Abstract
Lithium (Li) metal is considered to be the most ideal anode for rechargeable Li-metal batteries due to its high theoretical specific capacity and low redox potential. However, the Li dendrite problem associated with Li metal anodes limits its practical application. Herein, a new chemical dealloying method that adjust the pore structure formed during chemical dealloying at room temperature to prepare a porous Copper current collector which was to enable dendrite-free Li deposition. Benefiting from the naturally formed suitable size and uniform porous structure, this current collector provides larger surface area to homogenize the Li ion distribution and promote dendrite-free Li metal anode with improved Coulombic efficiency (96% for over 210 cycles at the current density of 0.5 mA cm−2 and 95% for 80 cycles at 1.0 mA cm−2). This work provides a novel way to suppress Li dendrite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the explosive growth of demand for lithium-ion batteries (LIB) in recent years, people’s requirements for high-density energy storage have been higher [1]. The theoretical specific capacity of the commercial graphitic anode is only 372 mAh g−1, which greatly limits the capacity of LIBs [2, 3]. As the most ideal anode material, Li metal holds quite a higher theoretical specific capacity (3860 mAh g−1) and lower redox potential (− 3.04 V vs. the standard hydrogen electrode) [4]. Lithium metal batteries (LMBs) with lithium metal as the anode, including lithium–sulfur (Li–S) batteries and lithium–oxygen (Li–O2) batteries, show extremely high specific energy densities [5,6,7]. However, the unstable Li dendrite growth and low Coulombic efficiency (CE) in LIB have prevented its real application [8, 9]. Unlike the commercial LIB, the LMB undergoes a striping/plating process on the planar substrate without any “host”, thus the morphology of the surface of the Li metal anode inevitably becomes rough and forms some protuberant tips [2, 8]. And the Li dendrite is formed eventually in the repeated cycling process [10]. With the growth of Li dendrite, it can break down the solid electrolyte interphase (SEI) and cause continuous interfacial reactions, further leading to the CE reduced and capacity decay [11, 12]. Also, Li dendrite can penetrate the separator and cause an irreversible volume expansion even safety hazards [13].
In recent years, porous materials with a high specific surface area have been studied in the field of energy storage [14, 15], such as LIB [16, 17], Li–S batteries [18] and supercapacitors [19]. Solving the Li dendrite problem is the key to realizing the commercial application of LMBs, for which multifarious strategies have been proposed by researchers [20]. Such as the modification of the separator [21], electrolyte [19, 22], solid electrolyte [23, 24], and planting a protective layer [25, 26]. As for the Li metal battery, according to the Sand’s model as shown below [27]:
Sand’s time (T) can be used to indicate the time when Li dendrites begin to form. It is therefore that as the effective electrode current density (J) increases, the longer it takes for the dendrites to growth. Therefore, using porous materials with high specific surface area to inhibit the generation of lithium dendrites has become a topic of interest to researchers [28, 29]. Carbon-based materials with high specific surface area were coated on the anode collector and acted as the deposition carrier of lithium, which effectively inhibited the generation of lithium dendrites [30, 31]. And the current collector has been widely concerned about solving the problem of Li metal anode [32, 33]. Using the current collector with 3D conductive framework is considered to be an effective way to suppress the growth of dendrites [34, 35]. The main reason is that the 3D porous structure provides a large specific surface and low local current density [4, 36].
Chemical dealloying is a universal method for the preparation of porous metallic materials due to its simplicity and accessibility [37,38,39]. In this paper, the Cu–Zn alloy is used as raw material. Because of the large potential difference between Cu and Zn (φCu2+/Cu = + 0.337 V, φZn2+/Zn = − 0.762 V), a simple chemical dealloying is prepared a porous Cu and application as a current collector for Li metal anode are explored. Then the effects of sulfuric acid, zinc ion and ultrasonic treatment on the pore structure of porous Cu prepared by the dealloying Cu–Zn alloy were studied. On the joint action of sulfuric acid, zinc ion, and ultrasonic treatment are used together, larger and more uniform holes can be obtained compared to sulfuric acid only. The advantages of using the porous Cu as a current collector are as follows. (1) The interconnected pores provide a large internal surface area to accommodate Li and a low local current density to induce uniform deposition of Li. (2) The Cu skeleton which has suitable and more uniform holes can 0promote the Li deposition due to its lithiophilic property and avoid the local accumulation of Li due to its rough structure. The porous–Cu/Li cell with porous Cu as electrode were assembled to characterize the electrochemical performance of porous Cu. The results show that at the current density of 0.5 mA cm−2, the porous–Cu/Li cell can cycle stably with a high CE (> 96%) for 210 cycles. Such excellent performance proves that the prepared porous Cu current collector inhibits the growth of lithium dendrites to a certain extent, and demonstrates a great potential for commercialization of Li metal battery.
2 Experimental
2.1 Materials preparations
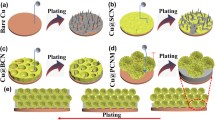
The schematic illustration for the formation process of the porous Cu is shown in Fig. 1a. To begin this process, the brass (Cu0.62Zn0.38) sheet was punched into disc with a diameter of 14 mm and successively washed with acetone and deionized water 3 times. After completely drying, the brass disc was immersed into the prepared 120 g/L H2SO4 and 90 g/L ZnSO4 mixture solution for 15 h. And the ultrasonic device (KQ-100DE) was used to activates the Zn on the alloy and control temperature. When the sample turned reddish brown completely after 15 h, took it out from the solution and put it into a vacuum drying oven (DZF-2B) for 24 h at low temperature. Then, the porous Cu was obtained, which can be used directly in a glove box.
Schematic illustration for the formation process of the porous Cu and the reaction principle. a Porous Cu obtained from Cu–Zn in the aqueous solution of 120 g/L H2SO4 + 90 g/L ZnSO4. b The reaction principle. The porous copper c obtained by chemical dealloying of sulfuric acid and ZnSO4 solution with ultrasonic intensification contains, d obtained compared to sulfuric acid only
2.2 Material characterizations
The surface morphology of the samples was characterized by field-emission scanning electron microscope (GEMINI SEM 300). The elemental distribution of the samples was characterized by energy-dispersive spectroscopy (EDS). In addition, X-ray diffraction (XRD, D8 ADVANCE A25) was used to characterize the composition change of the samples during the process of chemical dealloying, and the scanning range was 20°–80°(2θ).
2.3 Electrochemical characterizations
The commercial Cu foil and the prepared porous Cu with a diameter of 14 mm were used respectively to assemble Cu/Li and porous–Cu/Li cells (CR2025) with Li metal as the counter electrodes. In order to compare the confinement difference of the deposited/stripped Li between these two kinds of current collector, the Cu/Li and porous–Cu/Li cells were tested at different current density. 1 M lithium bis(trifluoromethanesulphonyl)imide (LiTFSI) in 1,3-dioxolane (DOL) and 1,2- dimethoxyethane (DME) (volume ratio 1:1) with 2% lithium nitrate (LiNO3) was used as electrolyte. Celgard-2325 polypropylene film was used as separator. The cells were first cycled from 0 to 1 V at 50 µA for five cycles to remove surface impurities and stabilize the SEI. After the activation process, the cells were examined with a capacity of 1 mAh cm−2 at the current densities of 0.5 mA cm−2 and 1 mA cm−2, respectively. All of the cells were assembled and disassembled in the glovebox filled with argon. The LANHE CT2001A was used as a testing system to characterize the electrochemical performance of the cells. The electrochemical impedance spectroscopy (EIS) measurements were performed on PARSTAT 4000A Electrochemical System (AMETRK, USA) with an amplitude of 5 mV at frequencies range from 0.01 Hz to 100 kHz. For the symmetrical cell test, planar Cu@Li and porous Cu@Li electrodes were cycled at a current density of 1 mA cm−2 with 1 mA h cm−2 Li plating/stripping.
3 Results and discussion
3.1 Morphology and composition analysis of porous Cu
The porous Cu was obtained from the chemical reaction of Cu–Zn alloy in the prepared 120 g/L H2SO4 and 90 g/L ZnSO4 mixture solution. The XRD patterns of initial Cu–Zn alloy and the porous Cu are shown in Fig. 2a. Comparing the two kinds of materials, it can be seen that the characteristic peak of the Cu0.62Zn0.38 phase in the initial Cu–Zn alloy disappears after 15 h of dealloying. While the characteristic peak of the pure Cu phase becomes distinct significantly, also without any impurities, indicating that almost all Zn phase in the initial Cu–Zn alloy dissolves in the mixture solution. From the pictures of initial (Fig. 2b) and prepared material (Fig. 2c), it can be observed that the surface of the Cu–Zn alloy is golden yellow, but the surface of the sample after dealloying becomes brownish red in accordance with Cu. Macroscopically, it shows that the Zn phase in the Cu–Zn alloy has dissolved and the Cu phase is retained. The reaction mechanism of Cu–Zn alloy in a mixture solution of 120 g/L H2SO4 and 90 g/L ZnSO4 is shown in Fig. 1b. When Cu and Zn are immersed in the dilute sulfuric acid (H2SO4) at the same time, it can be considered that a Cu–Zn primary battery is formed, Cu and Zn work as the two electrodes. In regard to the mechanism of anodic dissolution for brass, according to the dissolution–redeposition mechanism [40,41,42,43], the primary reactions involved in the corrosion of brass are as follows:
Because Zn is much more active than Cu, Zn will preferentially lose electrons and generate Zn2+ into the solution as presented in Eq. (2), while H+ in the solution will get electrons near the Cu electrode to generate H2 as presented in Eq. (3). Chemical dealloying of Zn occurs around the grain boundary, Zn dissolution process leaving behind a metallic Cu layer. At the same time, under the influence of the solution potential and pH a small amount of Cu2+ exists at the surface [44] and which were soluble in this electrolyte as presented in Eqs. (4) [40], then it is redeposited on the brass surface when copper in solution reaches a certain concentration as presented in Eq. (5) [41].
Figure 3a–c show the results of SEM for the dealloying of brass conduced in sulfuric acid. It can be seen that chemical dealloying occurs only on the surface of the alloy, the sizes of pores varying from 100 to − 2 μm (Fig. S2), which are less dense and some regions have not yet become porous. These pores are the result of selective dealloying zinc. At the same time, there is lattice misfit due to the difference between the lattice constant of the alloy and copper. Therefore, chemical dealloying of zinc results in the joint effect of alloy volume change and corrosion stress, resulting in cracks on the surface of the alloy belt during the de-alloying process [45]. Because the crack has a higher surface energy, the hole always appears near the crack. Figure 3d–f show the results of SEM for the dealloying of brass conduced in sulfuric acid with the process strengthening by ultrasonic. It can be seen that the chemical dealloying products have more uniform but smaller pores. The reason may be that the ultrasound can generate oscillating bubbles, and the ion mass transfer process is strengthened, as well as pressures of up to several hundred mega-pascals in transient cavities while the bulk conditions of the liquid solution remain at ambient temperature [46]. At the same time, the process strengthening by ultrasonic reduces the concentration gradient between the solid–liquid interface and activates the Zn and Cu on the alloy surface, which makes the reaction more uniform, a large number of particles protruding from the surface can be considered as the dissolution-redeposition copper. Figure 3g–i show the results of SEM for the dealloying of brass conduced in sulfuric acid with ZnSO4. The structure of the pores is disorderly distributed in a honeycomb shape. According to the Gibbs free energies as shown below:
Increased concentration of Zn2+ in solution will inhibit the dissolution of Zn in the alloy to some extent, so the number of pores on the faces goes down. The Cu in the vicinity of the pore is activated due to the peeling of adjacent Zn atoms, under the dissolution of Cu and Zn, the structure of the pore becomes a 1–2 μm large pore, but the metal as a whole is bright yellow which color similar to the brass, indicating that there is a large amount of Zn leave over on the surface.
The process strengthening by ultrasonic make chemical dealloying products have more uniform but smaller pores, and ZnSO4 makes the structure of the pores is large and distributed in a honeycomb shape. So, we guess that when the sulfuric acid with ZnSO4 and process strengthening by ultrasonic are used together, larger and more uniform porous can be obtained compared to sulfuric acid only. The SEM images for the surface of initial Cu–Zn alloy are shown in Fig. 4a–c, while, that for the surface of prepared porous Cu are shown in Fig. 4d–f. Comparing the figures mentioned above, it can be seen that the initial Cu–Zn exhibits a very smooth and uniform surface structure at different amplification. As we would have guessed, the porous Cu obtained by chemical dealloying of sulfuric acid and ZnSO4 solution with process strengthening by ultrasonic contains a large number of pores with uneven shapes and large sizes (Fig. 1c, d). The diameter of the formed pore is less than 2 μm and the formed Cu skeleton is relatively rough compared with others porous in this paper. In Fig. S1, we shot SEM with top view and lateral view about porous Cu. Fig. S1a–d shows the top view of porous copper. As shown in Fig. S1a, there are a large number of uniform pore structures on the porous copper, and in Fig. S1e, we can see that the pore structure of porous copper is not only on the surface, but also has a large number of pore structures inside. As higher magnification images shown in Fig. S1b–d and Fig. S1f–h, the size of the holes is 200–500nm. The N2 adsorption and desorption isotherms of porous copper are shown in Fig. S6, the results show that the specific surface area is 0.5 m²/g. If the pores is too small, the current collector cannot effectively the growth of deposited Li and most of the Li metal is deposited on the surface of the current collector [47]. Through further analysis of the figures with larger amplification for the surface of porous Cu (Fig. 4g) and the corresponding result of EDS (Fig. 4h), only one element of Cu appears in the porous Cu and is almost uniformly distributed, proving that the Zn in the initial Cu–Zn alloy has been removed completely. And the obtained porous Cu can maintain a stable and independent structure after chemical dealloying.
We added XPS tests to more clearly show their samples’ properties. Compared with porous Cu (Figs. 3a and S4a), the intensity of Cu–Zn alloy (Figs. S3b and S4b) on the Zn peaks was strong. A significant decrease of the Zn peak was found after dealloying process. This proves that a large amount of zinc is dissolved in the solution, and a small amount of Zn is not dissolved inside the matrix. In Fig. S5a, the peaks of Cu (II) 2P3/2 BE of 9332.8 eV and Cu (II) Cu2P3/2 BE of 935.1 eV are both very high, which is because the alloy surface was covered by a passive film. As shown in Fig. S5b, a significant decrease of the peak Cu (II) was evidenced, and the color of the solution did not change to blue during the dealloying process. This also proves the dissolution-redeposition process.
3.2 Electrochemical performance of porous Cu and Li+ deposition behavior
Figure 5 shows the CE for Cu/Li and porous–Cu/Li cells at a deposition capacity of 1 mAh cm−2 under the current density of 0.5 mA cm−2 and 1 mA cm−2. As shown in Fig. 5a, the Cu/Li and porous–Cu/Li cells exhibit initial CE of 77.76 and 87.62% respectively at 0.5 mA cm−2, while the initial CE at 1 mA cm−2 is 62.3 and 75.12% respectively (Fig. 5b). The results indicate that the porous Cu improves the reversibility of the Li metal anode. When the current density is 0.5 mA cm−2, the CE of porous–Cu/Li cell reaches 90% after the initial 5 cycles, and when the cycle proceeds to the 20th cycle, the CE can reach more than 96% and maintains stable cycling over 210 cycles. As the cycle continues to increase, after 240 cycles, the CE of porous–Cu/Li cell gradually drops to below 90%. Under the same cycling conditions, as for the Cu/Li cell, its CE can maintain more than 95% for 90 cycles, then the test data begins to appear significant fluctuations and drops to below 80% after 150 cycles. When the current density increases to 1 mA cm−2, the CE of porous-Cu/Li remains as high as 95% and sustains for 80 cycles, then the CE begins to decrease significantly. While the Cu/Li cell maintains stability for 40 cycles with a CE of 90%, after that, the CE appears a significant drop and reduces to below 80% at the 50th cycle. Comparing the cycling properties of the porous–Cu/Li and Planar Cu/Li, the stability of porous Cu during cycling is significantly more outstanding than that of commercial Cu foil, proving that the porous Cu current collector effectively inhibits the growth of Li dendrites. The application of porous Cu is expected to extend the lifespan of Li metal anode.
In order to characterize the distribution and growth of Li dendrites on the surface of Cu foil and porous Cu current collectors, the Cu/Li and porous–Cu/Li cells after cycle test were disassembled in the glove-box and the SEM images for the morphology of them are shown in Fig. 6a–f with different amplification. Figure 6a–c illustrate the surface morphology of commercial Cu foil after cycle test. It can be seen that a large amount of dendritic Li and mossy Li distribute on the planar unevenly. Due to excessive Li accumulation, the surface structure of the Cu foil becomes loose, then forming a large number of mossy lithium distributing on the surface independently. As shown in Fig. 6g, it can be seen that when protuberances are formed on the surface of the Cu foil, Li is more likely to deposit on the protuberance due to the difference in current density. The accumulation of Li increases with the proceeding of cycle and eventually grows into the dendrites. Figure 6b is a partial enlarged view of the independent structure on the surface of the planar Cu foil, it can be seen that the growth of Li dendrites still exists on the local structure. The rough surface of planar Cu foil can be observed from Fig. 6c. The planar Cu foil with numerous Li dendrites is easy to pierce the separator, causing an internal short circuit even the security risks. This part of Li cannot continue to participate in the reaction and will form “dead Li”. The Li dendrite and “dead Li” result in the irreversible loss of capacity directly. At the same cycling conditions, the surface of the porous Cu is inordinate smooth and the SEM images are shown in Fig. 6d–f. The pores of porous Cu are gradually filled with Li after cycle test compared with the initial morphology of it (Fig. 4d–f), eventually forming a planar structure. Moreover, Fig. 6f shows the deposition of Li among the pores is also particularly uniform. As shown in Fig. 6h, compared with the planar structure of Cu foil, the porous structure provides a larger specific surface area, which effectively reduces the local current density and promotes the uniform deposition of Li. The Cu exists as the form of skeleton, which can promote the Li deposition due to its lithiophilic property and avoid the local accumulation of Li due to its rough structure. After a long cycle test, the surface of porous Cu may begin to appear protuberances, and a small amount of dead Li emerges in the pores. However, the inhibition effect of porous Cu for Li dendrite is much better than that of the planar Cu foil. The application of porous Cu current collector for Li metal batteries is expected to exhibit excellent performance.
The electrochemical impedance spectroscopy (EIS) can investigate the interface stability of planar Cu/Li and porous Cu/Li cell. The Nyquist plots(1 mA cm−2, 1 mAh cm−2) of the planar Cu/Li and porous Cu/Li cell referring to before the cycle, 10th cycle and 100th cycle are shown in Fig. 7a−c, and the equivalent circuit is illustrated in Fig. 7d. As shown in Fig. 7a, before the cycle, the interfacial charge transfer resistance of planar Cu is about 332.1 Ω cm2, and porous Cu is about 154.4 Ω cm2. It is shown that the excellent conductivity of porous copper current collector. After 10 and 100 cycles, the interfacial charge transfer resistance for the planar Cu is about 29.11 Ω cm2 and 22.21 Ω cm2, but the porous Cu is about 18.25 Ω cm2 and 11.25 Ω cm2 as is shown in Fig. 7b, c. The porous Cu has much lower interfacial charge transfer resistance than planar Cu. For the symmetrical cell test to probe the cycling stability, from the Fig. 7e we can see that there are severe fluctuations in the curve for the planar Cu electrode, which is assigned to the nonuniform deposition of Li and irregular growth of dendrites. Compared with the overpotential of planar Cu electrode (70 mV), porous Cu electrodes maintains a stable and low overpotential (20 mV) with low and no fluctuation voltage hysteresis in 440 h. The reason is that the porous copper current collector inhibits the growth of lithium dendrites and “dead Li”, which improves the stability of SEI.
4 Conclusion
In this paper, the brass foil was chosen as the experimental material, the mixed solution of 120 g/L H2SO4 and 90 g/L ZnSO4 was selected as the reaction solution, and a porous Cu current collector was prepared by dealloying method with ultrasonic treatment. On the joint action of sulfuric acid, zinc ion and ultrasonic treatment are used together, larger and more uniform holes can be obtained compared to sulfuric acid only. The Zn from the brass is completely dissolved in the solution and the residual Cu with porous structure as current collector can promote the uniform deposition of Li+, further inhibiting the growth of Li dendrites. In the experimental results, the assembled porous–Cu/Li cell exhibited excellent electrochemical performance during cycling. The porous Cu current collector performed a high and stability CE, excellent conductivity and low overpotential. More importantly, the surface of porous Cu still maintains a very smooth state after cycling and without obvious Li dendrites. All the results show that the performances of the porous Cu are significantly improved compared to ordinary Cu foil, proving that this porous Cu can inhibit the growth of Li dendrites and is expected to be applied to Li metal electrodes to further improve the stability of LMBs.
Data availability
The data that support the findings of this study are available from the corresponding author: Zhao Fang fangzhao@xauat.edu.cn, upon reasonable request.
References
Z.P. Cano, D. Banham, S. Ye et al., Nat. Energy 3, 279–289 (2018)
Q. Li, S. Zhu, Y. Lu, Adv. Func. Mater. 27, 1606422 (2017)
S. Flandrois, B. Simon, Carbon 37, 165–180 (1999)
X. Wang, W. Zeng, L. Hong et al., Nat. Energy 3, 227–235 (2018)
Y. Zhao, Y. Ye, F. Wu, Y. Li, L. Li, R. Chen, Adv. Mater. 31, e1806532 (2019)
M. Li, Int. J. Electrochem. Sci. 15, 526–534 (2020)
C. Deng, Z. Wang, S. Wang, J. Yu, J. Mater. Chem. A 7, 12381–12413 (2019)
R. Zhang, N.W. Li, X.B. Cheng, Y.X. Yin, Q. Zhang, Y.G. Guo, Adv. Sci. (Weinh) 4, 1600445 (2017)
F. Ding, W. Xu, G.L. Graff et al., J. Am. Chem. Soc. 135, 4450–4456 (2013)
M.S. Whittingham, Proc. IEEE 100, 1518–1534 (2012)
W.G. Lim, S. Kim, C. Jo, J. Lee, Angew. Chem. Int. Ed. Engl. 58, 18746–18757 (2019)
Y. Gu, W.W. Wang, Y.J. Li et al., Nat. Commun. 9, 1339 (2018)
H. Yan, C. Shen, K. Yuan et al., ACS Sustain. Chem. Eng. 6, 4776–4783 (2018)
M.A. Rahman, Y.C. Wong, G. Song, C. Wen, J. Porous Mater. 22, 1313–1343 (2015)
X. Li, Y. Tang, F. Zhang, L. Chen, X. Wang, J. Porous Mater. 26, 987–993 (2019)
A. Xiao, J. Yang, W. Zhang, J. Porous Mater. 17, 583–588 (2010)
X. Zhang, J. Hu, X. Chen et al., J. Porous Mater. 26, 1821–1830 (2019)
H. Chen, P. Xia, W. Lei, Y. Pan, Y. Zou, Z. Ma, J. Porous Mater. 26, 1325–1333 (2019)
J. Peng, K. Zhong, W. Huang et al., Vacuum 191, 110331 (2021)
M. Bai, K. Xie, K. Yuan et al., Adv. Mater. (2018). https://doi.org/10.1002/adma.201801213
M. Li, Z. Zhang, Y. Yin et al., ACS Appl. Mater. Interfaces 12, 3610–3616 (2020)
N. Piao, X. Ji, H. Xu et al., Adv. Energy Mater. 10, 1903568 (2020)
F. Han, A.S. Westover, J. Yue et al., Nat. Energy 4, 187–196 (2019)
Q. Liu, Z. Geng, C. Han et al., J. Power Sources 389, 120–134 (2018)
K.R. Adair, C. Zhao, M.N. Banis et al., Angew. Chem. Int. Ed. Engl. 58, 15797–15802 (2019)
G. Wang, C. Chen, Y. Chen et al., Angew. Chem. Int. Ed. 59, 2055–2060 (2020)
M. Rosso, C. Brissot, A. Teyssot et al., Electrochim. Acta 51, 5334–5340 (2006)
H.K. Kang, S.G. Woo, J.H. Kim, S.R. Lee, Y.J. Kim, Electrochim. Acta 176, 172–178 (2015)
J. Sun, H. Wang, Y. Li, M. Zhao, J. Porous Mater. 28, 889–894 (2021)
S.W. Li, Y. Ma, B.Q. Wei, Energy Environ. Mater. 4, 103–110 (2021)
K. Yan, Z.D. Lu, H.W. Lee et al., Nat. Energy 1 (2016)
Y. He, H. Xu, J. Shi et al., Energy Storage Mater. 23, 418–426 (2019)
G. Wang, X. Xiong, P. Zou et al., Chem. Eng. J. 378, 122243 (2019)
J. Sun, C. Guo, Y. Cai et al., Electrochim. Acta 309, 18–24 (2019)
Y. Shi, Z. Wang, H. Gao et al., J. Mater. Chem. A 7, 1092–1098 (2019)
Y. Tang, K. Shen, Z. Lv et al., J. Power Sources 403, 82–89 (2018)
Z.Y. Hu, P. Wang, E.G. Fu et al., J. Alloy. Compd. 754, 26–31 (2018)
S. Singh, N. Bhatnagar, J. Porous Mater. 25, 537–554 (2018)
T. Song, M. Yan, M. Qian, J. Porous Mater. 22, 713–719 (2015)
R.H. Heidersbach, E.D. Verink, CORROSION 28, 397–418 (1972)
A.P. Pchelnikov, A.D. Sitnikov, I.K. Marshakov, V.V. Losev, Electrochim. Acta 26, 591–600 (1981)
A.V. Polunin, A.P. Pchelnikov, V.V. Losev, I.K. Marshakov, Electrochim. Acta 27, 467–475 (1982)
H. Sugawara, H. Ebiko, Corros. Sci. 7, 513–523 (1967)
P. Zhou, M.J. Hutchison, J.W. Erning, J.R. Scully, K. Ogle, Electrochim. Acta 229, 141–154 (2017)
L.W. Ding, H.R. Geng, J.H. Xu, Adv. Mater. Res. 1566 (2012)
Y. Chen, H.J. Feng, Y.H. Wang, Z. Tang, D. Chua, Mater. Lett. 226, 8–12 (2018)
Q.B. Yun, Y.B. He, W. Lv et al., Adv. Mater. 28, 6932 (2016)
Acknowledgements
This work was supported by the National Key Research and Development Project (2018YFE0203400), the National Natural Science Foundation of China (51974219), Natural Science Basic Research Plan in Shaanxi Province (2018JM5135).
Author information
Authors and Affiliations
Contributions
Conceptualization: LL, KZ; Methodology: LL, KZ, YD; Formal analysis and investigation: LL, JL and MR; Writing—original draft preparation: LL; Writing—review and editing: KZ, ZF.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, L., Zhong, K., Dang, Y. et al. Chemical dealloying pore structure control of porous copper current collector for dendrite-free lithium anode. J Porous Mater 28, 1813–1822 (2021). https://doi.org/10.1007/s10934-021-01130-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01130-z