Abstract
Mesoporous silica (SBA-16) was synthesized by the hydrothermal synthesis route. The structural properties of the synthesized SBA-16 were found out by a small-angle X-ray diffraction technique. Morphological characterization was characterized by scanning electron microscope (SEM) and high-resolution transmission electron microscope (HRTEM). Nitrogen adsorption–desorption isotherm was used to find out surface area, pore size, and pore volume. Aripiprazole was used as a model drug to calculate drug loading efficiency of prepared SBA-16 and their release kinetics was also calculated with the response to three different pH solutions. UV–Visible spectrophotometer was used to find out the absorbance of the sample. The results indicated that the synthesized SBA-16 has high drug loading efficiency with a control release profile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent researches, it is found that polymers are structurally and thermally not stable to carry the drug. Some polymers are toxic [1], that’s why they may be responsible to cause adverse effects in the body of living organisms. Polymers show slow biological degradation hence can be responsible for long term environmental pollution. To overcome all the drawbacks of polymer-based drug delivery there is a need to find out alternatives to conventional drug delivery vehicles.
SBA-16 (Santa Barbara Amorphous-16) belongs to MCM-48 (Mobil Composition of Matter 48) family and is mesoporous silica with cubic symmetry, high surface to volume ratio, pore size, and pore volume can be modified accordingly. In the past decades, SBA-16 and other mesoporous silica attracted the researchers of various disciplines. SBA-16 used in variety of applications such as catalyst [2], molecular imaging [3], chromatography [4], drug delivery [5] etc. SBA-16 is a three-dimensional cubic mesoporous with high thermal, chemical and physical stability. The size of SBA-16 can be tuned from 50 nm to sub-micron scale [6] as well as its pore size [7] depending upon the application. The surface area of SBA-16 can be modified [8] to introduce large amount of molecule for a wide variety of applications. SBA-16 does not show any cellular toxicity [9] and also has no adverse effect on the environment therefore it does not cause any type pollution. Mesoporous silica is biocompatible and is removed from the body through urine through basic metabolic process of life [10]. Moreover mesoporous silica especially SBA-16 has high drug loading capacity than other carriers [11]. Not only does mesoporous silica have high drug loading efficiency, but drug release kinetics can be controlled by functionalizing their inner wall.
So far, according to the literature survey and recent publications, no one has studied loading and releasing efficiency of aripiprazole in SBA-16. Some conventional aripiprazole carriers were used in this application such as solid lipid nanoparticles [12], poly(caprolactone) nanoparticle [13] and silicosan particles [14]. But these nanoparticles were much not impressive as their aripiprazole loading efficiency was 9.28–31.54% for solid lipid nanoparticle, 72.4 ± 3.16% for poly(caprolactone) nanoparticle and 10.1–58.3% for silicosan particle. Aripiprazole “7-[4-[4-(2, 3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril” is a third-generation drug originally used to treat schizophrenia and bipolar I disorder. The chemical formula of aripiprazole is C23H27Cl2N3O2 and 448.38 is the molecular weight. The chemical structure of aripiprazole is given in Fig. 1. Uncontrolled and high doses of aripiprazole may cause Akathisia (a condition of feeling restlessness and nervousness), anxiety and increase suicidal thoughts [15]. Whenever aripiprazole is administered orally, it can cause several side effects such as difficulty in speaking, drooling, loss of balance, muscle trembling, jerking or stiffness, etc. Therefore, the release required to overcome the side effects of aripiprazole need to be controlled. Only a few piece of research available on loading and controlled release of aripiprazole by various carrier, among them one of the research articles shows aripiprazole with poly(lactic acid) polymer with 73–80% drug loading efficiency [16]. It is the highest loading capacity for aripiprazole by any carrier till date.
In this research work, aripiprazole was loaded into SBA-16 matrix to ascertain its loading capacity as well as to find out how its release profile is affected at different pH condition. The results are very impressive with higher drug loading efficiency and show sustainable release with respect concerning particular time duration. This in vitro loading and release study of aripiprazole through SBA-16 would play a significant role to overcome its side effects and improve its efficacy.
2 Experimental
2.1 Materials
Pluronic F127 (PEO106PPO70PEO106), tetraethoxysilane (TEOS, 98%) and aripiprazole (98% purity) were purchased from Sigma Aldrich, hydrochloric acid (HCl, 37%) and 1-butanol from Fisher Scientific. Aqueous solution for this experiment was made up into double distilled water (DDW). Teflon coated stainless steel autoclave was used for the hydrothermal reaction process.
2.2 Synthesis of SBA-16 matrix
The hydrothermal synthesis of SBA-16 was accomplished by following the process explained in [17]. In a typical hydrothermal synthesis process of SBA-16, 3 g of Pluronic 127 (P127) was dissolved in 144 g of DDW. To maintain high acidic conditions for proper dissolution of P127 into DDW, 5.94 g of 2 M hydrochloric acid was added drop wise into stirring solution. 9 g 1-butanol (co-surfactant) was added after obtaining a uniform solution of P127 and remained it vigorously stirred for next 3 h. 14.2 g TEOS (as silica precursor) was added drop wise into the stirring solution and kept it for the next 24 h at room temperature (i.e. 38 °C). The white color precipitate was obtained after 24 h treatment; it was further placed into Teflon coated autoclave and kept it into hot air oven at 100 °C for next 24 h to treat hydrothermally. The product obtained after hydrothermal treatment was washed 5 times in ethanol with the help of centrifugation and dry it at 80 °C. At last, to obtain the dry, white and impurity-free powder, it was calcined at 550 °C for 5 h.
2.3 Preparation of aripiprazole standard curve
First of all, aripiprazole was dissolved into ethanol (1 mg/ml) to make a stock solution. Afterward, various amount of drug solutions (aripiprazole solutions) i.e. 1 µg/ml to 10 µg/ml was prepared by serial dilution method. The standard curve then created into MS Excel software by obtaining the absorbance of each diluted solution at 255 nm (λmax for aripiprazole) wavelength. The regression equation between the absorbance (y) and concentration (x) was obtained by creating a standard curve where R2 (regression coefficient) was 0.9968, m (slope) was 0.0659, c (intercept) was + 0.3142. The drug loading efficiency of SBA-16 was determined by putting the absorbance value of supernatant into the Eq. (1) [18].
where “y” is absorbance of the obtained solution and “x” is concentration of the drug into the given solution.
2.4 Aripiprazole loading into SBA-16
Aripiprazole was load into SBA-16 by post impregnation method at room temperature. In this drug loading process, 200 mg of aripiprazole was dissolved into 20 ml of ethanol thereafter 250 mg of SBA-16 were added into the aripiprazole/ethanol solution. The aripiprazole/ethanol/SBA-16 composite solution was continuously stirred at 800 rpm for 12 h. After 12 h of stirring, the obtained composite solution was centrifuged at 10,000 rpm for 2 h to separate the supernatant and solid. The obtained solid pellet was dried overnight into hot air oven at 50 °C and the obtained supernatant was use to find out the aripiprazole loading efficiency of SBA-16.
To calculate the drug loading efficiency of SBA-16, 1 ml of supernatant was diluted 100 times (converted the concentration of supernatant from mg/ml to µg/ml) and found out their absorbance at 255 nm wavelength. Ethanol was used as a reference solution into respective blank cuvette to create baseline in the UV–Vis spectrophotometer. The aripiprazole loading efficiency (LE%) of SBA-16 is calculated by Eq. (2) [19]
where LE% is loading efficiency (mg/ml), Ci is initial concentration of aripiprazole (mg/ml), Cf is final concentration of aripiprazole in the supernantent (mg/ml).
2.5 Aripiprazole release study
The dialysis method (also known as open ended tube method) was employed to study aripiprazole release from SBA-16 carrier. In this method, the open end of the tube tied with a 12 k molecular mass cut off dialysis membrane (semipermeable membrane). Three different dialysis membrane-bounded tube filled with aripiprazole-SBA-16 was immerged into three different solutions, viz. 0.1 N HCl (pH 1.2), phosphate buffer (pH 7.2) and phosphate buffer (pH 6.8) which mimic simulated gastric juice (SGJ), simulated body fluid (SBF), and simulated intestinal juice (SIJ) respectively. The release study performed at body temperature (37 °C) and sample aliquots were taken at definite time intervals to obtain their absorbance. Subsequently, solution media was replenished with their relevant fresh solution media. Thereafter, aripiprazole release study from aripiprazole loaded SBA-16 matrix was plotted using Eq. (3) [20]
where Ct is the concentration measured from released drug at time t, Ct−corrected is the real concentration of aripiprazole released at time t, v is the volume of the sample taken at different time intervals and V is the total volume of release drug.
2.6 Characterization
The structural property of hydrothermally prepared SBA-16 was determined by using small-angle X-ray diffraction (SAXRD) technique (Bruker D8). SAXRD pattern of SBA-16 was obtained at wavelength (λ) 1.5418 Å using Cu Kα radiation. The diffraction angle of SAXRD was 2θ and the XRD pattern was taken between 0.5° and 5° with 0.02° step size. Surface area, pore size, and pore volume were examined by N2 adsorption–desorption isotherm with the help of surface area analyzer (Quantachrome Nova Win version 11.02) at cryogenic temperature i.e. 77 K and degassing carried out at 200 °C for 6 h under vacuum condition. The surface area was calculated using the Brunauer–Emmett–Teller (BET) model while pore size distributions were calculated by the Barrett–Joyner–Halenda (BJH) model. Morphological characterization of SBA-16 was carried out using a scanning electron microscope (SEM; Zeiss EVO 50) and high-resolution transmission electron microscope (HRTEM) (Tecnai G20). The aripiprazole loading and release study was performed with the help of a UV–Visible spectrophotometer (Varian Cary-5000).
3 Result and discussion
3.1 SAXRD
Since SBA-16 is amorphous at the atomic level hence no reflections can be obtained at wide-angle XRD. SBA-16 is an ordered mesoporous nanostructure; therefore SAXRD technique is useful to find out diffraction pattern between 2θ angles 0.5° and 5° after fulfilling the Bragg’s condition [21]. Figure 2 shows the SAXRD graph of SBA-16 which reflects well-resolved peaks near to 2θ angle 0.96°, 1.28° and 1.38° which correspond to (110), (200) and (211) planes respectively [22]. The d spacing for the plane (110) is calculated as ~ 1.02 Å and SAXRD patterns indicate the Im3m cubic structure of SBA-16.
3.2 Nitrogen adsorption/desorption isotherm
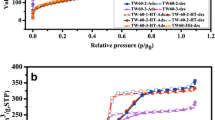
Figure 3a represents the nitrogen adsorption/desorption isotherm for SBA-16 which was observed at low temperature (77 K). Nitrogen adsorption/desorption technique is very helpful to find out surface area, pore size, and volume of the desired material. According to the International Union of Pure and Applied Chemistry (IUPAC), the obtained isotherm could be classified into type IV isotherms and H1 hysteresis loop which indicates an ordered cubic mesoporous structure. The pore size (Dp) and pore volume (Vp) of the SBA-16 (calculated by the BJH model) was derived as 5.743 nm and 0.195 cm3/g, respectively while the surface area (S) of SBA-16 was (calculated by BET model) derived as 781.551 m2/g.
3.3 SEM
Figure 4 shows the scanning electron microscope (SEM) image of SBA-16. The average size of the particle is observed around 400 nm which is an ideal size for a drug delivery system. The SEM image of prepared SBA-16 is evident in the almost spherical structure that show agreement with previous studies held on SBA-16. The small pores could be visualized in the obtained SEM image of spherical SBA-16 nanoparticle.
3.4 HRTEM
Uniform mesopores, ordered cubic structure of SBA-16 can be observed from the HRTEM image shown in Fig. 5. HRTEM image supported the N2 adsorption/desorption isotherm in the context of the mesoporous, cubic structure of prepared SBA-16. The distance between two subsequent channels is ~ 10 nm which supported the “d” space value obtained from SAXRD diffractogram.
3.5 Drug loading
Figure 6 represents the standard curve of aripiprazole. The aripiprazole loading efficiency of SBA-16 matrix was calculated with the help of Eqs. (1) and (2). The absorbance value of supernatant at the wavelength 255 nm was obtained which was put into the Eq. (1) at the place of y (absorbance) and hence x (concentration) of the drug was obtained in the supernatant. The achieved concentration of drug in supernatant converted into mg/ml and then put this value into the Eq. (2) to find out drug loading efficiency (LE%) of aripiprazole in SBA-16 matrix. 92.2% Aripiprazole loading efficiency of SBA-16 had been achieved after the final calculation.
The maximum aripiprazole drug loading amount of SBA-16 was calculated by using following equation;
where PE is the amount of aripiprazole load into the SBA-16 (mg/g), Ci and Cf is the initial and final concentration of drug in the final solution (mg/ml) respectively, V is the volume of the reaction solution and W is the weight of the SBA-16 (g). The total amount of aripiprazole loaded into SBA-16 calculated by using Eq. (4) and it was found to be 79.64 mg/g.
3.6 In vitro drug release study
Since aripiprazole is poorly soluble in water but readily solubilizes into ethanol similarly SBA-16 can be easily dispersed into ethanol. The pore size of the SBA-16 was nearly 5 nm and it is supposed that the molecular size of aripiprazole is less than 5 nm after dissolution into ethanol so, aripiprazole insert into SBA-16 easily but their release kinetic depends upon their bonding with an amine and silanol group. Figure 7 represents the in vitro aripiprazole release study concerning different solution medium i.e. SGJ (pH 1.2), SBF (pH 7.2) and SIJ (pH 6.8). The in vitro release profile of aripiprazole from SBA-16 was varied according to pH. In the solution of pH 1.8 which mimics to simulated gastric juice, show the sustainable release of aripiprazole which can be seen before 5 h thereafter, aripiprazole was released comparatively faster. At the end of 12 h, only 62.2% of total drug release was calculated. In the solution of pH 6.8 which mimics to simulated intestinal juice, aripiprazole show continuous sustainable release but their release gets saturates after 11 h. No more further significant release has observed at 12 h of their release and the total drug release content was 68.7%. Aripiprazole show continuous, sustainable and complete release into the solution of pH 7.2 which mimics the SBF. At the 10 h of their release time, 96.6% of the total drug was released and their 12 h of release study 99.6% aripiprazole is released.
The stability of drug in physiological condition and release profile basically depends upon the interaction between drug with their carrier and pH of the solution. Here, the carrier i.e. SBA-16 functionalized with APTES, which interact with silanol group present in the wall of SBA-16. The free amine groups (–NH) of APTES interacts with drug molecules through hydrogen bond. Aripiprazole is slightly alkaline therefore, it release faster in SGJ solution medium as compare to SBF and SIJ. In case of SBF solution and SIJ solution medium probably cations are formed by aripiprazole, consequently longer drug release time in the above medium. Since the in vitro release study of aripiprazole indicated that the solution of pH 7.2 which corresponds to SBF is best suited to their release so, aripiprazole loaded SBA-16 would be most effective to intravenous route than the oral route.
4 Conclusion
SBA-16 had been successfully synthesized with the help of the hydrothermal route. The amorphous, cubic Im3m structure of prepared SBA-16 was observed by SAXRD which with 1.02 Å lattice space. Nitrogen adsorption/desorption isotherm revealed ordered mesoporous structure with high surface area, ideal pore size and better pore volume of the prepared sample. Morphological characterization of the prepared samples was performed by SEM analysis which showed the almost spherical shape and 400 nm average particle sizes. HRTEM image showed ordered mesopores with cubic symmetry in a long chain. The channel space agreed with the lattice space of prepared SBA-16. 92.2% of drug loading efficiency had been achieved with a sustainable in vitro release profile of aripiprazole from the SBA-16 matrix.
References
R.-Y. Li, Z.-G. Liu, H.-Q. Liu, L. Chen, J.-F. Liu, Y.-H. Pan, Am. J. Transl. Res. 7, 1357 (2015)
X. Zhang, H. Yang, Y. Huo, J. Li, J. Ma, J. Ma, Dalton Trans. 45, 8972 (2016)
A.L.B. de Barros, K.S. de Oliveira Ferraz, T.C.S. Dantas, G.F. Andrade, V.N. Cardoso, E.M.B. de Sousa, Mater. Sci. Eng. C 56, 181 (2015)
I. Sierra, D. Pérez-Quintanilla, S. Morante, J. Gañán, J. Chromatogr. A 1363, 27 (2014)
C. Bharti, U. Nagaich, A.K. Pal, N. Gulati, Int. J. Pharm. Investig. 5, 124 (2015)
W.J.J. Stevens, K. Lebeau, M. Mertens, G. Van Tendeloo, P. Cool, E.F. Vansant, J. Phys. Chem. B 110, 9183 (2006)
T.-W. Kim, R. Ryoo, M. Kruk, K.P. Gierszal, M. Jaroniec, S. Kamiya, O. Terasaki, J. Phys. Chem. B 108, 11480 (2004)
A.T. Shah, M.I. Din, F.N. Kanwal, M.L. Mirza, Arab. J. Chem. 8, 579 (2015)
S. Chauhan, G. Manivasagam, P. Kumar, R.K. Ambasta, Pharm. Nanotechnol. 6, 245 (2018)
R. Narayan, U.Y. Nayak, A.M. Raichur, S. Garg, Pharmaceutics 10, 118 (2018)
S. Jangra, P. Girotra, V. Chhokar, V.K. Tomer, A.K. Sharma, S. Duhan, J. Porous Mater. 23, 679 (2016)
Silki, V.R. Sinha, AAPS PharmSciTech 19, 1264 (2018)
K. Sawant, A. Pandey, S. Patel, Mater. Sci. Eng. C 66, 230 (2016)
A.A. Mahmoud, A.H. Salama, R.N. Shamma, F. Farouk, AAPS PharmSciTech 19, 3751 (2018)
M.P. Pondé, A.C.C. Freire, Case Rep. Psychiatry (2015). https://doi.org/10.1155/2015/419746
S. Hiraoka, S. Uchida, N. Namiki, Chem. Pharm. Bull. 62, 654 (2014)
E. Poonia, M.S. Dahiya, V.K. Tomer, K. Kumar, S. Kumar, S. Duhan, Physica E 101, 284 (2018)
Q.-Z. Zhai, Mater. Sci. Eng. C 32, 2411 (2012)
H. Van de Ven, M. Vermeersch, A. Matheeussen, J. Vandervoort, W. Weyenberg, S. Apers, P. Cos, L. Maes, A. Ludwig, Int. J. Pharm. 420, 122 (2011)
F. Rehman, P.L.O. Volpe, C. Airoldi, Colloids Surf. B 119, 82 (2014)
A. Kumar, S. Gahlyan, R. Thakur, S. Devi, S. Duhan, J. Nanosci. Nanotechnol. 20, 4210 (2020)
S.S.E. Ghodsinia, B. Akhlaghinia, Green Chem. 21, 3029 (2019)
Acknowledgements
Author is thankful to UGC, India for the award of SRF to Mr. Atul Kumar and Mr. Narender Ranga for NFSC Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Ranga, N., Duhan, S. et al. In vitro study of aripiprazole loading and releasing efficiency of SBA-16. J Porous Mater 27, 1431–1437 (2020). https://doi.org/10.1007/s10934-020-00910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00910-3