyltrimethoxysilane (APTES) grafting, respectively, presenting best uptake of 2.4 mmol/g at CO2 partial pressure of 1 atm and 45 °C as reported by Sanz-Pérez et al. [24]. In another report, Raúl Sanz et al. [25] impregnated TEPA onto the one-dimensional hexagonal MCM-41 grafted by (3-aminepropyl) trimethoxysilane (APTMS) and found that new adsorbent could promote CO2 adsorption, with the maximum adsorption capacity of 2.37 mmol/g at 45 °C and a CO2 partial pressure of 100 kPa. What more, P. D. Jadhav et al. [26] used impregnation technology for fabrication of amine-containing 13X zeolites with monoethanolamine (MEA) as modifier. The results showed that the adsorbents show improvement in CO2 adsorption capacity over the unmodified zeolite by a factor of ca. 1.6 at 30 °C. Most of these are amine-modified with pure microporous zeolite or mesoporous silica as the carrier while the CO2 adsorption by amine-bifunctionalized micro-/mesoporous molecular sieves is still exploratory stage. The effects of the factors such as amine species, uniform dispersion of amine, combination of molecular sieves with different pore sizes, CO2 adsorption performance and regeneration performance should be widely studied.
ZSM-5 zeolite is a widely used molecular sieve with high SiO2/Al2O3 ratio [27, 28]. It has a homogeneous pore structure, hydrophobic properties and excellent hydrothermal stability. SBA-16 mesoporous material is a new type of silica material with a symmetrical oversized cage cubic mesoporous structure. The connectivity of its three-dimensional pore channels facilitates the transport of substances and diffusion of reactive molecules. Additionally, the SBA-16 scaffold presents high thermal stability, thick walls and enhanced resistance to local pore blockage, making it very attractive for CO2 dispersion [29]. It should be pointed out that SBA-16 is an ideal mesoporous silica support for potential applications due to its hydrothermally stable cubic cage structure with multidirectional and large pore systems allowing good accessibility for functionalization and adsorption [30].
The micro-/mesoporous materials have a synergistic effect to improve performance and the amine grafting provides a stable site for amine impregnation (to form a stable mesh structure) to further improve adsorption performance and thermal stability. This modification strategy has prepared adsorbents with high adsorption capacity, high stability and other advantages. And according to the grafting modification with or without the involvement of water the methods are divided into dry grafting and wet grafting, which have different reaction principles. Here the more complex reaction of wet grafting is chosen in anticipation of improving the adsorption performance of CO2. In addition, SAB-16 is less common in the literature due to the more difficult synthesis conditions compared to SBA-15, and moreover, the use of SBA-16 to bifunctionalize amine modification of micro-/mesoporous material has not been reported. Herein, ZSM-5/SBA-16 composite was prepared by embedding method followed by grafting APTES and impregnating TEPA on ZSM-5/SBA-16 using the “two-step method”, which has been rarely reported for CO2 adsorption. Characteristics of composite ZSM-5/SBA-16, ZSM-5/SBA-16-APTES and ZSM-5/SBA-16-APTES-TEPA, and the adsorption mechanism of CO2 were investigated, including amine efficiency, kinetics and regeneration performance.
2 Experimental
2.1 Preparation of support
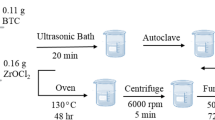
For the fabrication of ZSM-5 (Si/Al = 30) microporous molecular sieve, 0.143 g sodium hydroxide (NaOH, Aladdin, China), 0.50 g sodium metaaluminate (NaAlO2, Aladdin Industrial Co., Shanghai, China) and 10 g ultra-pure water (UP H2O) were stirred well at 25 °C for 1 h (500 rpm). The mixed solution was designated as X. 0.30 g NaOH and 2.65 g tetrapropyl ammonium bromide (TPABr, Sigma Aldrich Industrial Co., Shanghai, China) were mixed and stirred in 8 g ultra-pure water until dissolved at 25 °C for 15 min (500 rpm), then 10 g colloidal silica was added dropwise to the mixed solution and stirred at 25 °C for 1 h, and the mixed solution was designated as Y. X was added dropwise to Y and stirred at 25 °C for 3 h (500 rpm). Subsequently, the resulting solution was transferred to a polytetrafluoroethylene-lined autoclave and kept at 170 °C for 32 h. Afterward, the ZSM-5 molecular sieve was obtained by cooling, filtration, washing, drying at 100 °C and calcination at 550 °C for 6 h in ambient air, with a heating rate of 1 °C/min.
Under atmospheric pressure, the embedding method was used to synthesize the ZSM-5/SBA-16 composite molecular sieve [31]. Frist, for the SBA-16, 4.0 g F127 (AR, Sigma Aldrich Industrial Co., Shanghai, China) were dissolved in 8.4 g HCl (36.5 wt%, Sinopharm Chemical Reagent Co., Shanghai, China) and 190.0 g UP H2O and stirred at 45 °C for 45 min until F127 was dissolved completely (500 rpm). Next, 12.0 g BuOH (Xilong Chem. Reagent Co., Shantou, China) was slowly added and stirred for 2 h; 20.0 g TEOS was added to the solution and stirred for 24 h. Then, the pure SBA-16, was obtained by hydrothermal reaction (100 °C, 24 h), vacuum suction filtration, washing, drying (80 °C, 6 h) and calcination (550 °C, 6 h). Second, for the ZSM-5/SBA-16 composite molecular sieve, which was recorded as ZS, according to the above production steps of SBA-16, after F127 was completely dissolved, ZSM-5 molecular sieve prepared in advance were added and stirred for 24 h, and the rest of the steps were the same.
2.2 Preparation of amine-bifunctionalized samples
APTES was anchored onto the ZS support by grafting. The typical grafted steps carried out were depicted as follows. 1 mL APTES (Aladdin Industrial Co., Shanghai, China) was added in 100 mL toluene followed by addition of 1 g ZS support. Next, 0.4 mL UP H2O slowly added dropwise and subsequently stirred for 30 min. Then, the mixture was heated to 85 °C under reflux for 16 h, filtered, washed with toluene, and dried at 100 °C for 2 h. The grafted sample was denoted as ZS-A-1. TEPA was anchored onto the ZS-A-1 support by impregnating. A certain amount of TEPA (Sinopharm Chemical Reagent Co., Shanghai, China) was dispersed in 25 mL ethanol and stirred for 30 min until completely dissolved, next 0.5 g dried ZS-A-1 was added and stirred for 6 h. After evaporation (60 °C, 6 h) to remove ethanol, the bifunctionalized samples were obtained by drying in a natural convection oven at 105 °C for 2 h, which were referred to ZS-A-T-X (X = 30, 50, 55 or 60), where X stands for mass fraction of TEPA. For instance, ZS-A-T-30 indicates that the grafted sample ZS-A was further loaded with 30 wt% TEPA. The specific calculation method is shown in the following equation:
where ω is mass fraction; mZS and mTEPA are the weight of ZMS-5/SBA-16 and TEPA, respectively.
A schematic representation of the process for the preparation of APTES-grafting and TEPA-loaded samples (ZS-A-T-X) is shown in Fig. 1.
2.3 Characterization of samples
X-ray diffraction (XRD) tests were carried out with an XʹPert PRO powder diffractometer (PANalytical, Holland) using Cu-Kα radiation (40 kV and 40 mA) in 2θ range of 0.6–5° (scanning speed is 1°/min) and 5–50° (scanning speed is 5°/min). The morphology of each sample was obtained by scanning electron microscopy (SEM) with a JSM-7900F scanning electron microscope (Hi-tech Corporation, Japan) under accelerating voltage of 10 kV and 15 kV. Fourier transform infrared spectra (FTIR) were detected with a Nexus 470 IR spectrometer (Nicolet, USA) in wavenumber interval of 4000–400/cm. Detected translucent wafers were made by mixing the samples and KBr with a mass ratio of 1:100. Thermal gravimetric and relevant differential analysis (TGA/DTG) was performed on a microcomputer differential thermal balance (HCT-1, China) in nitrogen flow up to 800 °C for each sample. Nitrogen adsorption/desorption tests were carried out at − 196 °C with a JW-BK200C automatic surface analyzer (JWGB Sci & Tech, Beijing, China). Each sample was outgassed at 105 °C in vacuum atmosphere for 8 h before test. The surface area was calculated by Brunauer–Emmett–Teller (BET). Total pore volume was calculated from N2 adsorption capacity at P/P0 = 0.99. Pore size distribution was derived from adsorption branch of N2 curve using Barrett–Joyner–Halenda (BJH) method. The nitrogen contents of the samples were detected by EA 2400 II elemental analyzer (Perkin-Elmer, USA), and the average value of three experiments was taken as the test result.
2.4 Experimental method
The CO2 adsorption experiment was carried out on a thermogravimetric instrument. A microcomputer differential thermal balance with a sensitivity of 0.1 μg was used to determine the CO2 adsorption performance of the material. The adsorption capacities of CO2 on ZS and ZS after grafting and bifunctional modification were measured, respectively. About 6 mg of samples was desorbed at 110 °C for 1 h in a nitrogen atmosphere, cooled it down to the required adsorption temperature, then switched to the mixture of N2 and CO2, and recorded the change in sample mass. The CO2 adsorption capacity of the sample is calculated based on the mass measured by the thermogravimetric instrument. The equation is as the following:
where qe is the saturated adsorption capacity of CO2 (mmol/g); me and md are the weight of adsorption saturated and dewatered adsorbent (mg), respectively; 44.01 is the molar mass of CO2 (g/mol).
The cyclic performance of the sample was tested as follows. The adsorption temperature of the cycle experiment was set to 60 °C, a mixture of 15 vol% CO2 (99.999%) and the balance of N2 (99.999%) was used in the adsorption process, and the inlet flow rate was set to 60 mL/min. The desorption temperature was set to 110 °C, pure nitrogen is passed through the desorption process, and the inlet flow rate is 100 mL/min. The cycle was carried out for five times to explore the cyclic performance.
The pseudo-first-order kinetic equation, pseudo-second-order kinetic equation and Avrami model were used to fit the adsorption data to explore its adsorption mechanism.
The pseudo-first-order kinetic equation is as following:
The pseudo-second-order kinetic equation is as following:
The Avrami model is as following:
where qt and qe are the CO2 adsorption capacity of the adsorbent at time t and saturated adsorption capacity of CO2, respectively, mmol/g; k1, k2 and kA are the rate constants of three models, respectively, /min, g/(mmol·min) and /min, and nA is the Avrami model series.
3 Results and discussion
3.1 Characterization
The XRD patterns of ZSM-5, SBA-16, ZS and ZS-A-1 are presented in Fig. 2. As shown in the small-angle XRD pattern of the synthetic material (Fig. 2b), SBA-16 shows a sharp diffraction peak at 2θ = 8°, corresponding to SBA-16 with a 3D cubic (Im3m) mesostructured [31] and the (110) crystal plan [32]. The composite molecular sieve ZS shows the similar characteristic peak as SBA-16, but intensity of peak is weakened, which shows that the order of the mesoporous structure of ZS decreases. As seen from the wide-angle XRD pattern of the synthetic material (Fig. 2a), the as-synthesized ZSM-5 shows intense XRD peaks at 2θ = 7.9, 8.8, 20.9, 23.1, 23.9, and 29.9° corresponding well to the (101), (200), (103), (501), (303), and (503) planes of ZSM-5 [33]. Besides XRD patterns from ZSM-5, ZS and ZS-A-1 showed extra-reflections appearing as shoulders at slightly lower 2θ angles than the main peaks. The ZS and ZS-A-1 show a lower intensity in the characteristic peaks compared with ZSM-5 due to the existence of SBA-16 phase. These results suggested the well combination of dual pore structures in the composites. Besides, modified by APTES, the mesoporous and microporous structures of ZS were maintained, but the degree of order decreased.
SEM images of SAB-16, ZSM-5 and ZS are shown in Fig. 3. SBA-16 is a hard rock-like crystal with rough surfaces and hollow hole are observed (Fig. 3a, b), accompanied by the formation of a small amount of almost spherical SBA-16, and its morphology is similar to the report of Atul kumar et al. [34]. It can be seen from Fig. 3c, d that the shape of ZSM-5 is spherical, with a layer of crystal filaments sort of fluffy crystals attached to the surface and a cluster of long crystals, which is similar to the result reported by Gomes Elisa S et al. [35]. Figure 3e, f show that ZS is a clumpy agglomerate with a slightly uneven surface, and it appears that ZSM-5 is wrapped in SBA-16 molecular sieve. This indicates that ZS was successfully synthesized, which is consistent with the XRD results.
Figure 4 shows the FTIR spectra of the SBA-16, ZSM-5, ZS, APTES grafted ZS and double functionalized ZS with various amounts of TEPA impregnation. All materials showed broad bands near 3460/cm and 3480/cm due to the physically absorbed water and the stretching vibration mode of silanol –OH groups present on surface [36, 37]. At the same time, the bands around 1650/cm and 950/cm were found, which were attributed to Si–OH bending vibrations mode of identical bond as H2O described above [38, 39]. The T–O–T (T = Si or Al) vibration of the five-membered rings, the symbol of ZSM-5 [40], was found at 550/cm in the spectrum of ZS and at lower wavenumber in the spectra of the modified samples. The asymmetric and symmetric stretching vibration bands of Si–O–Si in the skeleton were displayed around 1220/cm and 793/cm [36, 37], respectively, and the bending stretching vibration band was shown around 455/cm [40]. Furthermore, the peak at 2360/cm was corresponding to band of CO2, probably related to incomplete background deduction. After APTES modification, the new bands of ZS-A-1 at 2920/cm and 660/cm were associated with C–H of methyl stretching and bending vibration, respectively, which indicates that the modifier APTES was successfully grafted onto the ZS sample.
In all cases, new bands appear in the FTIR spectra of the mine-modified ZS, thus confirming the presence of APTES and TEPA. New bands were identified around 2940, 2880, 1570, 1475, 1390 and 679/cm. The C–H asymmetric, symmetric stretching and bending vibrations were observed around 2940, 2880 and 1475/cm, respectively [41]. The new bands that arouse around 1570 and 1390/cm are ascribed, respectively, to bending and stretching vibrations modes of –NH2 bonds [42] present in C–N groups of the two amine compounds used: TEPA and APTES. Besides, the shoulder peak of ZS corresponded to the free silanol group Si–O stretching vibration at 962/cm disappeared and the peak of the group Si–O–Si appeared at 679/cm due to the reaction with the amine group on the modifier [43]. The reaction between silanol groups and APTES is depicted as follows:
The appearance of –CH2 group and the disappearance of Si–OH group after grafting also suggest that the APTES was successfully grafted on to the framework of the ZS. On the other hand, compared with the ZS modified by grafting alone, it can be seen that with the increase of TEPA impregnation, the peaks at 1570/cm and 2940/cm were significantly enhanced, and the peak at 1660/cm of physically adsorbed water was also enhanced, which was mainly due to the introduction of TEPA improving the ability of the materials to adsorb water. Infrared analysis results showed that TEPA was successfully loaded onto ZS grafted by APTES and the modified material and CO2 mainly react as follows:
Figure 5a shows the TGA weight loss curves of ZS and double functionalized ZS with different loadings of TEPA; Fig. 5b shows the corresponding DTG curves. It can be seen from the TGA curve that the bifunctionalized ZS materials mainly have three weight loss stages. The first weight loss appeared below 130 °C for each sample because of removal of moisture, impurity gases and residual solvents in the synthesis process. The amine evaporation and decomposition caused the second and third weight loss in the temperature range of 130–350 °C, 350–600 °C for the samples of double functionalized ZS-A-T-X, respectively. Among them, the second and third weight loss were mainly caused by TEPA and APTES, respectively. ZS-A-T-X demonstrated a sharp weight loss peak near 205 °C and ZS-A-T-60 appeared the maximum weight loss peak. In this stage, with the same APTES grafting amount, when the TEPA impregnation content increases, the mass loss gradually increases, the intensity peak obviously increases, and the stability of materials decreases. With impregnating 30, 50, 55 and 60% TEPA, the weight losses of the materials were 3.8, 4.8, 5.1 and 8.5%, respectively (Fig. 5a). These weight loss values were all lower than the theoretical value, mainly because APTES and TEPA were not fully loaded on ZS during the bifunctionalization process. In addition, the lower weight loss compared to the theoretical loading amount can be explained by that the partial amines loaded on the surface of the support were evaporated from the support during the drying process. No obvious weight loss was found in the temperature range of 600–800 °C for each sample. The amine-bifunctionalized ZS exhibited excellent thermal stabilities in nitrogen flow of up to 130 and 200 °C, respectively. This suggests that the regeneration temperature of 110 °C was suitable for the cyclic adsorption/desorption operation in all of the samples.
The N2 adsorption/desorption isotherms of the bifunctionalized ZS materials and pore size distribution of SBA-16, ZS and ZS-A-1 were shown in Fig. 6, and the textural properties such as specific surface area (SBET) and total pore volume (Vtotal) were summarized in Table 1. In Fig. 6a, SBA-16 and ZS belonged to typical IV isotherms, showing the H2 hysteresis ring, which were the adsorption characteristic of mesoporous [29]. However, ZSM-5 showed the type I isotherm, which was the adsorption characteristic curve of microporous materials. The hysteresis loop height of SBA-16 was higher than that of ZS, indicating that its pore volume was larger than that of ZS. The results were consistent with the data in Table 1. In Fig. 6b, when the amount of modifier added was lower, the modified sample initially shows high nitrogen uptake, and at a relative pressure of P/P0 = 0.92–1.00, it shows a hysteresis loop attributed to the capillary condensation of N2. Unfortunately, with the increase of TEPA impregnation amount, the N2 adsorption/desorption isotherm of the composite material ZS gradually decreased, and the hysteresis loop also gradually shrank to disappear. The reason was that the amine groups loaded onto the pores and surfaces of ZS increased with the load increased, resulting in the decrease of pore size and N2 adsorbed amount, the disappearance of hysteresis rings, and the pores filling. In Fig. 6c, the pore diameter distribution of SBA-16 was mainly centered around 6.99 nm, while the pore diameter distribution of ZS was mainly centered around 5.10 nm. This was mainly due to the embedding method adopted in this experiment, ZSM-5 micropore zeolite was imbedded into the pore channels of the mesoporous molecular sieve SBA-16, resulting in the decrease of pore diameter. However, ZSM-5 doesn’t have mesoporous pore size, which indicated that the prepared composite ZS had ordered mesoporous pore channels. In addition, due to the presence of ZSM-5, the pore diameter distribution and pore size of ZS materials decreased. After grafting ZS with APTES, organic amines were loaded onto the pores and surfaces of the material, blocking some of the pores and making the materials aperture smaller, which agrees with the data in Table 1, and with the increase of the amine loading capacity, the specific surface area and pore volume of the bifunctional materials decreased rapidly, from 6.304 to 5.063 m2/g and 0.022 to 0.020 cm3/g, respectively.
Table 1 also listed the nitrogen content of each sample measured by the element analyzer, and the amine efficiency was calculated by Eq. (13).
where \(n_{{{\text{CO}}_{2} }}\) is saturated adsorption capacity of CO2, mmol/g; nN is the molar content of element N, mmol/g.
As can be seen from the Table 1 that with the increase of the impregnated amount of TEPA, the nitrogen content of the impregnated modified sample ZS-A-T-X also gradually increases, while the amine efficiency and CO2 adsorption capacities increases first and then decreases with the increase of the impregnated amount due to the high concentration of the modifier. On one hand, at low levels, the modifier TEPA can be uniformly dispersed on the surface of the carrier, so the amine efficiency was higher. On the other hand, with the increase in the amount of modifier, the modifier was excessively agglomerated, clogging the pores, causing the amine inside the pores to fail to contact and react with CO2, and decreasing the amine efficiency. It should be noted that ZB-A-T-55 exhibits the highest CO2 adsorption capacity and amine efficiency which was attributed to the excellent structural order, the high nitrogen contents and the outstanding porosity. Therefore, it is essential to maintain excellent porosity and a high degree of amine dispersion based on an increased loading of amine. The CO2 saturated adsorption capacity of adsorbents, ZS, ZS-A-1 and ZS-A-T-55, were 1.238, 1.370 and 1.630 mmol/g, respectively. The results were expected as the synergy of APTES and TEPA on ZS carrier was resulted in greater CO2 adsorption. Although the CO2 adsorption capacity of the bifunctionalized material was not very high, it proved that the bifunctionalized adsorbent was higher than that of grafting alone, indicating that the double functionalization route was successful. There exist some possible reasons that: (a) the steric hindrance between some amine groups and other amine groups makes it impossible for them to react with CO2, resulting in the decrease of their adsorption capacity, (b) APTES grafting quantity is not so much as the theoretical quantity, (c) when bifunctionalised materials was loaded with large amounts of amine groups, good porosity should be ensured to prevent excessive pore blockage, and (d) in general, the higher pressure results in the more rapid kinetics of adsorption, but our experimental condition is at the ambient pressure. In summary, the application of the adsorbent was widened by using the bifunctionalized modification strategy.
3.2 Adsorption performance of samples
Figure 7a shows the CO2 adsorption curves for the bifunctionalized ZS with different loadings of TEPA at 90 °C. Figure 7b and c show the CO2 adsorption capacity curves for ZS-A-1 and ZS-A-T-55 at different temperatures (30, 45, 60, 75 and 90 °C), respectively. The data were presented in Table 1. Under the constant grafting amount of APTES, with increasing TEPA loading, the CO2 adsorption capacity increased first and then decreased. The CO2 saturated adsorption capacity of bifunctionalized adsorbents, ZS-A-T-30, ZS-A-T-50, ZS-A-T-55 and ZS-A-T-60, were 1.02, 1.27, 1.63 and 1.02 mmol/g, respectively, and the maximum adsorption capacity was obtained for ZS-A-T-55. Amine functional groups could react with CO2, theoretically the higher the load of impregnated amine group, the higher the adsorption capacity of CO2. Unexpectedly, excessive amine loading on the bifunctionalized ZS would cause the obstruction of the carrier channel, hindered the diffusion of CO2 in the material channel and the amine reaction in the pore.
In Fig. 7b, similarly, with increasing temperatures, the CO2 adsorption capacity increased first and then decreased, and the maximum adsorption capacity was obtained for ZS-A-1 at 60 °C. Besides, the CO2 adsorption capacity for ZS-A-T-55 increased with increasing temperatures which was described in Fig. 7c. Compared with the ZS modified by grafting alone, the bifunctionalized ZS exhibited higher CO2 adsorption capacity of 1.63 mmol/g and thermal stabilities of 90 °C. The reason was that the silicon hydroxyl groups on ZS can react with APTES, which promoted the dispersion of TEPA, making TEPA more uniformly dispersed in the pores of carriers and providing channels for CO2 diffusion. The synergy of APTES and TEPA on ZS carriers improved the CO2 adsorption performance of the adsorbents. Moreover, with the increase of temperature, the kinetic energy of CO2 molecules increased, benefiting the overcoming of kinetic obstacles, reduction of diffusion resistance, increment of the collision probability with amine active sites, thereby increasing the amount of CO2 adsorption.
It can be expected to be used as an efficient adsorbent in a wide range of flue gas temperatures (30–75 °C) without significant loss of CO2 adsorption capacity. The main reason why higher temperatures were not measured was that by continuing to increase the temperature, the desorption temperature would be reached and the CO2 previously adsorbed on the surface of the sample would start to desorb. At the same time, it was approached the actual flue gas desulfurization temperature. In other words, as the temperature increases, the CO2 adsorption capacity of ZS-A-T-55 were similar in a wide range (30–75 °C), and the best adsorption capacity was achieved at 90 °C.
It is interesting to note that this temperature was related to the diffusion limitation model with two different effects. The positive effect was that the kinetic limitation was diminished and the uniform diffusion of grafted amines was accelerated at higher temperatures, favoring the chemisorption of CO2 due to increment of the collision probability with active sites for CO2-amine interactions. On the other hand, the negative effect was that the high thermal energy contributed to the high activity of CO2 molecules at relatively high temperature, thereby resulting in the partial desorption of CO2 from chemical interaction and van der Waals’ force. Thus, the equilibrium between dynamics and thermodynamics is extremely important for CO2 adsorption performance.
The CO2 adsorption mechanism for bifunctionalized samples was further discussed. For example, the reaction in Eq. (7) can be divided into two steps as follows:
An intermediate zwitterion (RNH2+COO−) was formed by the attack of unpaired electrons in nitrogen to the electrophilic carbon atom of CO2 in the first step [Eq. (14)]. In the second step, the carbamate was formed from the charge compensation with a close neighbor amine group [Eq. (15)]. The amine impregnated into the grafted sample enhanced the interaction in Eq. (15) owing to the high mobility of impregnated amine molecules. In other words, due to the high mobility of the impregnated amine, it can be dispersed within the grafting amine, facilitating the second step of the reaction. The synergistic effect of fixed amines and mobile amines improved the CO2 adsorption capacity because the impregnated amines could affect the adsorption performance of grafted amines.
Table 2 summarizes the CO2 adsorption capacities of this experiment and other materials. As can be seen from the data in Table 2, although the adsorption capacities of four adsorbents were higher than that of this work, the advantage of amine-bifunctional micro-/mesoporous molecular sieve ZS cannot be ignored. Under the same conditions, the CO2 adsorption properties of these materials were found to depend on the kind, amount and viscosity of the organic compound loaded. However, the higher the concentration of CO2, the better the adsorption effect, while in this work, 15 vol% CO2 was closer to the CO2 content in the actual flue gas, which can better represent the adsorption performance in the actual application.
To determine the cyclic adsorption performance, the ZS-A-T-55 modified material with the highest adsorption capacity was performed 5 cycles of CO2 adsorption/desorption experiments (Fig. 8). After 5 cycles of adsorption/desorption, the fifth CO2 adsorption amount (1.496 mmol/g) of ZS-A-T-55 reduced by 8.2% compared with the first adsorption amount (1.630 mmol/g). There are three main reasons for this: one is that after the grafting modification, the TEPA that can be impregnated on the surface of the material was less than that in theory; the second is that during the grafting process, APTES were combined with the silanol on the ZS carrier; the last is due to the hydrogen bonding between the amine groups in the APTES and TEPA modifiers and the silanol groups in the ZS composite molecular sieve. At the same time, attribute to the combined action of covalent bonds and hydrogen bonds, the loss of TEPA during the heating process reduced [44]. Therefore, the bifunctional modified material showed eminent thermal stability and regeneration performance and is promising in potential applications in actual flue gas after desulfurization.
To investigate the adsorption mechanism of CO2, the experimental data of the adsorption of CO2 on the bifunctional modified sample ZS-A-T-55 at different temperatures were fitted and analyzed by the pseudo-first-order kinetic, pseudo-second-order kinetic and Avrami model. The fitting results are shown in Fig. 9a, b and c, respectively. The specific fitting parameters are shown in Table 3.
It can be seen from the fitting curve of the pseudo-first order kinetic adsorption that the correlation coefficient R2 fitted to the data of the ZS-AT-55 sample at 30, 60, 75 and 90 °C are all greater than 0.990. However, at 45 °C, the correlation coefficient R2 is 0.985, which is slightly lower than 0.990, indicating that the pseudo-first order kinetic equation cannot describe well the ZS-AT-55 at 45 °C and the CO2 adsorption process is not purely physical adsorption.
Then the data was simulated with the pseudo-second-order kinetic model, and found that the correlation coefficient R2 of ZS-AT-55 at all temperatures was less than 0.990, and the fitness are low, indicating that the pseudo-second-order kinetic equation cannot describe well CO2 adsorption reaction process of ZS-A-T-55. Therefore, its CO2 adsorption process is not purely chemical adsorption. Finally, the Avrami model was used to fit the adsorption data. The results show that the fitted correlation coefficients R2 are all greater than 0.990. Thus, the Avrami model can describe the adsorption process well, which shows that the adsorption process of CO2 by the material is a combination of physical and chemical adsorption process.
4 Conclusions
The ZSM-5/SBA-16 support was modified with grafted amine (APTES) and impregnated amine (TEPA) by bifunctionalization. XRD analysis shows that the micro/mesoporous composite ZS has the cage cubic mesoporous ordered structure of SBA-16 and the microporous structure of ZSM-5 zeolite at the same time, and the structure is not damaged after grafting, which is in close agreement with the results of XRD. The CO2 adsorption performances of the before and after ZS bifunctionalization were investigated in simulated flue gas conditions. With higher nitrogen content and better texture properties, ZS-A-T-55 achieved a highest CO2 uptake of 1.630 mmol/g and a highest amine efficiency of 0.25 at 90 °C while 1.373 mmol/g and 0.26 for ZS-A-1, indicating that the double functionalization route was successful. The adsorption process is the synergy between physical and chemical adsorption. The adsorption capacity of ZS-A-T-55 exhibited just 8.2% attrition of the original (1.630 mmol/g) after five adsorption/desorption recycles (1.496 mmol/g). The amine-bifunctionalized ZSM-5/SBA-16 adsorbent presented excellent CO2 adsorption/desorption performance, which is expected to show feasibility for industrial application.
References
Z. Lin, W. Shen, X. Chen, J.-P. Corriou, H. Xi, Appl Surf Sci 529, 147130 (2020). https://doi.org/10.1016/j.apsusc.2020.147130
E. Perez-Botella, R. Martinez-Franco, N. Gonzalez-Camunas et al., Front Chem 8, 588712 (2020). https://doi.org/10.3389/fchem.2020.588712
K.S. Lakhi, A.V. Baskar, J.S.M. Zaidi et al., RSC Adv. 5, 40183 (2015). https://doi.org/10.1039/c5ra04730g
H.A. Patel, J. Byun, C.T. Yavuz, Chemsuschem 10, 1303 (2017). https://doi.org/10.1002/cssc.201601545
J. Adánez, A. Abad, T. Mendiara, P. Gayán, L.F. de Diego, F. García-Labiano, Prog. Energy Combust. Sci. 65, 6 (2018). https://doi.org/10.1016/j.pecs.2017.07.005
J. Wang, L. Huang, R. Yang et al., Energy Environ. Sci. 7, 3478 (2014). https://doi.org/10.1039/c4ee01647e
S. Choi, J.H. Drese, C.W. Jones, Chemsuschem 2, 796 (2009). https://doi.org/10.1002/cssc.200900036
Q. Shen, X. Li, R. Li, Y. Wu, ACS Sustain. Chem. Eng. 8, 17608 (2020). https://doi.org/10.1021/acssuschemeng.0c06849
D.A. Khuong, H.N. Nguyen, T. Tsubota, Biomass Bioenergy 148, 106039 (2021). https://doi.org/10.1016/j.biombioe.2021.106039
L. Borchardt, Q.-L. Zhu, M.E. Casco et al., Mater. Today 20, 592 (2017). https://doi.org/10.1016/j.mattod.2017.06.002
Y. Zhao, J. Zhou, L. Fan et al., Ind. Eng. Chem. Res. 58, 19465 (2019). https://doi.org/10.1021/acs.iecr.9b03338
X. Ren, H. Li, J. Chen et al., Carbon 114, 473 (2017). https://doi.org/10.1016/j.carbon.2016.12.056
H.J. Choi, J.G. Min, S.H. Ahn et al., Mater. Horiz. 7, 1528 (2020). https://doi.org/10.1039/d0mh00307g
Z. Song, Q. Dong, W.L. Xu, F. Zhou, X. Liang, M. Yu, ACS Appl. Mater. Interfaces 10, 769 (2018). https://doi.org/10.1021/acsami.7b16574
K. Li, J.D. Kress, D.S. Mebane, J. Phys. Chem. C 120, 23683 (2016). https://doi.org/10.1021/acs.jpcc.6b08808
D.S. Mebane, J.D. Kress, C.B. Storlie, D.J. Fauth, M.L. Gray, K. Li, J. Phys. Chem. C 117, 26617 (2013). https://doi.org/10.1021/jp4076417
S. Kim, K.B. Lee, Chem. Eng. J. 356, 964 (2019). https://doi.org/10.1016/j.cej.2018.08.207
Y. Xiao, M. Zhou, G. He, Ind. Eng. Chem. Res. 58, 14380 (2019). https://doi.org/10.1021/acs.iecr.9b02670
S.C.G. Santos, S.W.M. Machado, A.M. Garrido Pedrosa, M.J.B. Souza, J. Porous Mater. 22, 1145 (2015). https://doi.org/10.1007/s10934-015-9990-0
H. Thakkar, A. Issa, A.A. Rownaghi, F. Rezaei, Chem. Eng. Technol. 40, 1999 (2017). https://doi.org/10.1002/ceat.201700188
Y. Belmabkhout, R. Serna-Guerrero, A. Sayari, Chem. Eng. Sci. 65, 3695 (2010). https://doi.org/10.1016/j.ces.2010.02.044
Z. Lin, J. Wei, L. Geng, D. Mei, L. Liao, Energy Technol. 6, 1618 (2018). https://doi.org/10.1002/ente.201700780
P. Kubisa, S. Penczek, Prog. Polymer Sci. 24, 1409 (1990)
E.S. Sanz-Pérez, A. Arencibia, R. Sanz, G. Calleja, Adsorption 22, 609 (2015). https://doi.org/10.1007/s10450-015-9740-2
R. Sanz, G. Calleja, A. Arencibia, E.S. Sanz-Pérez, Microporous Mesoporous Mater. 209, 165 (2015). https://doi.org/10.1016/j.micromeso.2014.10.045
P.D. Jadhav, R.V. Chatti, R.B. Biniwale, N.K. Labhsetwar, S. Devotta, S.S. Rayalu, Energy & Fuels 31, 3555 (2007)
T. Liu, H. Wang, Z. Hu, F. Wei, Microporous Mesoporous Mater. 316, 110968 (2021). https://doi.org/10.1016/j.micromeso.2021.110968
C. Bläker, C. Pasel, M. Luckas, F. Dreisbach, D. Bathen, Microporous Mesoporous Mater. (2020). https://doi.org/10.1016/j.micromeso.2020.110205
D. Carta, T. Montini, M.F. Casula et al., J. Mater. Chem. A 5, 20024 (2017). https://doi.org/10.1039/c7ta03640j
J. Wei, J. Shi, H. Pan, W. Zhao, Q. Ye, Y. Shi, Microporous Mesoporous Mater. 116, 394 (2008). https://doi.org/10.1016/j.micromeso.2008.04.028
X. Wang, J. Mei, Z. Zhao et al., ACS Catal. 8, 1891 (2018). https://doi.org/10.1021/acscatal.7b04147
N. Zucchetto, M.J. Reber, L. Pestalozzi, R. Schmid, A. Neels, D. Brühwiler, Microporous Mesoporous Mater. 257, 232 (2018). https://doi.org/10.1016/j.micromeso.2017.08.046
M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki, R. Ryoo, Nature 461, 246 (2009). https://doi.org/10.1038/nature08288
A. Kumar, N. Ranga, S. Duhan, R. Thakur, J. Porous Mater. 27, 1431 (2020). https://doi.org/10.1007/s10934-020-00910-3
E.S. Gomes, D.A.G. Aranda, M.M. Pereira, B. Louis, Microporous Mesoporous Mater. 263, 251 (2018). https://doi.org/10.1016/j.micromeso.2017.12.022
H. Chaudhuri, S. Dash, A. Sarkar, RSC Adv. 6, 99444 (2016). https://doi.org/10.1039/c6ra21480k
M. Naghiloo, M. Yousefpour, M.S. Nourbakhsh, Z. Taherian, J. Sol-Gel. Sci. Technol. 74, 537 (2015). https://doi.org/10.1007/s10971-015-3631-6
A. Feliczak-Guzik, B. Jadach, H. Piotrowska, M. Murias, J. Lulek, I. Nowak, Microporous Mesoporous Mater. 220, 231 (2016). https://doi.org/10.1016/j.micromeso.2015.09.006
Y. Jiang, Q. Gao, H. Yu, Y. Chen, F. Deng, Microporous Mesoporous Mater. 103, 316 (2007). https://doi.org/10.1016/j.micromeso.2007.02.024
A.A. Ahmed, Z.H. Yamani, Mater. Chem. Phys. (2021). https://doi.org/10.1016/j.matchemphys.2020.124181
N. Horri, E.S. Sanz-Pérez, A. Arencibia, R. Sanz, N. Frini-Srasra, E. Srasra, Appl. Clay Sci. (2019). https://doi.org/10.1016/j.clay.2019.105195
J. Yu, Y. Zhai, S.S.C. Chuang, Ind. Eng. Chem. Res. 57, 4052 (2018). https://doi.org/10.1021/acs.iecr.7b05114
X. Jiang, Y. Kong, Z. Zhao, X. Shen, RSC Adv. 10, 25911 (2020). https://doi.org/10.1039/d0ra04497k
W. Wilfong, B. Kail, B. Howard, T. F. Aquino, S .T. Estevam, M. Gray, Energy Technol. 5, 228 (2016). https://doi.org/10.1002/ente.201600319
Acknowledgements
The authors sincerely acknowledge the support of National Natural Science Foundation of China (Grant No.51966002) and Natural Science Foundation of Guangxi Province (Grant No. 2020GXNSFAA159144).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, L., Wei, J., Geng, L. et al. Amine-bifunctionalized ZSM-5/SBA-16 composite for CO2 adsorption. J Porous Mater 29, 19–31 (2022). https://doi.org/10.1007/s10934-021-01146-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01146-5