Abstract
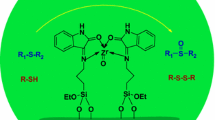
Using post grafting method, two hybrids composite materials were prepared by anchoring Nickel and Zinc Schiff base complexes onto amino-modified MCM-41(Ni-MCM-41 and Zn-MCM-41). The prepared catalysts have been characterized by FT-IR spectroscopy, thermogravimetric analysis, and transmission electron microscopy, powder X-ray diffraction and N2 adsorption–desorption isotherms. The catalysts showed excellent catalytic efficiency in oxidation reaction of sulfides to sulfoxides and oxidative coupling of thiols to their corresponding disulfides using urea hydrogen peroxide as oxidant under mild reaction conditions. The supported Schiff base complexes can be recovered easily and reused many times without significant loss in catalytic activity and selectivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the last decade, the fabrication and design of organic–inorganic hybrid porous materials, such as zeolite, microporous and mesoporous supported Schiff base complexes as heterogeneous catalysts have given widely attention in industries and catalyst filed [1–3]. The silica mesoporous material has two important properties: high surface area (>1000 m2 g−1), high pore volume (1.3 cm3 g−1), large pore size in the range 20–100 Å and great diversity in surface functionalization Compared to that of zeolites which allows them to be host for entry of bulkier guest molecules such as organometallic compounds and enzymes [4–6]. The lack of catalytic activities is the most significant disadvantage of the siliceous MCM-41. To overcome this problem the inner surfaces of MCM-41 are covered with nucleophilic silanols, which can be functionalized by post-Grafting method with various transition metals complexes. Schiff base complexes supported on MCM-41 as heterogonous catalysts have been used in various organic reaction such as hydrogenations, oxidations and polymerizations [7]. For example, Ni-Schiff base complex-MCM-41 and zinc Schiff base complex-MCM-41 have shown high selectivity in organic reactions such as: hydrogenations of benzene [8], hydrogen adsorption [9] and other reactions [10, 11], sulfoxidation reactions are of significant importance in organic chemistry, drug metabolism and medicinal chemistry [12] and as synthetic intermediates in the synthesis of biological compound [13]. Likewise, Disulfide synthesized from oxidative coupling of thiol plays an important role in synthetic organic chemistry [14], biology [15] and also found industrial applications as vulcanizing agent [16] thiols are oxidized to prevent oxidative damage due to control cellular redox potential in biological systems [17]. For this reason, many methods have been developed over the years to increase the efficiency of this kind of oxidation reactions. For example, oxidation of sulfides and oxidative coupling of thiols have been reported in the presence of various catalysts and using different oxidants (e.g. metal oxidants, organic oxidants, peroxides, halogens, and air). Although many methods have been reported for the oxidation of sulfides and conversion of thiols, the catalytic switching of the oxidation to meet green chemical requirements still remains a major challenge.
Herein, we report synthesis and characterization of ordered mesoporous silica MCM-41 containing a nickel and zinc Schiff base complexes immobilized to the internal surface of pores by using post-grafting method. The immobilized complexes were applied as efficient catalysts for chemo selective oxidation of sulfides and conversion of thiols with UHP as oxidant to give desired products in high yields.
2 Experimental section
2.1 General
Tetraethylorthosilicate (TEOS), cationic surfactant cetyltrimethylammoniumbromide (CTAB, 98 %), 3,4-dihydroxybenzaldehyde, 3-aminopropyltrimethoxysilane (3-APTES), sodium hydroxide (NaOH), Ni(NO3)2.6H2O, Zn(NO3)2·4H2O, organic sulfides, thiols, UHP and solvents were obtained from Merck, Aldrich or Fluka and were used without further purification. The physico-chemical characteristics were carried out using X-ray powder diffraction (XRPD) on a Philips diffractometer of X’pert company with monochromatized Cu Kα radiation under the conditions of 40 kV, λ = 1.5418 A and 30 mA. FT-IR spectra were recorded as KBr pellets using a VRTEX 70 model Bruker FT-IR spectrometer, thermogravimetric analysis (TGA) of the samples were carried out using a Shimadzu DTG-60 automatic thermal analyzer in the temperature range 30–900 °C at a heating rate of 10 °C min−1 in air. And N2 adsorption–desorption isotherm and BJH pore size distribution plot were recorded by (BELSORP-MINI).
2.2 Synthesis of the MCM-41
1.01 g of CTAB was added to the aqueous solution of NaOH (0.34 g in 30 mL deionized water).
5.78 g of TEOS was added drop wise to the resulting gel. After stirring for about 1 h at room temperature, then the mixture was transferred to a teflon lined autoclave and heated under static hydrothermal conditions at 383 K for 4 days. After cooling to room temperature the resultant solid product was collected by filtration, washed with distilled water and dried in air at 353 K. The white solid was calcined at 813 K for 5 h with a ramp 2 °C min. This mesoporous silica material is designated as MCM-41.
2.3 Amine group functionalization on MCM-41
4.8 g of calcined MCM-41 was mixed with 4.8 g solution of (3-aminopropyl)-triethoxysilane (APTES) in n-hexane (96 mL) then was stirred and refluxed under N2 atmosphere for 24 h. After completion of the reaction white precipitate of APTES-functionalized MCM-41 (APTES@MCM-41) was filtered, washed with n-hexane and dried under vacuum (scheme 1).
2.4 Ni or Zn Schiff base complexes immobilization on MCM-41
1.0 g of (APTES@MCM-41) was refluxed with 3, 4-dihydroxybenzladehyd (1 mmol, 0.134 g) in ethanol under N2 atmosphere for 3 h. Then the resulting yellowish solid (MCM-41-L) was collected by filtration washed with ethanol, and dried under vacuum. Eventually Ni or Zn@MCM-41 were prepared by dissolving (1 mmol) of Ni (NO3)2.6H2O or Zn (NO3)2.4H2O and above mentioned yellowish solid in ethanol (20 mL), this mixture was stirred for 24 h at 80 °C. The resulting solid was recovered by vacuum filtration, washed with ethanol using Soxhlet for 12 h to remove any unanchored metal ion species and dried under vacuum (Scheme 1).
2.5 General procedure for the preparation Sulfoxides catalyzed by Ni or Zn@MCM-41
To the mixture of sulfides (1 mmol) and Ni or Zn@MCM-41 (0.025 g) in ethanol, UHP (6 mmol) was added and stirred at room temperature for appropriate period of time as indicated in Table 4. The progress of the reaction was monitored by TLC. After the completion of the reaction, catalyst was separated by simple filtration and the product was extracted with CH2Cl2 (3 × 10 mL) and dried over anhydrous Na2SO4. Finally sulfoxide was obtained as the only product in very high isolated yields.
2.6 General procedure for the preparation of disulfides catalyzed by Ni or Zn@MCM-41
UHP (5 mmol) was added to the mixture of sulfide (1 mmol) and Ni or Zn@MCM-41 catalysts (0.025 g) in ethanol solvent. The mixture was stirred thoroughly for the required time at room temperature as indicated in Table 7. The progress of the reaction was monitored by TLC. After the completion of the reaction, catalyst was separated by simple filtration and the product was extracted with CH2Cl2 (3 × 10 mL) and dried over anhydrous Na2SO4. Finally disulfide was obtained in very high isolated yields.
3 Results and discussion
In continuation of our studies on the application of new heterogeneous systems in organic reactions [9, 18–20]. herein we report synthesis and characterization of Ni or Zn Schiff base complexes immobilized onto MCM-41. We studied their applications as heterogeneous and recoverable catalysts in oxidation of sulfides into sulfoxides and oxidative coupling of thiols into their corresponding disulfides in the presence of UHP as oxidant.
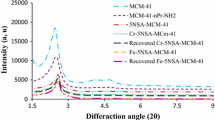
Low-angle XRD patterns calcined MCM-41, functionalized MCM-41, MCM-41– Ligand, Ni-MCM-41, Zn-MCM-41, recovered Ni-MCM-41 and Zn-MCM-41are shown in Fig. 1. The typical hexagonal phase of the MCM-41 exhibited three [(1 0 0), (1 1 0), and (2 0 0)] distinct diffractions which are clearly visible in calcined MCM-41. In other samples, intensity of diffraction peak of (1 0 0) decreased progressively when organic groups or metals (Ni or Zn) were introduced into MCM-41 pores. The intensity reduction may be mainly due to contrast matching between the silicate framework and organic moieties which are located inside the pores of mesoporous MCM-41. Obviously, increase in loading of groups over calcined MCM-41 makes this main peak (d100) broader or weaker. Also structural stability of the samples was retained after grafting of organometallic complex.
The nitrogen sorption isotherms of calcined MCM-41, functionalized MCM-41,Ni@MCM-41 and Zn@MCM-41 are shown in Fig. 2. According to the IUPAC classification all samples have type IV isotherms. The nitrogen sorption analysis exhibits that the surface area of MCM-41, Ni@MCM-41 and Zn@MCM-41 are 986.16, 170.18 and 220.10 m2 g respectively. Decreases in pore diameter, surface area, pore volume, and increase in wall thickness could be observed at each stage of modification which can be attributed to the introduction of grafting organic groups and metal ion inside the channels/onto the mesoporous wall of the supported MCM-41. The surface properties of the synthesized materials are summarized in Table 1.
Thermo gravimetric (TG) analysis of the samples is presented in Fig. 3. The lowest weight loss for calcined MCM-41 with the approximate amount of 6 % was observed, which is mainly attributed to the loss of physisorbed water and the TG spectrum of the functionalized MCM-41 indicated a three-step weight loss. The weight loss (3 %) below 120 °C was the result of the physically adsorbed water and the 8 % weight loss between 200 and 300 °C typically was related to desorption of physisorbed water, whereas the third weight loss is relevant to the decomposition of organic chemical. The TGA spectrum of grafted ligand to MCM-41 indicated 13 % weight loss above 350 °C that is mainly related to the decomposition of organic groups. The TGA curve of Ni@MCM-41 and Zn@MCM-41 shows a weight loss of 17.56 and 18 % respectively between 250 and 600 °C which is similar to the combustion of amine and decomposition of Schiff base complexes anchored on modified MCM-41.
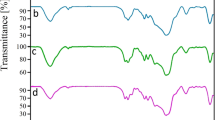
The FT-IR spectra of (a) calcined MCM-41, (b)MCM-41-NH2, (c) MCM-41-ligand, (d) Ni@MCM-41, (e) recovered Ni@MCM-41, (f) Zn@MCM-41 and (g) recovered Ni@MCM-41 in the range of 400–4000 cm−1 are depicted in Fig. 4. The absorption band around 1100–1200 cm−1 is due to Si–O asymmetric stretching vibrations of Si–O–Si bridges which indicate the presence of Silica in MCM-41 and band at 3000–3600 cm−1 region, can be attributed to the silanol OH groups. The spectra of functionalized MCM-41 show C–H stretching vibration at 2932 cm−1 and also two band (N–H) group appeared at 1642 and 1468 cm−1. Peak at 1657 cm−1 is assigned to the stretch vibration of the imine group (–C=N), which is the basic IR band of the Schiff base ligand. After complexion of Ni and Zn ions with MCM-41-ligand, the vibration peak (–C=N) shifts to lower frequency and appears at 1640 and 1638 cm−1 respectively. The above result parallels the fact of the formation of the coordination binding between the Schiff base ligands and the metal ions.
The ordered meso structure of calcined MCM-41 is further confirmed by TEM. Figure 5 shows that MCM-41 possesses clear well-order channels and confirms 2D hexagonal pore arrangement. This is in good agreement with the XRD and nitrogen adsorption–desorption results as discussed above.
3.1 Catalytic oxidation of sulfides to sulfoxides using Ni@MCM-41 or Zn@MCM-41 as catalyst
The catalytic performance of synthesized Ni@MCM- 41 and Zn@MCM-41 were evaluated for the oxidation of sulfides in presence UHP as oxidant (scheme 2). In order to optimize the reaction conditions, the effect of solvents on oxidation efficiency of the sulfides was tested using dibenzyl sulfide as a model compound. The results are listed in Table 2. It was observed that ethanol is the only solvent of choice for this purpose.
The progress of the reaction was also monitored with different catalyst loading. One can observe that there is increase in the conversion of sulfide if one increases the amount of catalyst from 5 to 25 mg. The results in Table 3 indicate that 25 mg of catalyst is sufficient for completion of the reaction.
With optimal conditions in hand, oxidation reactions of various types of structurally divergent aryl and alkyl sulfides to their corresponding sulfoxides have been carried out (Table 4). Oxidation reactions were performed under mild and completely heterogeneous conditions at room temperature with UHP as oxidant. After reaction completion, the product is extracted by simple filtration and dichloromethane is removed by evaporation.
Subsequently, the recyclability of two Ni@MCM-41 and Zn@MCM-41 heterogeneous catalysts in oxidation reaction of dibenzyl sulfide to dibenzyl sulfoxide were evaluated. The catalyst was also recovered from the oxidation reaction by centrifuge and washed exhaustively with ethanol, and dried in vacuum. The results shown in Fig. 6 indicate that this oxidation system is recyclable for five runs without any significant loss of catalytic activity.
3.2 Catalytic oxidative coupling of thiols to produce disulfides catalyzed by Ni@MCM-41 or Zn@MCM-41
The catalytic activity of Ni@MCM-41 and Zn@MCM-41 were evaluated for oxidative coupling of thiols with UHP as oxidant in mild condition (Scheme 3). To achieve optimum reaction conditions, various parameters such as: nature of solvent, amount of catalyst and UHP were studied.
A different solvent were screened for the oxidative coupling of 4-methyl thiophenol, and as the result ethanol was found to be the best solvent in terms of high yield and short time (Table 5).
To find the effect of the catalyst, blank experiment (4-methyl thiophenol (1 mmol) with UHP (5 mmol) in the absence of catalyst was conducted. It was found that the oxidation reaction occurs rather slowly (h). However when similar oxidation reaction was carried out in the presence of catalysts (Ni or Zn-MCM-41, 25 mg), the reaction was completed, gave an excellent yield of corresponding disulfides (Table 6).
Eventually, with optimal conditions, we investigated the coupling of several thiols with different functional groups (aromatic and aliphatic) into disulfides using Ni@MCM-41 and Zn@MCM-41 heterogeneous catalysts in ethanol at room temperature (Table 7).
From the viewpoint of practical applications of the catalyst, in this purpose, reusability and stability of our catalysts were studied through an oxidative coupling of 4-methyl thiophenol corresponding to disulfide. After completion of the reaction, catalyst separated by simple filtration and washed with ethanol and reused in the next run without any purification. As it can be seen from Fig. 7, the catalyst has been observed to be reusable for at least five times without a detectable catalyst leaching or an appreciable change in activity.
In order to investigation of efficiency this method, the catalytic activity of these catalysts were compared with some previous reported procedures for the oxidation of methyl phenyl sulfide (Table 8, entry 1–7) and oxidative coupling of 4-methyl benzene thiol (Table 8, entry 8–14). These catalysts showed good reactivity with short times and excellent yields.
4 Conclusion
Covalent binding of Ni or Zn complex over modified surface of MCM-41 was successfully achieved by post-grafting method. Ni or Zn @MCM-41 can function as a heterogeneous oxidation catalyst with UHP as oxidant for oxidation of organic compounds such as: sulfides and thiols. The designed catalytic system prevents effectively the over-oxidation of sulfides and thiols. Separation and recycling can also be easily done using a simple filtration without any loss in selectivity. The advantage of these synthesized catalyste: that it is cheap, selective, efficient and environmental friendly.
References
P. Chen, B. Fan, M. Song, C. Jin, J. Ma, R. Li, Catal. Commun. 7, 969 (2006)
A. Mobinikhaledi, M. Zendehdel, S.M.B. Hosseini-Ghazvin, P. Safari, Transit. Metal. Chem. 40, 313 (2015)
S. Bhunia, S. Koner, Polyhedron 30, 1857 (2011)
J.M. Thomas, R. Raja, J. Organomet. Chem. 689, 4110 (2004)
Y.C. Lee, S. Dutta, K.C.W. Wu, ChemSusChem 7, 3241 (2014)
Y. Li, Q. Ma, Z. Liu, X. Wang, X. Su, Anal. Chim. Acta 840, 68 (2014)
M. Ziolek, Catal. Today 90, 145 (2004)
R. Wojcieszak, S. Monteverdia, M. Mercy, I. Nowak, M. Ziolek, M.M. Bettahar, Appl. Catal. A Gen. 268, 241 (2004)
P. Carraro, V. Elıas, A.A. Garcıa, K. Blanco, M. Oliva, Int. J. Hydrogen Energy 39, 8749 (2014)
E. Rossetto, B.P. Nicola, R.F. de Souza, K. Bernardo-Gusmao, S.B.C. Pergher, J. Catal. 323, 45 (2015)
M. Silva, M.J.F. Calvete, N.P.F. Goncalves, H.D. Burrows, M. Sarakha, A. Fernandes, M.F. Ribeiro, M.E. Azenha, M.M. Pereira, J. Hazard. Mater. 233, 79 (2012)
A. Ghorbani-Choghamarani, J. Zeinivand, J. Iran. Chem. Soc. 7, 190 (2010)
M.C. Carreno, Chem. Rev. 95, 1717 (1995)
A. Supale, G. Gokavi, React. Kinet. Catal. Lett. 93, 141 (2008)
J.C. Lukesh, K.K. Wallin, R.T. Raines, Chem. Commun. 50, 9591 (2014)
M. Van Der Horst, K.G. Hendrikse, C.D. Woolard, J. Appl. Polym. Sci. 89, 47 (2003)
L. Bruelisauer, M.A. Gauthier, J.C. Leroux, J. Control. Release 195, 147 (2014)
M. Nikoorazm, A. Ghorbani-Choghamarani, H. Mahdavi, S.M. Esmaeili, Microporous Mesoporous Mater. 211, 174 (2015)
M. Nikoorazm, A. Ghorbani-Choghamarani, F. Ghorbani, H. Mahdavi, Z. Karamshahi, J. Porous. Mater. 22, 261 (2015)
M. Nikoorazm, A. Ghorbani-Choghamarani, N. Noori, J. Porous. Mater. 22, 877 (2015)
M.M. Lakouraj, M. Tajbakhsh, F. Shirini, M.V. Asady Tamami, Synth. Commun. 22, 261 (2005)
A. Shaabani, A. Bazgir, K. Soleimani, P. Salehi, Synth. Commun. 33, 2935 (2003)
M. Abdo, Z. Yong, V.L. Schramm, S. Knapp, Org. Lett. 12, 2982 (2010)
Y. Inbushi, M. Yoshihara, Phosphorus Sulfur 103, 101 (1995)
F.R. Bisogno, A. Rioz-Martnez, C. Rodguez, I. Lavandera, G. de Gonzalo, D.E. Torres Pazmino, M.W. Fraaije, V. Gotor, Chem. Catal. 2, 946 (2010)
A. Ghorbani-Choghamarani, G. Azadi, B. Tahmasbi, M. Hadizadeh-Hafshejani, Z. Abdi, Phosphorus Sulfur 189, 433 (2014)
A. Ghorbani-Choghamarani, H. Goudarziafshar, M. Nikoorazm, S. Yousefi, Lett. Org. Chem. 6, 335 (2009)
A. Ghorbani-Choghamarani, S. Sardari, J. Sulfur Chem. 32, 63 (2011)
A. Ghorbani-Choghmarani, M. Nikoorazm, H. Goudarziafshar, A. Shokr, H. Almasi, J. Chem. Sci. 123, 453 (2011)
B. Karami, M. Montazerozohori, M.H. Habibi, Molecules 10, 1358 (2005)
A. Rostami, B. Tahmasbi, F. Abedi, Z. Shokri, J. Mol. Catal. A: Chem. 378, 200 (2013)
A. Ghorbani-Choghamarani, Z. Darvishnejad, M. Norouzi, Appl. Organometal. Chem. 29, 170 (2015)
M. Mansour Lakouraj, M. Tajbakhsh, H. Tashakkorian, Monatsh. Chem. 138, 83 (2007)
A.G. Mei Wang, J. Shi, D. Wang, W. Tian, L. Sun, Appl. Organometal. Chem. 20, 830 (2006)
M. Lakshmi Kantam, B.V. Prakash, B. Bharathi, Ch. Venkat Reddy, J. Mol. Catal A: Chem. 226, 119 (2005)
K. Masayuki, A. Yasutaka, O. Shiho, N. Takuya, H. Akihiko, H. Yoshiro, Synthesis 21, 3286 (2007)
L. Menini, M.C. Pereira, A.C. Ferreira, J.D. Fabris, E.V. Gusevskaya, Appl. Catal A: Gen. 392, 151 (2011)
P.J. Chai, Y. Shu Li, C. Xia Tan, Chin. Chem. Lett. 22, 1403 (2011)
H. Imanieh, S. Ghamami, M.K. Mohammadi, A. Jangjoo, Russ. J. Gen. Chem. 77, 282 (2007)
A. Akdag, T. Webb, S.D. Worley, Tetrahedron Lett. 47, 3509–3510 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noori, N., Nikoorazm, M. & Ghorbani-Choghamarani, A. Synthesis and characterization of Ni and Zn Schiff base complexes supported on modified MCM-41 as reusable catalysts for various oxidation reactions. J Porous Mater 22, 1607–1615 (2015). https://doi.org/10.1007/s10934-015-0044-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0044-4






 ) calcined MCM-41, (
) calcined MCM-41, ( ) functionalized MCM-41, (
) functionalized MCM-41, ( ) Ligand-MCM-41, (
) Ligand-MCM-41, ( ) Ni@MCM-41 and (
) Ni@MCM-41 and ( ) Zn@MCM-41
) Zn@MCM-41




