Abstract
Neat polyurethane (PU) foams and polyurethane/nano tin oxide composites were prepared using in situ polymerization, blending methods. The produced nanocomposites were investigated by scanning electron microscopy, Fourier transform infrared spectra, thermal gravimetric analysis (TGA), and compression test. It was found that modification of tin oxide particles caused to make better distribution in PU foam nanocomposites. The results showed that increasing isocyanate content leading to enhance cross-link, density and compression strength of neat PU foams. Addition of SnO2 nanoparticles in different density of PU foam showed variety effects. In low density foam increasing amount of SnO2 nanoparticles from 0.5 to 1 wt% leaded PU foam to become soft with low strength. Moreover, TGA results of all PU foams exhibited one thermal decomposition step. Also, increasing isocyanate ratio and SnO2 nanoparticles improved thermal stability of PU foams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polyurethanes (PUs) are a significant class of polymers used to produce a large number of products. PUs are unique polymer materials with a wide range of physical, chemical and mechanical properties. PU materials exist in a variety of forms including flexible and rigid foams, chemical resistant coatings, specialty adhesives and sealants, and elastomers. The PU foam is popular for some of its suitable properties like good moisture resistance, excellent sound dampening, vibrational and oscillating environment tolerance. PU foams have a notably broad range of applications including cushioning, buoyancy, energy absorption (packaging) and thermal insulation. PU foams are produced by the reaction between a polyisocyanate and a polyol (polyether). There are two main important reactions in the production of flexible polyurethane foams: the blow reaction and the gelation reaction. Properties of the foam can be drastically altered by changing the isocyanate component [1–3]. The micro-phase separated to hard and soft blocks in PU foams lead to their elastic properties. Hard blocks are rigid structures that are physically cross-linked and give the strength of the polymer. soft blocks are flexible chains make the polymer to be elastic. By adapting the ratio of the polyol and polyisocyanate, PU can be customized to its application [4]. Over 90 % of all flexible foam slabstock is based on polyether polyols. Conventional polyether-based flexible foams made in variety of densities from 11 kg/m3 upwards to about 60 kg/m3 and in a wide range of hardness. The physical and mechanical properties of PU foam are dependent upon the cellular structure of the foam [5]. PU foams have some limitations, such as low thermal stability and low mechanical properties, etc. Recently researchers have investigated polymer nanocomposites containing nanoparticle to improve their physical, mechanical, and chemical properties [6, 7]. Polymer composites by second phase have a number of attractive material properties such as high specific stiffness and strength, fatigue behavior, and physical properties, which make them suitable for use in applications [7]. For PU foams, it was found that chemical composition significantly affected the structures because it led to different cellular morphology [8]. Gunashekar et al. [9] studied filtration behavior of PU foams in different chemical composition. In addition, particle-filled nanocomposites could effect on the cell size and density [10]. Cao et al. [7] reinforced PU foam with the inclusion of 5 % functional organoclay and reported enhancement of physical and mechanical properties of PU foam. The modified montmorillonite clay was dispersed in either polyol or isocyanate, before mixing to polymerization. Uddin et al. [11] studied ballistic performance of PU foam nanocomposite by TiO2 nanoparticles and showed that dispersion of 3 wt% of TiO2 into PU foam affected on cell structures and mechanical properties. Mahfuz et al. [6] were investigated PU foams/TiO2/SiC nanocomposites to observe their structural and mechanical characteristics. Nanoparticles have been dispersed into the virgin materials of PU foam by ultrasonic process. Different techniques are available to infuse nanoparticle in the polymer matrices.
According to literature survey done by the authors there is no any systematic work concentrated on the role of SnO2 nanoparticles on the physical, thermal and mechanical properties of PU foam. In the current research efforts have been made to improve the mechanical performance of PU foam by dispersing of nanoparticles in PU matrices.
2 Materials and methods
Polyether polyol (Part A) containing all of required additives, and a polymeric MDI isocyanate (Part B) (diphenylmethane 4,4′-diisocyanate) for flexible PU foam were from KUMHO PETROCHEMICAL Co. Technical data of polyether polyol are listed in Table 1. Tin oxide nanoparticles was used with purity and average particle size of about 99.9 % and 40–70 nm respectively.
The fabrication of polyurethane nanocomposite foam was carried out in three steps. The first and second steps were concentrated on the functionalizing of nanoparticles and the doping of liquid polyurethane with nanoparticles. In the third step it was tried to make standard foam specimens using casting method. The details of mentioned steps are summarized in flow chart appeared in Fig. 1.
Step1: 2.5 g of SnO2 nanoparticle in 100 ml toluene was disturbed with magnet stirring (1000 rpm) at 90 °C for 10 h. After isolation and washing with methanol; modified SnO2 powder was then cast in a Teflon mold as shown in Fig. 1. After about 1 h the cast PU foam was demolded and dried in an oven at 80 °C for 24 h.
Step 2: Liquid PU material has two parts, Part A and Part B. Part A was selected for distribution of nanoparticles since it is less reactive. Functionalized SnO2 nanoparticles, were first carefully measured along with Part A to have a specific percentage of weight. After infusion by ultrasonic, the modified Part A was mixed with Part B by using a mechanical stirrer at about 1270 rpm. In this study different ratio of Part A and Part B were chosen. Furthermore, different content of nanoparticles were added to PUs. These samples have listed in Table 2.
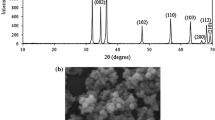
Step 3: The mixture was then cast in a Teflon mold as shown in Fig. 2a. After about 1 h the cast PU foam was demolded (Fig. 2b).
The Cylindrical test specimens (40 mm in diameter and 40 mm in thickness) were cut from samples. The specimen was weighted, and then the densities were calculated. The reported densities are the average of at least three measurements. The PU foams were cut in perpendicular directions to the foam growth and structure of samples was examined by scanning electron microscopy (SEM, Cambridge S360 model). The average cell sizes and strut thickness were measured by applying measurement software (manual microstructure distance measurement, Nahamin Pardazan Asia Co.) to the micrographs.
Uniaxial compression test was performed for PU foam with different densities. Mechanical properties were evaluated as a function of foam density using a conventional mechanical testing machine. Tests were conducted at cross head of speed 10 mm/min and at room temperature using Santam STA 150 machine. Fourier transform infrared spectra (FTIR) testing was performed using Spectrum RXI FTIR apparatus. Thermal analysis experiments were accomplished with a thermal gravimetric analysis (TGA) apparatus operated in the conventional TGA mode (BAHR: STA 503 Instrument) at the heating rate of 10 °C/min from 25 to 500 °C in a nitrogen atmosphere, and the sample weight was about 10 mg.
3 Results and discussion
3.1 FTIR results
Two samples of SnO2, both bare and functionalized, have been investigated by FTIR in order to study the type of the surface groups and the nature of the chemical bond. Figure 3 shows Fourier transformed spectrum of SnO2 nanoparticles at room temperature. The spectrum was recorded in the range of 4000–400 cm−1. The FTIR spectrum shows the characteristics peaks at 615.38, 669.95 and 3435.59 cm−1. The band at 669.95 cm−1 corresponds to O–Sn–O vibrations. Comparison of FTIR spectra obtained from SnO2 nanoparticle and its modification (Fig. 3) makes some useful data. In modification nanoparticles it is observed that the absorbance peaks of the Sn–O appears near 2520 cm−1. On the other hand, at about 1620 cm−1 the peak attributes to the bending mode of surface water molecules and –OH groups. The functionalized SnO2 sample shows peak between 1250 and 800 cm−1 that is probably due to the bending of Sn–OH bonds. The main reason of low peak intensity in the current research rather than appeared in Scipioni et al. [12] is dilution of OH group on nanoparticle surface. FTIR has been used widely and successfully to study type of bonded in PU groups [13]. Figure 4 shows FTIR spectra of neat PU foams with different density. The peaks at 2270–2280, 1680–1690 and 1600 cm−1 belong to –NCO vibration, urea –C=O vibration and aromatic v(C=C) vibration, respectively [14, 15]. The peak at 1702 cm−1 is attributed completely to the hydrogen bonded urethane carbonyl groups.
An increase in the amount of isocyanate leads to addition reaction of foam formation. However, due to the exothermic nature of the reaction, the temperature during the formation of foam samples PU6 and PU8 are more than PU4 sample. Higher temperature causes to formation of hydrogen bondings that lead to increase of hard segment. Thus PU6 and PU8 samples have higher strength than PU4 sample [13].
With looking at in more detail on FTIR results appeared in Fig. 5, one may conclude that the link between chain bands of PU enhances as the thickness of cell struts increases. It is well known that the higher cross-linking degree of PU leads to thicker cell wall structure (struts, ligaments and junctions). These changes in the cell structure may result in higher density of PU foams. In the other side, these changes may lead to higher density for PU foams [16]. On the other hand, the density of PU foam depends strongly on the SnO2 content. In this regard, according to obtained results the number of cross-link between chains of PU foams is relatively constant as the content of nanoparticles increases from 0.5 to 1 % in low density sample. In low density sample, due to lower reaction between isocyanate and polyol the cross-link between chains can be decreased. This phenomenon results to agglomeration of particles in PU4-1 and leading to be softened. The reverse behavior can be seen in samples with high density. In the other hand, in Fig. 6, the intensity peak at 3300–3500 cm−1 in comparison to PU6-0.5 and PU6-1 is significantly reduced. This peak relates to NH group showing the strength and number of hydrogen bonds declines from PU6-1 to PU6-0.5. On the other hand, NH group can be mixed with the carbonyl group to form hydrogen bond in hard PU micro-phase. The authors believe that the reason of this variation can be attributed to the fact that addition nano sized SnO2 causes to increase the ability of the PU to be cross-linked. This is because the intensity depends strongly on the carbonyl groups and their amount will be dictated by cross linking. This is why the intensity of PU6-1 is much lower than that of PU6-0.5. Therefore, PU6-1 sample has more cross-link and strength than PU6-0.5 [13–15]. Perhaps the most reason of lack of peak of SnO2-PU interface can be attributed to the fact that a thick PU film cover the interface of SnO2-PU and impede to appear the correspondence peak by FTIR analysis. The similar result explained in more details by Barrios et al. [17]. The same results of PU6 nanocomposites also were observed for PU8 nanocomposites.
3.2 Morphological analysis
SEM images of neat PU foams with different densities are shown in Fig. 7. As can be seen that the cell structure of neat PU foams show spherical shape. Cell sizes of PU4 and PU6 and PU8 are listed in Table 3. With this considerable growth in cell dimension, the cell structure still seems to be unscathed and uniform. The reaction of isocyanate and hydroxyl groups plays an important role in PU foam manufacture as it provides CO2 gas for blowing the foam [18]. Accordingly, increase of isocyanate content leads to more growth of cells in PU6 and PU8 [19].
The average strut thickness obtained from four points for each specimen are listed in Table 3. As illustrated in Fig. 7a, PU4 foam shows smaller cavities with thin strut thickness with respect to PU6 and PU8 foams (Fig. 7b, c). Low reaction of isocyanate and polyol causes to reduce hydrogen bond and then make thin strut in PU4. Due to decrease in struts thickness of PU4 foam, their bulk density is declined. These differences lead to different mechanical and physical properties of foams. These results approve with the TGA results and have been confirmed by previous studies [20].
The effect of incorporation of SnO2 nanoparticles on cellular morphology of PU foam specimens was studied. Figure 8 shows PU foams with 0.5 wt% of SnO2 nanoparticles, the cell dimension has indeed decreased but riddled with a number of collapsed cells. As SnO2 increases to 1 % shown in Fig. 9, the cell structure has almost intact. The SnO2 nanoparticles can act as nucleation sites and increase cell density of foams [21]. By comparison of images presented in Fig. 10, in nanocomposite of PU6 foams the average cell radius decreases with increasing amount of the SnO2 nanoparticles. Further study showed that increase amount of nanoparticles in low-density polyurethane foam (PU4) to about 0.5 wt% similar behavior to high-density but increase amount of nanoparticles to 1 wt% due to agglomeration and weak bond between nanoparticles and polymer chains showed opposite behavior. The effect of this phenomenon is clearly visible in reducing the mechanical properties of PU4 composites group.
3.3 Mechanical properties
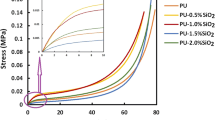
The properties of PU also depend on cell morphology for example cell size, cavity size, thickness of struts and etc. Compressive stress–strain curves for the variety of density of neat PU foams are shown in Fig. 11. All curves show three steps of deformation; linear behavior, plateau region and densification [22]. By comparison of curves, with increase the density in PU foams strength of step 1 increases but plateau region decreases. This is because more cross-linking taken placed with increase the density and so initial strength of struts increases. On the other hand, more isocyanate lead low and thick struts, therefore, plateau region is decreased [23, 24]. The stress–strain results of PU nanocomposites foams under quasi-static compression loading are shown in Fig. 12. It can be seen that compressive stress of nanocomposite PUs are higher than the neat Pus. In comparison to the neat PU foam, the PU4-1 nanocomposite foams show about 100 % increase in compression strength of plateau region, while the PU8-1 nanocomposite foams with 1 wt% SnO2 show about 1000 % increase in strength of plateau region. It is also seen that increase in strength can be attributed to stiffer cell faces and walls with addition nanoparticles. The exact reason of higher strength of PU8-1 than that of PU6-1 and PU4-1 refers to the load carrying by foam wall. It is clear the capability of thicker wall to carry the load is much higher than thinner. Mahfuz et al. [6] reported the strength of polyurethane reinforced with different additives depended on strut thickness due to their role on carrying load .
It is also noticed in the FTIR and SEM results that there are described in previous sections. Because of decrease of strut thickness, it is obvious that the strength of samples was decreased by addition 1 % nanoparticles in low density (PU4-1). But the opposite results were obtained for other nanocomposite samples. In the other word, the strength of specimens increasing by addition of nanoparticles from 0.25 to 1 wt% in high density nanocomposite PU foams. The compress strength of PU8 and PU6 series (including neat and their nanocomposites) shows similar behavior to rigid PU foams. Based on the literature survey done by the authors, increase in both –NCO/–OH ratio and nanoparticle content lead the system to be strengthen [25–27]. It is worth noting that the achieved results are confirmed by the consequences of researchers [10, 16, 19, 28, 29].
3.4 TGA results
TGA results were obtained in the form of percent weight loss as a function of temperature. During heating, degradation and decomposition of the PU foam can be occurred. Figure 13 shows decomposition behaviors of all neat foams are in the form of one step degradation process, as reported in the previous study [6]. Fig. 14 represents the temperature corresponding to 50 % decomposition of the nanocomposite samples. It is observed that the decomposition temperatures for nanocomposite PU foams are higher than neat PU foams. This improvement in thermal stability could also be due to the catalytic effect on the cross-linking of the PU foam caused by the presence of SnO2 nanoparticles. In the other word, the presence of nanoparticles enhances the number of cross link bond between urea chains [6, 16].
4 Conclusion
It has been observed that incorporation of a small weight percent of SnO2 nanoparticles into PU foams can change their cell structures. The following phrases are the summary of the current investigation:
-
Functionalized SnO2 nanoparticles dispersed in a good manner in polyol of PU foam.
-
An increase in isocyanate content leaded to increase the growth of cells and the density.
-
FTIR spectra of neat PU foams showed that an increase in isocyanate content caused to increase the bonding between the chains.
-
The dependence of mechanical properties of high density PU foams on the presence of SnO2 nanoparticles is much higher than that of low density PU foams.
-
Almost all Nanocomposite PU foams (except PU4-1) showed higher strength due to stronger cell struts because of presence of SnO2.
-
Thermal resistance of PU foams enhanced due to addition of SnO2 nanoparticles.
References
N. Adam, G. Avar, H. Blankenheim, W. Friederichs, M. Giersig, E. Weigand, M. Halfmann, F.-W. Wittbecker, D.-R. Larimer, U. Maier, S. Meyer-Ahrens, K.-L. Noble, H.-G. Wussow, Polyurethanes, Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2000), pp. 545–604
M. Sadeghi, M. Mehdi Talakesh, B. Ghalei, M. Shafiei, J. Membr. Sci. 427, 21–29 (2013)
L.M. Chiacchiarelli, I. Puri, D. Puglia, J.M. Kenny, L. Torre, Thermochim. Acta 549, 172–178 (2012)
P. Cinelli, I. Anguillesi, A. Lazzeri, Eur. Polym. J. 49, 1174–1184 (2013)
S. Gunashekar, K.M. Pillai, B.C. Church, N.H. Abu-Zahra, J. Porous Mater. 22, 749–759 (2015)
H. Mahfuz, V.K. Rangari, M.S. Islam, S. Jeelani, Compos. Part A-Appl. Sci. 35, 453–460 (2004)
X. Cao, L. James Lee, T. Widya, C. Macosko, Polymer 46, 775–783 (2005)
J.G. Gwon, S.K. Kim, J.H. Kim, J. Porous Mater. 23, 465–473 (2015)
S. Gunashekar, N. Abu-Zahra, J. Porous Mater. 23(3), 801–810 (2016)
G. Harikrishnan, T.U. Patro, D.V. Khakhar, Ind. Eng. Chem. Res. 45, 7126–7134 (2006)
H.M.M.F. Uddin, S. Zainuddin, S. Jeelani, J. Nanotechnol. 2009, 1–8 (2009)
R. Scipioni, D. Gazzoli, F. Teocoli, O. Palumbo, A. Paolone, N. Ibris, S. Brutti, M.A. Navarra, Membranes 4, 123–142 (2014)
P. Zhao, Y. Wang, J. Zhu, X. Hua, Q. Wen, Sci. China Ser. B 51, 58–61 (2008)
P. Zhao, X. Hua, Y. Wang, J. Zhu, Q. Wen, Mater. Sci. Eng. A-Struct. 457, 231–235 (2007)
C. Zhou, P. Wang, W. Li, Compos. Part B-Eng. 42, 318–325 (2011)
H. Mahfuz, M. Uddin, V. Rangari, M. Saha, S. Zainuddin, S. Jeelani, Appl. Compos. Mater. 12, 193–211 (2005)
A.E. Barrios, J.R.R. Méndez, N.V.P. Aguilar, G.A. Espinosa, J.L.D. Rodríguez, Infrared Spectroscopy—Materials Science, Engineering and Technology, in FTIR—An Essential Characterization Technique for Polymeric Materials, ed. by Th Theophile (InTech, Croatia, 2012), pp. 195–212
K.H. Choe, D.S. Lee, W.J. Seo, W.N. Kim, Polym. J. 36, 368–373 (2004)
R. Jahanmardi, B. Kangarlou, A. Dibazar, J. Nanostruct. Chem. 3, 1–6 (2013)
J.G. Gwon, S.K. Kim, J.H. Kim, Mater. Des. 89, 448–454 (2016)
L. Verdolotti, M. Lavorgna, R. Lamanna, E. Di Maio, S. Iannace, Polymer 56, 20–28 (2015)
M.E. Kabir, M.C. Saha, S. Jeelani, Mater. Sci. Eng. A-Struct. 459, 111–116 (2007)
H. Janik, M. Sienkiewicz, J. Kucinska-Lipka, H. Dodiuk, S.H. Goodman, 9-Polyurethanes, Handbook of Thermoset Plastics, 3rd edn. (William Andrew Publishing, Boston, 2014), pp. 253–295
M.M. Bernal, M. Martin-Gallego, L.J. Romasanta, A.-C. Mortamet, M.A. Lopez-Manchado, A.J. Ryan, R. Verdejo, Polymer 53, 4025–4032 (2012)
D.-H. Kim, O.-J. Kwon, S.-R. Yang, J.-S. Park, B.C. Chun, Fiber Polym. 8, 155–162 (2007)
A.R. Hamilton, O.T. Thomsen, L.A.O. Madaleno, L.R. Jensen, J.C.M. Rauhe, R. Pyrz, Compos. Sci. Technol. 87, 210–217 (2013)
P.-S. Joanna, C. Bogusław, L. Joanna, J. Porous Mater. 18, 631–638 (2010)
M.L. Pinto, J. Chem. Educ. 87, 212–215 (2010)
D.U. Shah, F. Vollrath, D. Porter, Polymer 56, 93–101 (2015)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmailzadeh, M., Danesh Manesh, H. & Zebarjad, S. Role of SnO2 nanoparticles on mechanical and thermal properties of flexible polyurethane foam nanocomposite. J Porous Mater 23, 1381–1388 (2016). https://doi.org/10.1007/s10934-016-0197-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0197-9