Abstract
The synthesis of highly efficient CO2 adsorbent derived from MOF coupled with graphene oxide, HKUST-1@GrO, is proposed at the room temperature to achieve the most desirability form an eco-environmental perspective. The modified Hummers method coupled with an ultra-fast MOF formation approach were explored to synthesis the superior CO2 adsorbent, i.e. HKUST-1@GrO. Then, the structure of adsorbent was deeply characterized by the application of different analyses including Fourier-Transform Infrared (FTIR) Spectroscopy, X-ray Diffraction (XRD), Brunauer–Emmett–Teller (BET), and Scanning Electron Microscopy (SEM). The optimization of CO2 adsorption was carried out under a broad range of temperatures (283–293 K) and pressures (1–10 bars). The N2 adsorption/desorption isotherms analysis indicated that loading of graphene oxide (3 wt%) on HKUST-1 increases its specific surface area from 1032 to 1354 m2/g. The maximum adsorption capacity of CO2 by HKUST-1@GrO composite at 283 K and 10 bars was evaluated equal to 12.44 mmol/g. Thermodynamic studies elucidated that the dominant CO2 adsorption was taken place as spontaneous, physisorption, and exothermic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, with the expansion of population and urbanization, however, the growth of industries, human demand for energy has increased. This issue has led to a significant increase in the exploitation of fossil fuels as the main source of greenhouse gases (GHGS) emissions [1]. According to proven data, GHGS had been identified as the major factor in climate change and global warming. The destructive effects of this issue are not currently hidden in the natural conditions of human life and organism. Of course, the rapid growth of greenhouse gas emissions has made a more deplorable situation compared with the past few decades [1, 2]. Regarding this, the continuous CO2 emissions must be declined, because the rising trend in energy consumption is ceaselessly ongoing and it is improbable that fossil fuels be widely and efficiently replaced by clean fuels in the coming years [3]. Therefore, it is imperative to choose and explore an efficient approach based on available technologies. Among all the technologies and routs comprising incineration, biological treatment, condensation, membrane, photochemical oxidation, chemical absorption, adsorption by solid absorbents such as activated carbon, zeolite, and metal–organic frameworks (MOFS) are widely developed due to ease of application and optimization potential concerning the cost and energy consumption [4, 5].
MOFs with possessing unique properties such as low density, high specific surface area, and pore tailoring capability are considered as a prior candidate for various applications, namely, gas storage, sequestration, and conversion [6]. Up to now, around more than 20,000 different types of MOFS has been identified and developed by various synthesis approaches [6]. Considering the aforementioned characteristics, MOFs have been efficiently applied for CO2 capture. Lin et al. [7] reported that MIL-101 had an adsorption capacity of 1.6 mmol/g for CO2 at 298 K. The CO2 capacity of Cu-BTC was attained up to 6.49 mmol/g at 1 bar and 273 K by Yan et al. [8]. To synthesis the different types of MOFs, various approaches are explored comprising Hydrothermal [9], slow evaporation [10], microwave [11], solvothermal [12], ultrasonic [13] and mechanochemical [14]. However, a high-energy demand and much time consuming can be considered as the most drawback of the mentioned approaches, which ultimately leads to the high costs of their production and application [15].
To overcome these disadvantages, some limited investigations were conducted. For instance, Zhao, Nunn [16] proved that the use of hydroxyl double salts (HDSS), as a moderator for the fabrication of some MOF kinds e.g. (Cu3 (BTC)2), can reduce the synthesis time from a few days to several hours. In addition, as another obvious disadvantage, MOFs are not able to capture the small molecules because of their crystal structures. Thus, the composition of MOFs with other nanomaterials is proposed as an attainable approach to amend this deficiency. Given this, applying graphene-derived materials such as graphene oxide (GrO) or reducing GrO as a substrate is the preferred option to amplify the adsorption capacity of MOFs [17, 18]. Graphene—derived materials due to their unique properties such as high surface area, extensive porosity, and specifically, their tendency to capture of gas molecules have been extensively utilized in various applications to achieve the green environment in the recent years [19]. Hence, among the methods used to synthesize graphene-derived materials, the Hummers method has been more considered because of its higher efficiency in manufacturing the final product [20]. Therefore, this approach was utilized to synthesis the high-quality graphene nanosheets in the present study.
Thus, the main objective of this investigation was the highly efficient CO2 capture by a novel nanocomposite, i.e. HKUST-1@GrO, synthesized via a facile and time-efficiency approach. Different characterization analysis was conducted to assess the properties of final synthesized adsorbent composing FTIR, BET, SEM, XRD, and the volumetric CO2 test.
2 Materials and methods
2.1 Materials
All chemical material was purchased from Merk company, Germany. These materials are included: graphite powder, hydrogen peroxide (30%), sodium nitrate (99%), sulfuric acid (98%), potassium permanganate (99.5%), 1,3,5-benzenetricarboxylic acid (BTC, 99%), N,N-dimethylformamide (DMF, 99.5%), zinc oxide (99%), copper nitrate hydrate (Cu(NO3)2.3H2O, 99%), and ethanol (99.7%).
2.2 Synthesis process
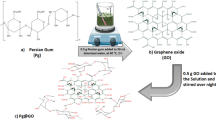
The synthesis process of adsorbents is schematically illustrated in Fig. 1 and briefly explained in the following:
2.2.1 GrO synthesis
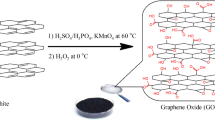
Graphite oxide was prepared according to the modified Hummers method by the oxidation of graphite powder [21]. In the first step, graphite powder (4 g) and sodium nitrate (3 g) were added into concentrated sulfuric acid (120 ml) and then, the solution was continuously stirred in an ice bath to prevent rising temperature. Secondly, potassium permanganate (15 g) was slowly added into the above solution and allowed the suspension was stirred at room temperature for 6 h. In the next step, 150 ml distilled water was gradually added to the solution. After passing 10–12 h, 50 ml distilled warm water accompanied by 30 ml hydrogen peroxide was respectively added into the suspension. Finally, the obtained GrO was washed several times with distilled water to remove the maintained acid and then filtered. However, to achieve a homogenous GrO powder, the filtered mixture was dried at 60 °C in the oven for 2 days.
2.2.2 HKUST-1 synthesis
The metal–organic framework (HKUST-1) was prepared in five steps [16, 22]. First, the zinc oxide (0.5 g) was dissolved in distilled water (10 ml). Then, the solution obtained from the previous step was first sonicated for 10 min. and then dissolved in dimethylformamide (16 ml) and stirred. Subsequently, copper nitrate (2 g) was added to the above solution. After this, benzene tricarboxylic acid (1 g) was dissolved in ethanol and added to the sample. Finally, the sample was washed with ethanol several times and filtered, then placed in a vacuum oven for 6 h at 120 °C to obtain a powder of HKUST-1.
2.2.3 HKUST-1@GrO synthesis
The steps of HKUST-1@GrO composite preparation are similar to HKUST-1 synthesis [23]. First, the zinc oxide (0.5 g) was dissolved in distilled water (10 ml) and sonicated for 10 min. and then dissolved in dimethylformamide (16 ml). After that, a certain amount of graphene oxide powder (3 wt %) was dissolved in distilled water (8 ml) and sonicated for 20 min. In continuation, copper nitrate (2 g) was added to the solution of the previous step. Subsequently, benzene tricarboxylic acid (1 g) was dissolved in ethanol (20 ml). In this step, first the zinc oxide solution and then copper nitrate/GrO solution was added. The resulting solution was washed with ethanol several times, filtered and dried in a vacuum oven for 6 h at 120 °C.
2.3 Characterization
The X-ray diffraction pattern was recorded on a PW1730 diffractometer (PHILIPS, US) using Cu-Kα radiation (λ = 1.5405 Å). The samples were scanned from 10 to 80° 2θ with a step size of 0.05°. Scanning electron microscopy (SEM) images were obtained using FEI NOVA NanoSEM 450, US. The N2 adsorption/desorption isotherms (BET) at 77 k were measured by using Belsorp mini ǁ, Japan. The infrared (IR) spectra were taken on a Thermo Nicolet Avatar 360.
2.4 Adsorption of CO2
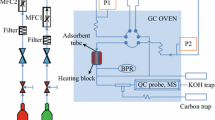
In this study, a volumetric method was used to investigate the CO2 adsorption capacity. The schematic diagram of the CO2 adsorption apparatus is illustrated in Fig. 2. Two precision barometers were used to measure CO2 adsorption at 283, 288, and 293 K and in the pressures range from1 to 10 bars. The applied samples were degassed under a simultaneous vacuum (Rocker 420, China) and heating at 253 K for 2 h. However, helium gas was used as a non-adsorbent gas to control the process. The adsorption capacity was calculated by using Soave–Redlich–Kwong (SRK) equation of state in MATLAB software [24].
2.5 Adsorption isotherm
Langmuir and Freundlich were utilized to describe the experimental data. Langmuir model assumes that CO2 adsorption is carried out as monolayer onto an energetically uniform surface [25] and defined as follows:
where q is the amount of adsorbed CO2 (mmol/g), qm is the maximum of CO2 adsorption capacity (mmol/g) and b is the Langmuir constant. Freundlich isotherm is an experimental model that is assumed that the adsorption is taken place as a multilayer onto a non-uniform surface and defined by the following non-linear equation [26].
where k and n stand the adsorption capacity and the adsorption intensity, respectively.
2.6 Thermodynamic of adsorption
Thermodynamic parameters represent the feasibility and spontaneity of the adsorption process and the entropy changes during the adsorption process [5]. Gibbs free energy (∆G), enthalpy (∆H), and entropy (∆S) are three substantial thermodynamic parameters that are used to gain a better understanding regarding the adsorption mechanisms. The following equations were used to calculate the thermodynamic data [27].
In the above equation, ∆Hads is isosteric heat of adsorption, R stands the gas constant (J/K·mol), P and T are the pressure (bar), and temperature (K), respectively. Furthermore, the following equations were used to obtain the Gibbs free energy and the entropy where, P is the equilibrium pressure of the adsorbate, Ps is the equilibrium pressure of adsorption at the standard pressure.
3 Result and discussion
3.1 Characterization results
The results of the X-ray diffraction pattern of GrO, HKUST-1, and HKUST-1@GrO are shown in Fig. 3. Regarding the GrO pattern (Fig. 3a), the appearance of peaks around 20 and 40°, indicating that the oxidation and purification process of the sample were carried out. The observation of diffraction peaks at around 2θ = 26.5 and 2θ = 42.8 degrees showed that some of the graphite is not fully oxidized, as well [28]. The crystalline structure is caused to appear the intense peaks in Fig. 3b. A comparison of these two patterns illustrates that the peak height of HKUST-1@GrO is lower than HKUST-1 because the addition of graphite oxide has prevented the growth of HKUST-1 crystals [29]. In addition, the effect of the activation method is well known on the formation of crystalline structure. Wee et al. [30] proved that the activation methods i.e. hydrothermal, room temperature, and freeze dyer possess an important effect on the crystal structure of Cu3(BTC)2, and however, the extent of crystal structure would be reduced by decreasing the applied time treatment.
Figure 4 shows the FTIR spectra for the synthesized adsorbents. In close agreement with the literature [14, 22, 31], the functional groups in GrO adsorbent appear at 1032 cm−1 (C-O), and 1387 cm−1 (C=O). However, the peaks at 1225, 1619, and 1724 cm−1, respectively, were assigned to the epoxy (C–O–C), the hydroxyl (OH) and the carboxyl functional groups (C=O). The peak observed at around 3427 cm−1 is usually associated with the hydroxyl functional group. The other two functional groups related to the HKUST-1 and HKUST-1@GrO have appeared at 730 cm−1 (C–H), and 1108 cm−1 (C–O). In addition, the observed peaks at around 1376 and 1645 cm−1 can be ascribed to the stretching vibration of C=O bonds. In general, the peaks detected between the wavenumber 700 to 1700 cm−1 confirmed the presence of benzene tricarboxylic acid (BTC) as the organic ligand in the structure. This result was related to the study conducted by Xu et al. [23]. In both spectra dealing with HKUST-1 and HKUST-1@GrO, the peaks were analogous, indicating the chemical similarity of the two adsorbents [32]. The presence of GrO in the HKUST-1@GrO is observable at 3440 cm−1 which was slightly more intense than that in the HKUST-1 [14]. The morphology of the synthesized adsorbents is depicted in Fig. 5. the effect of preparation methods on the quality of the structure of the adsorbent has been well documented in the previous studies [24, 27]. Therefore, reducing the synthesis time can prevent the formation of regular hexagonal crystals [33]. Accordingly, the adsorbents have illustrated no similar structural arrangement. However, the HKUST-1@GrO morphology shows that GrO had impeded to further growth of the HKUST-1 crystals [23]. Furthermore, GrO morphology distinguished that all the applied graphite is not attended in the GrO formation, because the non-oxidized graphite particles are still visible in its structure [34]. Figure 6 shows the N2 adsorption–desorption isotherms of HKUST-1, and HKUST-1@GrO. The IUPAC classification system is generally used for the physical adsorption analysis [35]. Regarding this, the study adsorbents demonstrate a type IV isotherm and hysteresis loop at P/P0 > 0.4, indicating the predominance of the mesoporous cavity in their structure [36]. On the other hand, the results of the textual parameters in Table 1 showed that the SBET and Vtotal of HKUST-1@GrO were relatively higher than those in comparison with the GrO and HKUST-1. It could be inferred that the interaction of GrO with HKUST-1 improved the HKUST-1@GrO structural properties [23, 37].
3.2 CO2 adsorption
The influence of temperature on the CO2 adoption capacity was assessed. As shown in Fig. 7 and Table 2, the adsorption capacity of adsorbents gradually decreased with increasing temperature. The enhancement of temperature leads to the increment of CO2 molecules kinetic energy, resulting in the desorption of adsorbed molecules [38]. The effect of pressure on the adsorption capacity can be analyzed as follows: the adsorption sites are initially vacant and CO2 molecules are adsorbed easily and rapidly. By progressing the adsorption process, less available unoccupied sites remained and the amount of CO2 adsorption would decrease until reaching zero. Therefore, the higher adsorption could not be attained by increasing the pressure in this situation [39]. The obtained results in agreement with the last section proved that the HKUST-1@GrO possesses the higher potential for CO2 adsorption due to the better structural characteristics [37].
3.3 Adsorption isotherm
The simulation results of Langmuir and Freundlich isotherms based on the experimental adsorption process are shown in Table 3. Accordingly, the amount of CO2 adsorption decreased with increasing temperature and it has shown an uptrend with increasing pressure to a certain extent. This was exactly consistent with the results of the experimental data. As a result, the maximum simulated uptake capacity (qm) was equal to 12.39 mmol/g at 283 K which is attributed to HKUST-1@GrO absorbent. The n constant in all cases was higher than one and in the range of 1 to 10, which clearly indicates the desirability of adsorption at different temperatures [25]. Furthermore, the obtained n values for HKUST-1@GrO were higher than those for HKUST-1, indicating the more favorability of CO2 adsorption by HKUST-1@GrO. Moreover, regarding the estimated R2 by Langmuir and Freundlich equation, the Langmuir model demonstrated the best fitness with the experimental data. Thus, a monolayer adsorption behavior was observed onto a homogeneous surface [27].
3.4 Thermodynamic of adsorption
To figure out the nature of reactions that occurred during the CO2 adsorption process, a thermodynamic study was performed. Figure 8 Shows the changes in the isosteric heat of adsorption for certain amounts of adsorbed CO2 (9, 10, 11 and 12 mg/g). The results showed that the slope of all curves is negative, thus the nature of the CO2 adsorption process was exothermic. Table 4 shows the values of the thermodynamic parameters. Among the adsorbents, HKUST-1@GrO had the highest enthalpy. The higher enthalpy is due to the higher adsorption potential, which is further enhanced by the presence of GrO [23]. In physical adsorption, the enthalpy value is expected to acquire less than 80 (kJ/mol), which is in accordance with the observed results in the present study [24].
The positive entropy (∆S) indicates that there is a disorder in the adsorbent surface. Table 4 shows the enhancement of entropy is due to the increased interaction between the adsorbent surface and the adsorbed molecules. It is because, initially more adsorption sites are empty and the interaction between the adsorbent surface and the CO2 molecules is strong, but in the following, the number of active sites would be reduced, leads to the decrease in the entropy. As a result, the entropy decreases due to the decrement of adsorbent-adsorbate interactions [40]. In addition, the Gibbs free energy was negative, indicating that the adsorption action was random and spontaneous.[41].
4 Conclusion
The facile and highly efficient CO2 adsorbent, HKUST-1@GrO, was synthesized by the combination of the modified Hummers method with the ultra-fast MOF formation approach. According to the obtained results, the modification of GrO by the synthesized HKUST-1, i.e. HKUST-1@GrO, resulting in a significantly positive impact on the adsorption capacity and increased the capacity of HKUST-1 from 4.1 to 5.09 mmol/g, in respect with the HKUST-1@GrO at 293 K and at 1 bar. In addition, the SBET of HKUST-1@GrO composite was equal to 1354 m2/g, considerably higher than that in its pre-structures i.e. GrO, HKUST-1, indicating the favorability of applied method to synthesis. However, the maximum adsorption capacity of HKUST-1@GrO was assessed by the Langmuir isotherm equal to 12.39 mmol/g at 283 K and 1 bar. Furthermore, thermodynamic studies revealed that the adsorption process of CO2 was carried out physically and spontaneously. Summing up, regarding the explored properties of HKUST-1@GrO as theoretically and experimentally, it could be offered as a novel candidate for a highly efficient CO2 adsorption from an eco-environmental point of view.
Abbreviations
- MOFs :
-
Material organic frameworks
- K:
-
Kelvin
- HDSs :
-
Hydroxy double salts
- P:
-
Pressure
- RGO:
-
Reduced graphene oxide
- Ps :
-
Equilibrium pressure of adsorption at standard pressure, bar
- GrO:
-
Graphene oxide
- q:
-
Amount of adsorbed, Mmol/g
- BTC:
-
1,3,5-Benzenetricarboxylic acid
- qm :
-
Maximum of CO2 adsorption capacity, mmol/g
- DMF:
-
N, N-dimethylformamide
- b:
-
Langmuir constant, 1/bar
- SEM:
-
Scanning electron microscopy
- KF :
-
Freundlich constant, [(mmol/g)(1/bar)(1/n)]
- BET:
-
Brunauer–Emmett–Teller
- n:
-
Freundlich exponent, dimensionless
- FTIR:
-
Fourier-Transform infrared spectroscopy
- ∆G:
-
Gibbs free energy, kJ/mol
- SRK:
-
Soave–Redlich–Kwong
- ∆Hads :
-
Isosteric heat of adsorption, kJ/mol
- SBET :
-
Surface-specific area, m2/g
- ∆S:
-
Entropy changes, J/K·mol
- Vtotal :
-
Total pore volume, cm3/g
- T:
-
Temperature, K or °C
- Vmeso :
-
Mesopore volume, cm3/g
- R:
-
Gas constant, 8.314 J/K·mol
- Vmicro :
-
Micropore volume, cm3/g
- R2 :
-
Determination coefficient
References
J.L. Míguez et al., Evolution of CO2 capture technology between 2007 and 2017 through the study of patent activity. Appl. Energy 211, 1282–1296 (2018)
F.M. Stuardi, F. MacPherson, J. Leclaire, Integrated CO2-capture and utilization: a priority research direction. Curr. Opin. Green Sustain. Chem. 16, 71–76 (2019)
C. Song et al., Alternative pathways for efficient CO2 capture by hybrid processes—a review. Renew. Sustain. Energy Rev. 82, 215–231 (2018)
K. Kamarudin, N. Zaini, N. Khairuddin, CO2 removal using amine-functionalized kenaf in pressure swing adsorption system. J. Environ. Chem. Eng. 6(1), 549–559 (2018)
A. Heidari et al., Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem. Eng. J. 254, 503–513 (2014)
V.V.E. Butova et al., Metal-organic frameworks: structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 85(3), 280–307 (2016)
Y. Lin et al., Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO 2 capture. Sci. Rep. 3, 1859 (2013)
X. Yan et al., Extremely enhanced CO2 uptake by HKUST-1 metal–organic framework via a simple chemical treatment. Microporous Mesoporous Mater. 183, 69–73 (2014)
H. Zhao et al., In situ hydrothermal synthesis of tetrazole coordination polymers with interesting physical properties. Chem. Soc. Rev. 37(1), 84–100 (2008)
T. Gadzikwa et al., Selective bifunctional modification of a non-catenated metal− organic framework material via “click” chemistry. J. Am. Chem. Soc. 131(38), 13613–13615 (2009)
G. Zhu et al., Microwave assisted synthesis of reduced graphene oxide incorporated MOF-derived ZnO composites for photocatalytic application. Catal. Commun. 88, 5–8 (2017)
R. Zou et al., Storage and separation applications of nanoporous metal–organic frameworks. CrystEngComm 12(5), 1337–1353 (2010)
F. Israr et al., High yield synthesis of Ni-BTC metal–organic framework with ultrasonic irradiation: role of polar aprotic DMF solvent. Ultrason. Sonochem. 31, 93–101 (2016)
Y. Li et al., Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity. Chem. Eng. J. 298, 191–197 (2016)
Y. Chen et al., High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. 28(5), 584–589 (2018)
J. Zhao et al., Facile conversion of hydroxy double salts to metal–organic frameworks using metal oxide particles and atomic layer deposition thin-film templates. J. Am. Chem. Soc. 137(43), 13756–13759 (2015)
Y. Chen et al., A new MOF-505@GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 308, 1065–1072 (2017)
S.-C. Wu et al., Synthesis of aluminum-based MOF/graphite oxide composite and enhanced removal of methyl orange. J. Alloys Compd. 724, 625–632 (2017)
H. Hsu et al., Application of graphene oxide aerogel to the adsorption of polycyclic aromatic hydrocarbons emitted from the diesel vehicular exhaust. J. Environ. Chem. Eng. 7, 103414 (2019)
D.C. Marcano et al., Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
L. Shahriary, A.A. Athawale, Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2(01), 58–63 (2014)
H. Li et al., Ultrafast room temperature synthesis of novel composites Imi@ Cu-BTC with improved stability against moisture. Chem. Eng. J. 307, 537–543 (2017)
F. Xu et al., Ultrafast room temperature synthesis of GrO@HKUST-1 composites with high CO2 adsorption capacity and CO2/N2 adsorption selectivity. Chem. Eng. J. 303, 231–237 (2016)
M. Nowrouzi, H. Younesi, N. Bahramifar, High efficient carbon dioxide capture onto as-synthesized activated carbon by chemical activation of Persian Ironwood biomass and the economic pre-feasibility study for scale-up. J. Clean. Prod. 168, 499–509 (2017)
X. Zhou et al., Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem. Eng. J. 200, 399–404 (2012)
N. Can, B.C. Ömür, A. Altındal, Modeling of heavy metal ion adsorption isotherms onto metallophthalocyanine film. Sens. Actuators B 237, 953–961 (2016)
M. Nowrouzi, H. Younesi, N. Bahramifar, Superior CO2 capture performance on biomass-derived carbon/metal oxides nanocomposites from Persian ironwood by H3PO4 activation. Fuel 223, 99–114 (2018)
S.N. Alam, N. Sharma, L. Kumar, Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6(01), 1–18 (2017)
J. Cheng et al., Preparation of a Cu(BTC)-rGO catalyst loaded on a Pt deposited Cu foam cathode to reduce CO2 in a photoelectrochemical cell. RSC Adv. 8(56), 32296–32303 (2018)
L.H. Wee et al., Fine tuning of the metal–organic framework Cu 3 (BTC) 2 HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 22(27), 13742–13746 (2012)
S. Homayoonnia, S. Zeinali, Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sens. Actuators B 237, 776–786 (2016)
W. Huang et al., Preparation and adsorption performance of GrO@Cu-BTC for separation of CO2/CH4. Ind. Eng. Chem. Res. 53(27), 11176–11184 (2014)
M. Gimeno-Fabra et al., Instant MOFs: continuous synthesis of metal–organic frameworks by rapid solvent mixing. Chem. Commun. 48(86), 10642–10644 (2012)
C. Fu et al., Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 8(5), 6269–6280 (2013)
Z. ALOthman, A review: fundamental aspects of silicate mesoporous materials. Materials. 5(12), 2874–2902 (2012)
M. Thommes et al., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87(9–10), 1051–1069 (2015)
X. Zhou et al., A novel MOF/graphene oxide composite GrO@MIL-101 with high adsorption capacity for acetone. J. Mater. Chem. A 2(13), 4722–4730 (2014)
Z. Asadi-Sangachini et al., The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 26(26), 26542–26552 (2019)
H. Frost, T. Düren, R.Q. Snurr, Effects of surface area, free volume, and heat of adsorption on hydrogen uptake in metal− organic frameworks. J Phys. Chem. B 110(19), 9565–9570 (2006)
S. Rangabhashiyam, N. Selvaraju, Efficacy of unmodified and chemically modified Swietenia mahagoni shells for the removal of hexavalent chromium from simulated wastewater. J. Mol. Liq. 209, 487–497 (2015)
L.A. Rodrigues et al., Phenol removal from aqueous solution by activated carbon produced from avocado kernel seeds. Chem. Eng. J. 174(1), 49–57 (2011)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zarei Mohammadabad, M., Moeinaddini, M., Nowrouzi, M. et al. Facile and cost-efficient synthesis of highly efficient CO2 adsorbents: a pathway towards a green environment. J Porous Mater 27, 1659–1668 (2020). https://doi.org/10.1007/s10934-020-00945-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00945-6