Abstract
A hard anodization (HA) technique is employed using different mixtures of phosphoric/oxalic acid for fast fabrication of alumina nanopore arrays in voltages higher than 200 V. The mixtures enable to avoid the breakdown of porous anodic alumina (PAA) in the high voltages. It is revealed for the first time that continuously tunable pore intervals (Dint) from 500 to 750 nm can be controlled by varying the concentrations of oxalic acid at anodization voltages (Uanod) from 230 to 360 V, far beyond the Uanod in the single electrolyte of phosphoric acid or oxalic acid. The ratios of interpore distance, pore diameter and barrier layer thickness to anodization voltage are in the range of conventional HA process for each acid mixture. In this approach, the PAA film growth rate is 26 µm/h, being 7 times larger than that in typical mild anodization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anodic alumina membrane with self-organized hexagonal packed pore arrays in nanometer scale has been intensively utilized as the starting material to synthesize diverse nanostructures [1–5]. To facilitate various practical applications, simple fabrication of the porous anodic alumina (PAA) films with different ranges of pore size and pore intervals on large scales is a challenge. The self-ordering process is one of the most favorable approaches to fabrication of the PAA [6, 7]. Self-ordered PAA has been achieved based on the mild anodization (MA) in a limited condition [8–12]. However, the MA is a slow process in which the oxide growth rates about 9–6 µmh−1. It needs several days of processing time to produce proper PAA film for the application. Recently, a new method based on the hard anodization (HA) process was introduced for the fast fabrication of the PAA film in a wide range of pore sizes and interpore distances [13–15]. The high current density (100–80 mA/cm−2) and oxide growth rate (25–50 µmh−1) of HA are key factors in the use of this technique for various industrial applications. However, at a voltage higher than 200 V, the catastrophic flow of electric current leads to breakdown of the oxide film using HA [15]. Such limitations in HA may decrease the applicability of this method to fabricate the PAA film in a wider interpore distance. It is generally believed that in a high voltage anodization, the barrier layer temperature and ionic mobility are the key controlling factors ruling the catastrophic flow [17–19]. As it was shown in a previous report, the ionic mobility in the barrier layer can be modified by the anodization electrolyte mixture [19].
When the anodization is performed in electrolytes such malonic, citric, boric, and chromic acid, the growth rate and regularity of PAA decreases drastically and far inferior to those formed by conventional MA or HA processes using sulfuric, oxalic, or phosphoric acid [11, 16, 20]. From this point of view by using a mixture of sulfuric and oxalic acid, PAA with appropriate regularity was obtained in MA and HA condition [21, 22]. The ionic mobility and permittivity of the barrier layer formed in oxalic acid are higher than it in phosphoric acid [17, 19]. Therefore by mixing these acids, ionic mobility and permittivity of the barrier layer can be modified.
In the present study, an approach to fabrication of PAA film using oxalic/phosphoric acid mixture and anodization voltage over 200 V in HA condition is reported. The use of different electrolyte composition in anodization, ranging between 230 and 360 V is effectively demonstrated.
2 Experimental
High purity aluminum foil (99.999 %, 0.25 mm in thickness) was used as a starting material, degreased in acetone and washed in deionized water. The aluminum foil was then electro-polished at a constant voltage of 20 V in a 1:4 volume mixture of perchloric acid and ethanol in order to diminish the aluminum surface roughness.
The hard anodization technique was carried out using oxalic/phosphoric mixtures containing 0.3 M phosphoric acid and x M oxalic acid (x = 0.05, 0.1, 0.15, 0.2 and 0.3) as electrolyte. During the anodization process the electrolyte temperature of all the samples was kept below 278 K.
Powerful low temperature chillers were used to control the temperature of the electrolyte in the reactor. At first, to create a protective layer against burning the sample at high anodization voltages and currents, the process was started by conventional MA at 70 V for 10 min. The anodization voltage was then increased to a final value and kept constant. These anodization voltages and corresponding acid concentrations are presented in Table 1.
To facilitate the observation of cells arrangement at the bottom, the remaining aluminum substrate was detached by a saturated SuCl4 solution. The samples were then broken to consider the barrier layer configuration. The morphologies of the samples were investigated by a field emission scanning electron microscope.
3 Results and discussion
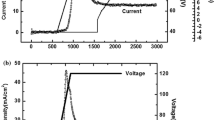
As seen in Table 1, five samples were fabricated with different phosphoric/oxalic acid mixtures. The phosphoric acid concentration was kept at 0.3 M and the oxalic concentrations were 0.3, 0.25 and reduced to 0.1 for the samples. The evolution of the current density and voltage of anodization process was considered. Typical curves of current density and its voltage are shown in Fig. 1. Following MA in 70 V, the anodization voltage is increased up to 180 V with a rate of 0.1 V/s and then the increment rate is changed to 0.15 V/s up to final anodization of 260 V. As seen in this figure while the voltage increases from MA to HA condition, at the certain voltages, the current starts to increase sharply. The current evolution is almost constant up to this voltage, after that, it increases rapidly until the maximum value where it decreased an approximately exponential decay as a function of time. As we know, in HA or MA, the interpore distance is linearly dependent on the applied voltage. According to this fact by increasing an anodization voltage, as it is indicated in Fig. 1b by arrowhead, some pores vanish and others continue growing. The anodizing time up to 2400 s (Fig. 1a) can be considered as MA due to the low anodizing current and after that anodizing current start to transit to HA. During the anodization increment, the pores begin to separate from each other until the anodization voltage reach to the final value. Then the interpore distance remains constant.
The current density–time transients show that the current increases by increment of final anodization voltage. The maximum current versus anodization voltage is plotted in Fig. 2a. As we know in HA or MA, the anodization current amplitude increases by rising the anodization voltage [14, 15]. In a given electrolyte, this increment of the anodization voltage form the spatial limit is accompanied by the catastrophic flow of electric current. Nevertheless, it was revealed that this phenomenon can be postponed to high voltage by decreasing the ionic mobility in barrier layer [23]. The ionic mobility and permittivity of the barrier layer formed in oxalic acid are higher than it in phosphoric acid [17, 19]. Decreasing the ionic oxalic contamination in the barrier layer leads to a reduction of permittivity and ionic mobility. This reduction conduces to anodization in higher voltage. As it is seen in Fig. 2, the HA in voltage higher than 200 V is possible by decreasing the oxalic concentration in oxalic/phosphoric acid mixture electrolyte. Although here the purpose of this article is fabrication of PAA film with using HA in Phosphoric/Oxalic Acid Mixtures and not to study about the reason of the how oxalic anion suppress the anodizing current during high voltage anodization. We mention some reasons for this phenomenon. Based on our knowledge until now no any articles have mentioned this effect. But it seems in mixed electrolyte this is complicated effect and may need many experimental and theoretical researches to realize about this phenomenon. It seem some physical and chemical parameters such as chemical reaction at the interface of oxide-electrolyte and ionic mobility of different onion (oxalate and phosphate) with various electric charge distribution, inside the barrier layer could response for the existence of this effect. But the contribution of these parameters is not clear.
Figure 3 shows typical SEM micrographs of the bottom surface of PAA formed at HA conditionin different mixtures. A low arrangement of pores was observed for all samples. Generally in aluminum anodization higher than 200 V, it was shown that in various electrolytes, anodization process leads to fabrication of PAA film with low regularity [11, 16, 24].
In order to gain insight into the effect of HA in a high voltage on a structure of PAA film, we focused on characterizing the microstructure of the barrier layer in this condition. As it was reported, in a high field anodization, for a given anodization potential (∆U), the barrier layer thickness (tbarr) is inversely proportional to the logarithm of the current density (j) via the following equation;
where j0 and β are material dependent constants and (∆U/tbarr) is the effective electric field strength (E) across the barrier layer [17]. According to SEM analyses (Figs. 3, 4), the barrier layer thickness (tbarr) was found to be linearly proportional the anodization voltage (see Fig. 5). The ratio of the tbarr to the anodization voltage was almost the same for all samples. The ratio was found to be 1, the same as that of the HA [14] which was about 20 % smaller than tbarr (1.3 nm/v) in MA condition [12]. This reduction can be ascribed to the high current density involved in the HA. Similarly, the interpore distance (Dint) and pore size (Dp) are found to be linearly proportional to the anodization voltage. The proportional constant obeys the HA process by the values of αinterpore = 2 nm/V and αpore = 0.4 nm/V for interpore distance and pore diameter, respectively. The porosity (p) of PAA films formed here is 4–5.5 % close to the reported range for HA condition [14, 15, 24].
It should be pointed out that PAA films grow slowly under the MA processes, whereas the film growth rate can be increased in HA condition due to the high anodization current. The growth rate of PAA film here was 7 times larger (26 µm/h) than that for typical MA (2–6 µm/h) [24]. According to SEM micrographs in Fig. 6 and their corresponding current density–time curves presented in Fig. 2, the thickness of PAA film is proportional to the charge transferring Q, during the anodization time interval, L = κQ, with a proportionality constant about 0.55 µm/C. As it is seen in Fig. 6c, d, the pore diameters can be adjusted by wet chemical etching in 0.5 M phosphoric acid. Therefore by this process, PAA film with a pore diameter of 450 nm is available.
4 Conclusions
The PAA films were fabricated by the HA technique in the phosphoric/oxalic acid mixtures with D int from 500 to 750 nm. A wide range of oxalic acid concentrations and anodization voltages were employed. The effect of oxalic acid concentration on the pore formation was investigated and the following conclusions were obtained.
-
1.
Phosphoric/oxalic acid mixtures were able to suppress breakdown of porous anodic alumina in voltages higher than 200 V.
-
2.
By increasing the anodization voltage, the ratio of oxalic in the acid mixtures was decreased.
-
3.
For each acid mixture, the ratios of interpore distance and pore diameter to anodization voltage were 2 nm/V and 0.4 nm/V, respectively which are in the range of conventional HA process. The porosity of the samples varied from 4 to 5.5 %.
References
M.T. Rahman, X. Liu, A. Morisako, J. Appl. Phys. 99, 08G9041 (2006)
T. Kikuchi, Y. Wachi, T. Takahashi, M. Sakairi, R.O. Suzuki, Electrochim. Acta 94, 269–276 (2013)
S. Zhang, L. Wang, C. Xu, A. Li, L. Chen, D. Yang, ECS Solid State Lett. 2(1), Q1–Q4 (2013)
W.J. Zheng, G.T. Fei, B. Wang, L.D. Zhang, Nanoscale Res. Lett. 4, 665–667 (2009)
C.J. Ingham, J.T. Maat, W.M. de Vos, Biotechnol. Adv. 30, 1089–1099 (2012)
V. Valtchev, S. Mintova, M. Tsapatsis, Ordered Porous Solids: Recent Advances and Prospects (Elsevier Science, Amsterdam, 2009)
A. Eftekhari, Nanostructured Materials in Electrochemistry (Wiley, Weinheim, 2008)
O. Jessensky, F. Muller, U. Gosele, J. Electrochem. Soc. 145(11), 3735–3740 (1998)
F.Y. Li, L. Zhang, R.M. Metzger, Chem. Mater. 10, 2470–2480 (1998)
H. Masuda, K. Fukuda, Science 268, 1466–1468 (1995)
S. Ono, M. Saito, M. Ishiguro, H. Asoh, J. Electrochem. Soc. 151, 473–478 (2004)
K. Nielsch, J. Choi, K. Schwirn, R.B. Wehrspohn, U. Gosele, Nano Lett. 2(7), 677–680 (2002)
S.Z. Chu, K. Wada, S. Inoue, M. Isogai, A. Yasumorib, Adv. Mater. 17, 2115–2119 (2005)
W. Lee, R. Ji, U. Gosele, K. Nielsch, Nat. Mater. 5, 741–747 (2006)
M. AlmasiKashi, A. Ramazani, M. Noormohammadi, M. Zarei, P.J. Marashi, J. Phys. D Appl. Phys. 40, 7032 (2007)
S.Z. Chu, K. Wada, S. Inoue, M. Isogai, Y. Katsuta, A. Yasumori, J. Electrochem. Soc. 153, 384–391 (2006)
G.E. Thompson, G.C. Wood, Nature 290, 230–232 (1981)
W. Lee, K. Nielsch, U. Gösele, Nanotechnology 18, 475713 (2007)
M. Noormohammadia, M. Moradi, Mater. Chem. Phys. 135, 1089–1095 (2012)
S. Ono, M. Saito, H. Asoh, Electrochim. Acta 51, 827 (2005)
S. Shingubara, K. Morimoto, H. Sakaue, T. Takahagi, Electrochem. Solid State Lett. 7, E15 (2004)
M.A. Kashi, A. Ramazani, M. Rahmandoust, M. Noormohammadi, J. Phys. D Appl. Phys. 40, 4625 (2007)
C. Sun, J. Luo, L. Wu, J. Zhang, ACS Appl Mater Interfaces 2(5), 1299–1302 (2010)
W. Lee, S.J. Park, Chem. Rev. 114(15), 7487–7556 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rayat Azimi, H.A., Zarei, M., Rafati, A. et al. Fabrication of self-ordered nanoporous alumina with 500–750 nm interpore distances using hard anodization in phosphoric/oxalic acid mixtures. J Porous Mater 23, 357–363 (2016). https://doi.org/10.1007/s10934-015-0088-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0088-5