Abstract
In this study, a facile hydrothermal method was used to prepare Fe3O4/MIL-101 composite as a photocatalyst. The resulting composite was characterized using X-ray diffraction, scanning electron microscopy, nitrogen adsorption/desorption isotherms, thermal analysis, X-ray photoelectron spectroscopy, and UV–Vis diffuse reflection spectroscopy. The Fe3O4/MIL-101 composite possesses a large surface area and mesoporous structure and exhibits a good photocatalytic activity for the MB degradation in the visible light region. A kinetic model for dye degradation over this heterogeneous catalyst was proposed by combining the parameters of the Langmuir isotherms and the kinetics of the unimolecular reaction. The proposed model fixes well with the experimental data. The mechanism of MB photocatalytic degradation is also addressed. The catalyst is stable after three recycles, which makes it a potential candidate for environmental restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, the semiconductors have widely used as photocatalysts to degrade the organic pollutants in aquatic sources because of unique advantages including recyclable ability, taking advantage of solar energy, and low cost [1,2,3,4,5,6,7]. Metal–organic frameworks (MOFs) are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are considered as the new generation semiconductors in photocatalytic application. Primary studies on MOF-5 (Zn-benzene-1,4-dicarboxylate) have proved that benzene-1,4-dicarboxylate in MOF-5 can act as antennas to absorb light and activate zinc, which is similar to inorganic semiconductor quantum dots, via a linker to metal cluster charge transfer [8]. MIL-101, which is one of MOFs materials, has received considerable attention owing to its high surface area and porosity, well-defined structure, and chemical stability. MIL-101, reported by Férey et al. has the formula as Cr3X(H2O)2O(BDC)3 where X can be F or OH; BDC is benzene-1,4-dicarboxylate [9]. It is applied in sensor technology [10], gas adsorption and separation processes [11], catalysis [12, 13], and ion exchange [14]. Recently, combining functional nano and microparticles with MIL-101 in order to fabricate nano-composites with specific functional properties has attracted the interest of many researchers. In particular, the introduction of magnetic materials into MIL-101 has received considerable attention because the composites can be easily positioned by an external magnetic field. From the catalysis and adsorption point of view, magnetic separation has many more advantages compared with filtration, centrifugation, and liquid–liquid extraction [15]. Fe3O4/MIL-101 is reported for the efficient removal of Acid Red 1 and Orange G [16], Acid Orange 7 [17] from aqueous solutions, and a selective and regeneratable adsorbent for the removal of arsenic species from water [18]. MIL-101 plays a crucial role as a carrier in the Fe3O4/MIL-101 catalyst in the catalytic conversion of benzyl alcohol to benzaldehyde by enhancing the catalytic activity of Fe3O4 nanoparticles [19]. Fe3O4/MIL-101 is a highly efficient heterogeneous catalyst for the dimerization reaction of o-phenylenediamine in the presence of H2O2 [20]. MOF-5 (zinc-terephthalate) was reported first for photocatalytic activity. Then, more and more MOFs have found utility as novel photocatalysts [21, 22]. Several papers reported visible-light photocatalytic activity of modified MIL-101 such as N–K2Ti4O9/MIL-101 composite [22], WO3/MIL-101 for enhancement charge carrier separation of photocatalyst [23], Ag3PO4/MIL-101/NiFe2O4 composite [24], MnOx/MIL-101 [25], and Bi25FeO40/MIL-101/PTH [26]. To the best of our knowledge, few papers have dealt with the study of the visible-light-driven photocatalytic properties of Fe3O4/MIL-101 up to now.
In the present paper, the synthesis of Fe3O4/MIL-101 is demonstrated, and the visible-light-driven-photocatalytic degradation of methylene blue using this material as a catalyst is also investigated.
2 Experimental
2.1 Materials and characterizations
Chromium nitrate hexahydrate (Cr(NO3)2·6H2O, Daejung, Korea, ≥ 99%), Acid Terephthalic (C8H6O4, Merck, 99%), Iron(III) chloride hexahydrate (FeCl3·6H2O, Daejung, Korea, 98%), Iron(II) chloride tetrahydrate (FeCl2·4H2O, Daejung, Korea, 99%), Methanol (CH3OH, Chemical Factory Co., Ltd. China, 99%), Methylene blue (MB) 1% (C16H18N5N3SCl, China) were used in this paper.
The X-ray diffraction (XRD) patterns were performed with a D8-Advance (Brucker, Germany) with Cu-Kα radiation of 0.154 nm. Infrared analyses were conducted on a BIO-RAD FTS-3000 spectrometer (Shimadzu, Japan). The textural properties of the samples were studied using nitrogen adsorption/desorption isotherms on Micromeritics ASAP 2020 (USA). The morphology was studied using scanning electron microscopy (SEM) and the elemental analyses were conducted using EDX spectroscopy on JSM Jeol 5410 LV (Japan). Transmission electron microscopy (TEM) was carried out using a JEOL JEM–2100F microscope. X-ray photoelectron spectroscopy (XPS) was performed on an ESCALab 250 (ThermoScientific Corporation, Japan) spectrometer. Magnetic measurements were conducted on a DMS 880 (ADE Technologies, USA) magnetometer with filed sweeping from − 20000 to 20000 Oe at room temperature. The photoluminescence (PL) was recorded by means of a spectrometer Horiba Jobin–Yvon HR800 LabRam using UV excitation. Raman spectra were recorded with a 750 nm laser excitation on a Horiba Raman Spectrometer. The concentration of MB was determined using a UV 1800 (Shimadzu, Japan) spectrophotometer at a maximal wavelength of 664 nm.
2.2 Synthesis of Fe3O4/MIL-101
MIL-101 was synthesized according to Férey et al. [9]. Typically, 1.66 g of terephthalic acid was mixed with 5 g of Cr(NO3)3·9H2O, 260 µL of HF, and 63 mL of distilled water. The mixture was transferred into a Teflon-lined autoclave, which was sealed and heated to 200 °C in 2 h and kept at this temperature for 8 h. After cooling to room temperature, the solid (MIL-101) was obtained by filtration, washed with ethanol, NH4F, and N,N-dimethylformamide at 60 °C for 24 h to remove the residual terephthalic acid. The Fe3O4/MIL-101 material was synthesized according to Saikia et al. [19]. Practically, 1 mmol of FeCl2·4H2O and 2 mmol of FeCl3·6H2O were added to 100 mL of distilled water containing 0.5 g of MIL-101 prepared in the first experiment. The resulting suspension was stirred vigorously under a nitrogen flow for 1 h; then, 14 mL of an NH3 solution was added. The resulting black suspension was stirred for another 30 min. After that, it was collected by centrifugation and dried at 100 °C for 24 h. The resulting product was denoted as Fe3O4/MIL-101. The Fe3O4 was synthesized for iron (II) and (III) chloride as above mentioned for the sake of comparision.
2.3 Photocatalytic degradation of MB
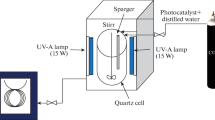
The photocatalytic activity of Fe3O4/MIL-101 was studied in the degradation of MB at ambient temperature. The adsorption of MB from aqueous solutions was performed in the dark for 300 min to ensure saturation. Then, the mixture solution continued to be stirred mechanically and visible light (60 W incandescent Lamp) was illuminated from the bottom with a cut-off filter (< 400 nm) as shown in Fig. 1. For each experiment, 0.05 g of Fe3O4/MIL-101 was placed in a 500 mL3 beaker containing 300 mL3 of dye solution (10–50 mg L−1), which was stirred magnetically at 30 °C. 3 mL of the sample was withdrawn at different time intervals and determined for the MB concentration. Each determination was repeated three times. The decolorization fraction is expressed as follows:
where C0 and Ct is MB concentration at initial and certain time.
The chemical oxygen demand (COD) of the MB solution was determined using the ASTM method [27]. The sample was oxidized by a boiling mixture of chromic and sulfuric acids and refluxed in a strong acid solution with a known excess of potassium dichromate. After digestion, the remaining unreacted K2Cr2O7 was titrated with ferrous ammonium sulfate to determine the amount of K2Cr2O7 consumed, and the oxidizable matter was calculated in terms of oxygen equivalent. The samples were analyzed in three measurement to yield the most reliable data.
3 Results and discussion
3.1 Characterization of Fe3O4/MIL–101
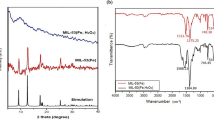
Figure 2a represents the XRD patterns of MIL-101 and Fe3O4/MIL-101. The characteristic diffractions of MIL-101 at 2θ of 5.12°; 5.85°; 8.5°; 9.1°, and 10.4° correspond to Miller index of (511), (822), (753), (1022) and (880), respectively [15, 16].These sharp peaks with high intensities indicate that the obtained MIL-101 exhibits high crystallinity [9, 15, 16]. Figure 2b presents XRD pattern of Fe3O4. The diffractions of Fe3O4 appear at Miller indices (220), (311), (400), (422), (511), and (440) (JCPDS No: 00-001-1111). The XRD pattern of Fe3O4/composite is composed of characteristic of Fe3O4 and MIL-101. The results show that Fe3O4 is encapsulated into MIL-101 and the MIL-101 structure possesses integrity in Fe3O4/MIL-101 composite.
Vibration bands of FT-IR spectra at around 1398 cm−1 are assigned to the framework (O–C–O)-groups, confirming the presence of the dicarboxylate moieties within MIL-101 or Fe3O4/MIL-101 [20, 21]. The bands at around 3427 cm−1, 2359 cm−1, and 1624 cm−1 are owing to the water molecules within the frameworks [11, 12]. No vibration at 632 cm−1 proves no phase of hematite in nanocomposite [17, 18]. The characteristic broad band at 586 cm−1 for the Fe3O4/MIL-101 proves the incorporation of Fe–O groups on MIL-101 [20] (Fig. 2c).
The nitrogen adsorption/desorption isotherms of MIL-101 and Fe3O4/MIL-101 are presented in Fig. 2d. The curves belong to the H4 type according to IUPAC classification. MIL-101 has a BET (Brunauer–Emmett–Teller) surface area of 3360 m2 g−1 and a pore volume of 1.43 cm3 g−1. Fe3O4/MIL-101 has a lower BET surface area and pore volume than the parent material because the pores of the parent material are filled with Fe3O4 particles. However, a significant BET surface area of 1860 m2 g−1 and pore volume of 0.72 cm3 g−1 are conserved. This also proves the encapsulation of iron oxides within the pores of the framework.
The TGA and DTA curves of Fe3O4/MIL-101 exhibit two main weight losses: the first in the temperature range of 25–118 °C corresponds to the loss of the physically adsorbed water and the second between 118 and 375 °C is associated with the decomposition of the organic framework with an exothermic peak at 375.89 °C (Fig. 2e).
The morphology of MIL-101 and Fe3O4/MIL-101 was observed using SEM (Fig. 3a, b). Pure MIL-101 shows 0.5 µm octahedral shape particles with smooth facets, while Fe3O4/MIL-101 provides the octahedral particles with rough facets because the fine particles of Fe3O4 with 10–20 nm size are incorporated into the MIL-101 surface (Fig. 3c). The elementary analysis by means of EDX shows the presence of iron and chromium in the Fe3O4/MIL-101 composite (Fig. 3d). Since the results of EDX only reflect the surface composition of the elements, the molar ratio of Fe/Cr (1.27/1) is significantly higher than that of the initially synthesized gel (0.38/1), indicating that iron is introduced into MIL-101 and located mainly on the surface the crystals. No typical hysteresis loop of the Fe3O4/MIL-101 nanocomposite is observed, suggesting the superparamagnetic behavior of the material owing to the aggregation of the small-sized spherical magnetic particles (Fig. 3e). The saturation magnetization value of the nanocomposite is around 15 emu g−1. This value is small compared with that of the Fe3O4 nanoparticles due to the small particle size effect and the presence of the non-magnetic MIL-101 support in the nanocomposite. This superparamagnetic behavior of the Fe3O4/MIL-101 composite allows the particles to aggregate rapidly in the presence of an external magnetic field. However, the particles disperse easily as soon as the external field is removed.
The elemental composition and oxidation states of the metals were studied using X-ray photoelectron spectroscopy (XPS). The XPS survey spectrum shows that C, O, Cr, and Fe exist in Fe3O4/MIL-101 (Fig. 4a). Figure 4d demonstrates the high-resolution XPS spectrum of C1s, which can be deconvoluted into two surface components, corresponding to the carbon components on the benzoic ring at a binding energy of 284.6 eV and the carboxylate (C=O) groups of the terephthalate linkers at a binding energy of 288.6 eV [21, 28]. The XPS spectrum of O1s could be fitted by two peaks at binding energies of around 531.7 and 530.4 eV (Fig. 4e), which are assigned to the oxygen components on terephthalate linkers and on the iron oxide, respectively [22, 23]. For the Fe2p spectrum (Fig. 4b), the binding energy peak around 714.4 eV is contributed to Fe2p3/2 and the peak at 725.8 eV is contributed to Fe2p1/2. All of these results clearly confirm the formation of Fe3O4/MIL-101. The binding energy values of 585.8 eV for Cr2p1/2 and 576.3 eV for Cr2p3/2 (Fig. 4c) are typically assigned to Cr(III) [29].
The photoluminescence (PL) emission spectrum has widely been employed to study the efficiency of charge carrier trapping, immigration, and transfer [30,31,32]. In order to study the effect of Fe3O4 on the recombination of electron–hole pairs produced by MIL-101. The PL presented in Fig. 5a compares electron–hole recombination of Fe3O4/MIL-101 and MIL-101. MIL-101 shows a broad PL emission band, which is similar to that in the literature [33]. Fe3O4/MIL-101 shows diminished PL intensity, implying lower charge recombination in comparison with pure MIL-101. Peak shifting from 392 nm for MIL-101 to 400 nm for Fe3O4/MIL-101 is resulted from the trapping of electrons at defect sites prior to recombination [34]. Raman spectra were employed to study the structure and surface defects of semiconductors [35]. We did not observe obvious peak shifts for the MOF bands in the Raman spectra. The two sharp peaks at 1618 and 1461 cm−1 correspond to the D and G bands, respectively [36, 37]. The intensity ratio of the D and G bands (ID/IG) is used to estimate the disorder in the materials. It is found that the ID/IG ratio of Fe3O4/MIL-101 (1.43) is higher than that of pure MIL-101 (1.37), which implies that magnetic iron oxide has successfully been tailored in Fe3O4/MIL-101 (Fig. 5b). The UV–Vis diffuse reflection spectrum is widely used to calculate the optical band gap energy [2, 3, 6, 7]. The band gaps of the Fe3O4/MIL-101 composite were studied by means of a UV–Vis diffuse reflection spectrum at room temperature (Fig. 5c). Fe3O4/MIL-101 shows an absorption edge around 200–600 nm (Fig. 5c). A very high absorption peak around 230 nm indicates the π → π* transitions in the terephthalate ring. Fe3O4/MIL-101 shows a remarkable absorption band shifting towards the longer wavelength region. The band gaps obtained from the Kubelka–Munk plot are 3.48 and 2.4 eV (Fig. 5d). The observed decrease in the band gap energy of the iron-doped MIL-101 may be attributed to the excitation of the 3d electrons of Fe(III) or Fe(II) to the conduction band level of Cr by a charge transfer transition. The low band gap at 2.4 eV enables Fe3O4/MIL-101 to exhibit the photocatalytic activity under visible light.
3.2 Visible-light-driven photocatalytic degradation of MB over Fe3O4/MIL-101
3.2.1 Catalytic kinetics
Figure 6a shows the decolorization fraction for MB in dark adsorption and under the visible light illumination with and without the catalyst. The MB solution was not decolorized under visible light in the absence of MIL-101 and Fe3O4/MIL-101, indicating that the photolysis of MB under this condition could be ignored. MIL-101 exhibits a higher adsorption capacity than Fe3O4/MIL-101 with the equilibrium decolorization fraction at 73.5% and 57.3% in dark adsorption, respectively. However, the MB solution decolorizes up to 95% with Fe3O4/MIL-101 after 650 min of illumination; meanwhile, the decolorization is not observed in the MB solution containing MIL-101 under the same condition. This suggests that MIL-101 is not a photocatalyst for MB degradation. The leaching experiment, in which Fe3O4/MIL-101 was filtered after 420 min, was also conducted. The decolorization of MB does not take place under illumination, confirming that Fe3O4/MIL-101 is a heterogeneous photocatalyst in the degradation of MB.
The UV–Vis spectra for the photocatalytic degradation of MB over Fe3O4/MIL-101show that the adsorption band peaks at 664 nm (electron transfer π → π* in the MB structure), and the concentration of MB in the solution decreases with illumination time (Fig. 6b).
To confirm the mineralization of MB over Fe3O4/MIL-101, the change of COD of the reaction products with time was analyzed. The initial COD of 82.6 mg L−1 decreases with the illumination reaching 35.2 mg L−1 after 720 min (Fig. 6c). This decrease proves that Fe3O4/MIL-101 is an efficient photocatalyst for MB degradation under visible light.
There are two common approaches for investigating formal kinetics of heterogeneous catalytic reactions: (i) the light illumination is performed as soon as the catalyst is included; (ii) the light illumination is performed only when the catalyst adsorbs at saturation in the dark. There is a difficulty to distinguish the adsorption and catalysis processes in the first approach. This problem is solved in the second approach, but it is difficult to determine the initial concentration of the catalyst in the kinetic equation. In this case, the initial concentration is considered as the equilibrium concentration in dark adsorption. Very few articles [38] have dealt with this issue so far.
It is known that the Langmuir–Heishellwood model is widely used to study the formal kinetics of dye degradation over heterogeneous photocatalysts. This model is as follows:
where k1 is rate constant (min−1); C0 and Ct are MB concentration at initial and time t (mg L−1).
In the present study, we propose a modified Langmuir–Heishellwood model, in which the Langmuir equilibrium constant and the kinetics are combined.
Figure 7a presents the kinetics of adsorption and photocatalytic decolorization of MB over Fe3O4/MIL-101. Fe3O4/MIL-101 exhibits a high adsorption capacity of MB. The adsorption is saturated between 240 and 300 min depending on the initial MB concentration. The higher the MB concentration causes the slower saturation. After 300 min, the adsorption ensures saturation.
The equilibrium adsorption capacity, qe, is expressed as follows:
where C0 and Ce are the MB concentration at initial and equilibrium time (mg L−1); V is the volume of the MB solution (L); m is the mass of catalyst (g).
The relationship between Ce and qe is expressed by the Langmuir isotherm model [38]:
where qm is the maximum monolayer adsorption capacity (mg L−1); KL is the Langmuir adsorption equilibrium constant (mg L−1).
The value of KL and qm can be obtained from the slope and intercept of the linear plot of 1/Ce versus qe. A high determination coefficient (R2 = 0.97) shows that this model could fix the experimental data well, and implies the monolayer adsorption on the adsorbents. The values of qm and KL are 243.6 mg g−1 and 0.013 L mg−1, respectively.
It is supposed that MB molecules adsorb on the catalyst and then they degrade on the catalyst surface under visible light illumination. The overall reaction could be illustrated as follows:
where k1 is the forward adsorption rate constant; k–1 is the back adsorption rate constant; k2 is the photocatalytic rate coefficient.
It is supposed that the photocatalytic degradation is slow and is the rate-determining step. Therefore, the law rate is expressed as follows:
where C is the dye concentration (mg L−1) at time t; k2 is the kinetics rate coefficient (mg L−1 min−1); θ is the fraction of the surface covered by MB. θ is expressed in the Langmuir model [39]:
Then,
Integrating Eq. (8) with the boundary conditions t → 0 and C → C0e gives
When t = 0, then
Substituting (10) into (9) gives
where KL is the Langmuir adsorption equilibrium constant (L mg−1), which is determined from the Langmuir isotherm model as above; C0e (mg L−1) is the equilibrium concentration of adsorbate at time t (min) taken from light illumination.
The plot of the \(\frac{1}{K}\ln C + C\) against t gives a straight line with slope k2 (Fig. 7b).
The kinetics data are fixed to the Langmuir–Hinshelwood model for the sake of comparison. The value of k2 at different initial MB concentrations is listed in Table 1. The coefficient of determination of the straight lines is higher than those of the Langmuir–Hinshelwood model (t(5) = 2.597, p value = 0.048 < 0.05), indicating the proposed modified Langmuir–Hinshelwood model has a goodness of fix for the experimental data. The value of k2 decreases with the increase of the initial MB concentration. A higher MB concentration can shield the light from interacting with the catalyst, resulting in a lower rate coefficient.
It is difficult to compare the catalytic activity of Fe3O4/MIL-101 composite in the present with that of other catalysts previously reported due to different reaction conditions. The pseudo-first-order rate constant of Langmuir–Hinshelwood model seems appropriate to compare the catalytic activity because several authors report this value (Table 2). The value of the pseudo-first -order rate constant for the degradation on the present catalyst in the visible region is lower than that for P25 (TiO2) in the UV region and compatible with Ce–TiO2, but higher than that for other catalysts reported such as Ag–ZnO and Ce–TiO2 in visible region. This result shows that the photocatalytic activity of Fe3O4/MIL-101 is relatively high.
3.2.2 Mechanism of heterogeneous catalyst
To figure out the mechanisms of photocatalytic activity, radical scavengers such as tert-butanol (TB), 1,4-benzoquinone (BQ), dimethyl sulfoxide (DMSO), and ammonium oxalate (AO) were used to quench hydroxyl radicals (\(^{ \cdot } {\text{OH}}\)), superoxide radicals (−·O2), e− and h+, respectively. The mixture of MB solution and the catalyst was stirred for 300 min to ensure adsorption/desorption equilibrium and then the scavenger solution was added as soon as the light was turned on. As seen in Fig. 8, the MB degradation rate tends to decrease as the corresponding radical scavenger is added to the reaction solution. AO and TB decrease the MB degradation rate significantly. After 600 min reaction, the MB decolorization fraction reaches 82% for the case without radical scavengers, and it only reaches 49.5% for AO and 43.8% for TB. Meanwhile, BQ and DMSO slightly slow down the degradation rate of MB. These findings imply that \(^{ \cdot } {\text{OH}}\) and h+ play a critical role in MB degradation although −·O2 and e− contribute to MB degradation as well.
The general coupled semiconductor mechanism [6, 7, 34, 45] could be manipulated to explain the MB degradation over the Fe3O4/MIL-101 catalyst. The edge of the valence band (VB) and conduction band (CB) for MIL-101 is + 0.49 eV, and − 1.57 eV, respectively [47], and for Fe3O4 is 0.48 and 2.08 eV [48], respectively. Firstly, MB molecules adsorb quickly onto Fe3O4/MIL-101 to form MBads (reaction (12)). Both MIL-101 and Fe3O4 could absorb visible light to generate the pairs of e− and h+ at CB and VB, respectively, according to reaction (13). CB of MIL-101 is more negative than that of Fe3O4, then it will transfer the excited electrons to CB of Fe3O4 that is believed to prevent the fast recombination of the photo-excited e− and h+ pairs. The LUMO (lowest unoccupied molecular orbital) of photo-excited MB· (–3.81 eV) is more negative than CB of Fe3O4 [49]. Therefore, MB could act as the photosensitizer to favorably provide additional photo-generated electrons into CBs of Fe3O4 through the formed downstream channel (reaction (14) and (15)). The energy of h+ of Fe3O4 (2.08 V) is more positive than the potential of H2O/OH· (+1.9 V) [50]. Then, h+ could be quickly converted to the hydroxyl radical upon oxidation of surface water, according to reaction (16). The potential of e− in MIL-101 (–1.57 V) is more negative than that of O2/−·O2 (–0.28 V), then introduced oxygen forms the free radicals −·O2, as reaction (17). These radicals are responsible for MB degradation (reaction (18) and (19)). According to the published articles [49, 50], the reactions could be illustrated as follows (see Fig. 9):
To evaluate the reusability of Fe3O4/MIL-101 for catalyzing MB degradation, the used Fe3O4/MIL-101 was regenerated by drying for 24 h at 100 °C and then reused. The catalytic activities of the reused Fe3O4/MIL-101 are presented in Fig. 10. The COD of the initial MB solution is 85.2 mg L−1. After 720 min of adsorption and illumination, COD reduces to 35.9 mg L−1. After the second, third and fourth cycle, COD reduces to 37.2, 39.8, and 39.9 mg L−1 (Fig. 10a). Although the photo-catalytic activity decreases gradually with an increase in cycles, the regenerated Fe3O4/MIL-101 still remained good performance for its catalytic activity. The Fe3O4/MIL-101catalyst after the fourth use shows an XRD pattern with the decreasing characteristic peaks in comparison with the original catalyst (Fig. 10b). This indicates the stability and true heterogeneous nature of the catalyst under the experimental conditions.
In addition, the Fe3O4/MIL-101 catalyst reveals excellent photocatalytic degradation of several other dyes (Congo Red (CR), Methyl Orange (MO), and Rhodamine-B (RD) (Fig. 11). The results found above show that Fe3O4/MIL-101 is a promising photocatalyst for the treatment of organic pollutants in aqueous solutions.
4 Conclusions
Highly active Fe3O4/MIL-101composite with large surface area, mesoporous heterostructures, and enhanced visible-light absorption was successfully prepared on the basis of hydrothermal strategy where MIL-101 acts as the semiconductor for the formation of catalytic heterostructures. Fe3O4/MIL-101 is a heterogeneous catalyst in nature and stable after the fourth reuse. We proposed a kinetic model of dye degradation over this heterogeneous catalyst by combining the parameters of Langmuir isotherms and kinetics of the unimolecular reaction. This model exhibits goodness of fit for the kinetics data.
References
H. Safajou, H. Khojasteh, M. Salavati-Niasari, S. Mortazavi-Derazkola, Enhanced photocatalytic degradation of dyes over graphene/Pd/TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 498, 423–432 (2017)
M. Ghanbari, M. Salavati-Niasari, Tl4CdI6 nanostructures: facile sonochemical synthesis and photocatalytic activity for removal of organic dyes. Inorg. Chem. 57, 11443–11455 (2018)
M. Ghanbari, F. Ansari, M. Salavati-Niasari, Simple synthesis-controlled fabrication of thallium cadmium iodide nanostructures via a novel route and photocatalytic investigation in degradation of toxic dyes. Inorgan. Chim. Acta 455, 88–97 (2017)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, M. Hamadanian, Praseodymium oxide nanostructures: novel solvent-less preparation, characterization and investigation of their optical and photocatalytic properties. RSC Adv. 5, 33792–33800 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Nanocrystalline Pr6O11: synthesis, characterization, optical and photocatalytic properties. New J. Chem. 39, 3948–3955 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Facile route to synthesize zirconium dioxide (ZrO2) nanostructures: structural, optical and photocatalytic studies. J. Mol. Liq. 216, 545–551 (2016)
S. Zinatloo-Ajabshir, M.S. Morassaei, M. Salavati-Niasari, Facile fabrication of Dy2Sn2O72 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminants. J. Colloid Interface Sci. 497, 298–308 (2017)
T. Tachikawa, J.R. Choi, M. Fujitsuka, T. Majima, MOF-5 Tachikawa.pdf, J. Phys. Chem, C 112, 14090–14101 (2008)
G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé, I. Margiolaki, A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042 (2005)
L.E. Kreno, K. Leong, O.K. Farha, M. Allendorf, R.P. Van Duyne, J.T. Hupp, Metal–organic framework materials as chemical sensors. Chem. Rev. 112, 1105–1125 (2012)
H.B.T. Jeazet, C. Staudt, C. Janiak, A method for increasing permeability in O2/N2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone. Chem. Commun. 48, 2140–2142 (2012)
A. Corma, H. García, F.X. Llabrés i Xamena, Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 110, 4606–4655 (2010). https://doi.org/10.1021/cr9003924
N.T.S. Phan, T.T. Nguyen, Q.H. Luu, L.T.L. Nguyen, Paal-Knorr reaction catalyzed by metal-organic framework IRMOF-3 as an efficient and reusable heterogeneous catalyst. J. Mol. Catal. A 363–364, 178–185 (2012)
K.S. Min, M.P. Suh, Silver(I)-polynitrile network solids for anion exchange: anion-induced transformation of supramolecular structure in the crystalline state. J. Am. Chem. Soc. 122, 6834–6840 (2000)
P.N. Dave, L.V. Chopda, Application of iron oxide nanomaterials for the removal of heavy metals. J. Nanotechnol. 2014, 1–14 (2014)
T. Wang, P. Zhao, N. Lu, H. Chen, C. Zhang, X. Hou, Facile fabrication of Fe3O4/MIL-101(Cr) for effective removal of acid red 1 and orange G from aqueous solution. Chem. Eng. J. 101, 1–50 (2016)
X. Yue, W. Guo, X. Li, H. Zhou, R. Wang, Core-shell Fe3O4@ MIL-101(Fe) composites as heterogeneous catalysts of persulfate activation for the removal of acid orange 7. Environ. Sci. Pollut. Res. 101, 1629–1658 (2016)
K. Folens, K. Leus, N.R. Nicomel, M. Meledina, S. Turner, G. Van Tendeloo, G. Du Laing, P. Van Der Voort, Fe3O4@MIL-101—a selective and regenerable adsorbent for the removal of as species from water. Eur. J. Inorg. Chem. 2016, 4395–4401 (2016)
M. Saikia, D. Bhuyan, L. Saikia, Facile synthesis of Fe3O4 nanoparticles on metal organic framework MIL-101(Cr): characterization and catalytic activity. New J. Chem. 39, 64–67 (2015)
Z.W. Jiang, Y.F.L. Fu Qiang Dai, C.Z. Huang, Facile synthesis of Fe3O4/MIL-101(Fe) composite with enhanced catalytic performance. RSC Adv. 1, 1–3 (2016)
L. Shen, S. Liang, W. Wu, R. Liang, L. Wu, CdS-decorated UiO–66(NH2) nanocomposites fabricated by a facile photodeposition process: an efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 1, 11473–11482 (2013)
Y. Xu, Q. Chen, H. Yang, M. Lv, Q. He, X. Liu, F. Wei, Enhanced photodegradation of Rhodamine B under visible light by N-K2Ti4O9/MIL-101 composite. Mater. Sci. Semicond. Process. 36, 115–123 (2015)
L. Wang, L. Zan, WO3 in suit embed into MIL-101 for enhancement charge carrier separation of photocatalyst. Sci. Rep. 9, 4860–4872 (2019)
T. Zhou, G. Zhang, H. Zhang, H. Yang, P. Ma, X. Li, X. Qiu, G. Liu, Highly efficient visible-light-driven photocatalytic degradation of rhodamine B by a novel Z-scheme Ag3 PO4/MIL-101/NiFe2O4 composite. Catal. Sci. Technol. 8, 2402–2416 (2018)
D. Ding, Z. Jiang, Q. Ouyang, L. Wang, Y. Zhang, L. Zan, Enhanced photocatalytic activity and mechanism insight of MnOx/MIL-101. J. Taiwan Inst. Chem. Eng. 82, 226–232 (2018)
M. Lv, H. Yang, Y. Xu, Q. Chen, X. Liu, F. Wei, Improving the visible light photocatalytic activities of Bi25FeO40/MIL-101/PTH via polythiophene wrapping. J. Environ. Chem. Eng. 3, 1003–1008 (2015)
A.E. Greenberg, Standard Methods for the Examination of Water and Wastewater (American Public Health Association, Washington, DC, 1985)
L. Shen, S. Liang, W. Wu, R. Liang, L. Wu, Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(vi). Dalt. Trans. 42, 13649–13657 (2013)
T. Van Vu, H. Kosslick, A. Schulz, J. Harloff, E. Paetzold, M. Schneider, J. Radnik, N. Steinfeldt, G. Fulda, U. Kragl, Selective hydroformylation of olefins over the rhodium supported large porous metal-organic framework MIL-101. Appl. Catal. A 468, 410–417 (2013)
S.M. Hosseinpour-Mashkani, F. Mohandes, M. Salavati-Niasari, K. Venkateswara-Rao, Microwave-assisted synthesis and photovoltaic measurements of CuInS2 nanoparticles prepared by using metal-organic precursors. Mater. Res. Bull. 47, 3148–3159 (2012)
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, A. Yeganeh Faal, S. Bagheri, Synthesis, characterization, and morphological control of ZnTiO3 nanoparticles through sol-gel processes and its photocatalyst application. Adv. Powder Technol. 27, 2066–2075 (2016)
H. Ait Ahsaine, A. El Jaouhari, A. Slassi, M. Ezahri, A. Benlhachemi, B. Bakiz, F. Guinneton, J.R. Gavarri, Electronic band structure and visible-light photocatalytic activity of Bi2WO6: elucidating the effect of lutetium doping. RSC Adv. 6, 101105–101114 (2016)
Y. Xu, M. Lv, H. Yang, Q. Chen, X. Liu, F. Wei, BiVO4/MIL-101 composite having the synergistically enhanced visible light photocatalytic activity. RSC Adv. 5, 43473–43479 (2015)
K. Fujihara, S. Izumi, T. Ohno, M. Matsumura, Time-resolved photoluminescence of particulate TiO2 photocatalysts suspended in aqueous solutions. J. Photochem. Photobiol., A 132, 99–104 (2000)
M. Ghanbari, S. Gholamrezaei, M. Salavati-Niasari, Ag2CdI4: synthesis, characterization and investigation the strain lattice and grain size. J. Alloys Compd. 667, 115–122 (2016)
A. Jarrah, S. Farhadi, K6P2W18O62 encapsulated into magnetic Fe3O4/MIL-101 (Cr) metal-organic framework: a novel magnetically recoverable nanoporous adsorbent for ultrafast treatment of aqueous organic pollutants. RSC Adv. 8, 37976–37992 (2018)
L. Nirumand, S. Farhadi, A. Zabardasti, A. Khataee, Copper ferrite nanoparticles supported on MIL-101/reduced graphene oxide as an efficient and recyclable sonocatalyst. J. Taiwan Inst. Chem. Eng. 93, 674–685 (2018)
F. Motahari, M.R. Mozdianfard, F. Soofivand, M. Salavati-Niasari, NiO nanostructures: synthesis, characterization and photocatalyst application in dye wastewater treatment. RSC Adv. 4, 27654–27660 (2014)
I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1403 (1918)
H. Fan, X. Zhao, J. Yang, X. Shan, L. Yang, Y. Zhang, X. Li, M. Gao, ZnO-graphene composite for photocatalytic degradation of methylene blue dye. Catal. Commun. 29, 29–34 (2012)
Y. Wang, R. Shi, J. Lin, Y. Zhu, Significant photocatalytic enhancement in methylene blue degradation of TiO2 photocatalysts via graphene-like carbon in situ hybridization. Appl. Catal. B 100, 179–183 (2010)
P. Du, A. Bueno-López, M. Verbaas, A.R. Almeida, M. Makkee, J.A. Moulijn, G. Mul, The effect of surface OH-population on the photocatalytic activity of rare earth-doped P25-TiO2 in methylene blue degradation. J. Catal. 260, 75–80 (2008)
A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard, J.M. Herrmann, Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 31, 145–157 (2001)
F. Jiang, T. Yan, H. Chen, A. Sun, C. Xu, X. Wang, A g-C3 N4 -CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation. Appl. Surf. Sci. 295, 164–172 (2014)
T.J. Whang, M.T. Hsieh, H.H. Chen, Visible-light photocatalytic degradation of methylene blue with laser-induced Ag/ZnO nanoparticles. Appl. Surf. Sci. 258, 2796–2801 (2012)
J.Z. Kong, A.D. Li, X.Y. Li, H.F. Zhai, W.Q. Zhang, Y.P. Gong, H. Li, D. Wu, Photo-degradation of methylene blue using Ta-doped ZnO nanoparticle. J. Solid State Chem. 183, 1359–1364 (2010)
Y. Wang, Y. Zhang, Z. Jiang, G. Jiang, Z. Zhao, Q. Wu, Y. Liu, Q. Xu, A. Duan, C. Xu, Controlled fabrication and enhanced visible-light photocatalytic hydrogen production of Au@CdS/MIL-101 heterostructure. Appl. Catal. B 185, 307–314 (2016)
D. Channei, Fe3O4/SiO2/CeO2 core-shell magnetic nanoparticles as photocatalyst. Environ. Sci. (2014) 1–9. http://environment.scientific-journal.com
H. Seema, K.C. Kemp, V. Chandra, K.S. Kim, Graphene–SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 355705, 1–8 (2012)
A. Elaziouti, Preparation and characterization of p–n heterojunction CuBi2O4/CeO2 and its photocatalytic activities under UVA light irradiation. J. King Saud. Univ. Sci. 27, 120–135 (2015)
Acknowledgements
This research was sponsored by Hue University under Decision No. 1208/QĐ-DHH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thanh, H.T.M., Tu, N.T.T., Hung, N.P. et al. Magnetic iron oxide modified MIL-101 composite as an efficient visible-light-driven photocatalyst for methylene blue degradation. J Porous Mater 26, 1699–1712 (2019). https://doi.org/10.1007/s10934-019-00767-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-019-00767-1