Abstract
Schiff base complex of copper-functionalized MCM-41 (Cu-complex@MCM-41) was synthesized and used as an efficient and novel heterogeneous catalyst for the oxidative coupling of thiols into corresponding disulfides and oxidation of sulfides to sulfoxides using hydrogen peroxide (H2O2) as the oxidant. An aliphatic and aromatic series of sulfides and thiols including various functional groups were successfully converted into corresponding products. The all products were obtained in good to excellent yields. The mesoporous catalyst is characterized by FT-IR spectroscopy, BET, XRD, SEM, EDS and TGA. Recovery of the catalyst is easily achieved by simple filtration and reused for several consecutive runs without significant loss of its catalytic efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, separation and recycling of heterogeneous catalysts is employed in the various area such as industrial and green chemistry. Heterogeneous supports allow efficient recycling by filtration, but the application of heterogeneous catalysts is limited because of their less active site than their homogeneous counterparts [1, 2]. In this regard, nano materials have emerged as useful and sustainable alternatives to the immobilization of homogeneous catalysts [3] since, when the size of the support is decreased to the nanometer scale, the surface area is substantially increased, which is combined with excellent accessibility of the surface-bound catalytic sites [4]. Among the various nano materials, Inorganic nanoporous materials have recently been used in various fields, such as catalysis [5], adsorption [6], extraction [7], energy [8], drug delivery systems [9], and for their luminescent character [10], and especially in the isolation and recycling of expensive homogeneous catalysts [5]. Among them, mesoporous MCM-41 have been widely used as catalysts in many organic reactions because of some unique properties like as large specific surface area (>1000 m2/g), homogeneous hexagonal pore arrays with pore diameters between 1.5 and 10 nm, relatively hydrophobic nature, ease of functionalization, high thermal stability (900 °C) and facile separation [11, 12]. Its high thermal stability allows perform of organic reaction in high temperatures and also its very large pore size allows passage of large molecules such as organic ligands and metal complexes through the pores to reach to the surface of the channel [13]. Furthermore, Existence of many silanol groups on the mesoporous MCM-41 surface leads to coupling with alkoxysilane compounds which support terminal functional groups available for immobilization of other substances [11]. These properties of MCM-41 has attracted considerable attention for its potential application as catalyst [5, 11].
Copper is an efficient and non-toxic metal moiety in many heterogeneous and homogeneous catalysts particularly for the synthesis of organic compounds. Briefly, among the copper catalyzed systems it can be noted, Anchored Cu (II) acetylacetonate onto amine functionalized mesoporous silica was produced and tested for aziridination of styrene [14]. The friedel–crafts hydroxyalkylation [15] and Suzuki–Miyaura-coupling reactions [16] in the presence of Cu (II) complexes have been reported. Also copper (II)-SBA-15 demonstrated as an efficient catalyst for 1,4-triazoles synthesis via azide alkyne cycloaddition [17].
Coupling reactions such as oxidative coupling of thiols into corresponding disulfides and as well as oxidation of organic functional groups such as oxidation of sulfides into sulfoxides are useful transformations in the synthesis of new molecules, as well as for various medical, biological, materials, and nanotechnological applications [18–20]. In particular, some of biologically active sulfoxides play an important role as therapeutic agents such as antifungal, antibacterial, anti-ulcer, anti-atherosclerotic, antihypertensive and as well as psychotropics and vasodilators [21, 22]. Furthermore, Omeprazole and the pesticide Fipronil are two typical examples of the extensive application of these intermediates in pharmaceutical and fine chemical industries [23, 24].
Likewise, disulfide bond formation is used in sulfenylation of enolates and other anions, industrial applications such as vulcanizing agents [25], bioactive molecules as well as oil sweetening processes and peptide or protein stabilization anions [26], while some disulfides have been found useful as vulcanizing agents for rubber and elastomers imparting them suitable tensile strength [27]. Moreover, they are also relatively more stable in organic reactions such as oxidation, alkylation and acylation compared to the corresponding free thiols, therefore the thiol group can be protected as a disulfide [26]. Additionally, in organic synthesis, S–S bonds are used for the synthesis of organo-sulfur compounds via C–S bond formation [28].
For these reasons, many methods have been developed over the years to find efficient these organic transformations [18–32]. For example, many methods have been reported in the presence of various catalysts and using different oxidant (e.g. metal oxidants, organic oxidant, peroxides, halogens, and air) [33]. For example, Cu(NO3)2·3H2O and Fe(NO3)3·9H2O [34], Cu(NO3)2·N2O4 [35, 36], melamine hydrogen peroxide [37] and urea hydrogen peroxide [38] have been used as oxidant for oxidation of sulfides and oxidative coupling of thioles previously. Concerning the green oxidant, H2O2 (with only H2O as a byproduct) in particular have received attention because of their environmental implications, readily available, high atom efficiency and lower costs than when used other oxidizing agents [39].
Owing to the inherent advantages of recovery, in the context of green chemistry and reuse, herein Cu-based heterogeneous catalyst have been reported for oxidative coupling of thiols into corresponding disulfides and also oxidation of sulfides to sulfoxides under mild condition.
2 Experimental
2.1 Materials
Chemicals and solvents used in this work were obtained from Sigma-Aldrich, Fluka or Merck chemical companies and used without further purification.
2.2 Preparation of MCM-41
Mesoporous MCM-41 was prepared according to the described procedure. Deionized water was added to 2 M NaOH and cetyltrimethylammoniumbromide (CTAB) as a surfactant template and stirred intense at 80 °C, after clarification tetraethylorthosilicate (TEOS) was added slowly and continuously. After 2 h stirring, the synthetic solution obtained with molar composition TEOS/CTAB/NaOH/H2O:60/3.0/1.0/1. After cooling to room temperature, the resulting solid was gathered by filtration, washed with deionized water, and dried at 343 K. Then followed by calcination at 823 K for 5 h with rate of 2 °C/min to remove the residual surfactant.
2.3 Preparation of catalyst
1.0 g of 3-aminopropyltriethoxysilane (APTES) was added to a suspension of MCM-41 (1 g) in n-hexane (30 mL), and allowed to reflux for 24 h under N2 atmosphere. Then, the reaction mixture was cooled down to room temperature, filtered and the resulting solid washed with n-hexane. The solid was dried under vacuum to get white solid (MCM-41 functionalized aminopropyl groups). Then, for the Preparation of MCM-41 functionalized isatin-Schiff base (Schiff base@MCM-41), the above mentioned solid (1 g) was refluxed with 0.147 g (1 mmol) of isatin and acetic acid in ethanol for 5 h under N2 atmosphere. The resulting solid (Schiff base@MCM-41) was filtered, washed with ethanol and dried in vacuum. Finally, for Preparation of Cu-complex@MCM-41, the Schiff base@MCM-41 (1.0 g) was mixed with 3.0 mmol of Cu(NO3)2·3H2O in 10 mL of ethanol. The mixture was stirred at under reflux condition for 10 h. The solid product was obtained by filtration, washed with ethanol and dried at 60 °C.
2.4 General procedure for the oxidation of sulfides to sulfoxides
A mixture of sulfide (1 mmol), hydrogen peroxide (1.2 mmol) and Cu-complex@MCM-41 (0.04 g) was stirred at 35 °C in ethanol (3 mL) and the progress of the reaction was monitored by TLC. After completion of the reaction, catalyst was separated by simple filtration and washed with dichloromethane, and next, the product was extracted with CH2Cl2 (5 mL × 4). The organic layer was dried over anhydrous Na2SO4. Finally, the organic solvents were evaporated, and products were obtained in good to high yield.
2.5 General procedure for the oxidative coupling of thiols into disulfides
Cu-complex@MCM-41 (0.02 g) was added to a mixture of thiol (1 mmol) and hydrogen peroxide (1.2 mmol) at 35 °C in ethanol (3 mL). Then the mixture was stirred for the appropriate time. The progress was monitored by TLC. After completion of the reaction, the catalyst was separated by simple filtration. The product was extracted with CH2Cl2 (5 mL × 4). The organic layer was dried over anhydrous Na2SO4. Finally, the organic solvents were evaporated, and products were obtained in good to high yield.
3 Results and discussion
3.1 Catalyst preparation
In continuation of our studies on the application of immobilized metal complex on MCM-41 in organic reactions [5], herein, we report the preparation and characterization of Schiff base complex of copper-functionalized on MCM-41 (Cu-complex@MCM-41). Also, we examined the catalytic activity of this catalyst for some organic oxidation reactions such as (1) oxidation of sulfides into sulfoxides, and (2) oxidative coupling of thiols into their corresponding disulfides using H2O2 as oxidant.
The Cu-complex@MCM-41 was prepared by the concise route outlined in Scheme 1. Initially, the MCM-41 mesoporous has been prepared using cetyltrimethylammoniumbromide (CTAB) as structure-directing agent and tetraethylorthosilicate (TEOS) as a source of silicon in NaOH solution according to the methods reported recently [5, 11], and subsequently were functionalized with 3-aminopropyltriethoxysilane (APTES). The immobilized salen on MCM-41 was performed via condensation of isatin and supported-aminopropyl groups. Ultimately, the Cu-complex@MCM-41 were prepared using reaction of Schiff base@MCM-41 with Cu(NO3)2·3H2O.
3.2 Catalyst characterization
Cu-complex@MCM-41 have been characterized by Fourier transform infrared spectroscopy (FT-IR), powder X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), thermogravimetric analysis (TGA), N2 adsorption–desorption isotherms, and by their comparisons with that of authentic samples [5, 11, 12, 40].
3.2.1 Scanning electron microscopy and energy-dispersive X-ray spectroscopy
The morphological and size of the catalyst was evaluated using scanning electron microscopy. The SEM image of the Cu-complex@MCM-41 was shown in Fig. 1. As shown in Fig. 1a, the catalyst was formed of nanometer-sized particles (20–50 nm). SEM images of the Cu-complex@MCM-41 shown a spherical particles with a homogeneous size about 35 nm in diameter. To investigate the catalyst characterization of the supported copper species, the prepared catalyst was analyzed by EDS. As shown in Fig. 1b, EDS spectrum of catalyst showed the presence of C, N, O, Si, and Cu species in the catalyst. Also the mass percent of C, N, O, Si and Cu is 42.75, 4.99, 31.94, 16.12 and 4.20 respectively.
3.2.2 Thermo gravimetric analysis (TGA)
The TGA was used to determine the percentage of chemisorbed organic functional groups onto the MCM-41 mesoporous. The TGA curve of MCM-41, MCM-41-NH2, Schiff base@MCM-41 and Cu-complex@MCM-41 shows the mass loss of the organic functional group as it decomposes upon heating (Fig. 2). The weight loss at temperatures below 200 °C is due to the removal of physically adsorbed solvent and moisture inside the MCM-41 channels [11]. The thermal gravimetric analysis of MCM-41-NH2 and Schiff base@MCM-41 showed a weight loss above 250 °C can be assigned mainly due to decomposition of (3-aminopropyl)triethoxysilane groups and Schiff base ligands grafting to the MCM-41. The three steps of weight losses at 250, 350 and 400 °C in TGA curve of catalyst may be associated to the removal of physically adsorbed solvent and water, the physisorbed 3-(aminopropyl)triethoxysilane groups and the well grafting of organic groups including copper complex into MCM-41 channels, respectively. Based on these results, the well grafting of copper complexe into MCM-41 channels is verified.
Thermal stability of the catalyst was also determined, since synthesis of many organic compound were usually carried out at high temperature. As shown in Fig. 2, Cu-complex@MCM-41 catalyst was stable even at 200 °C.
3.2.3 Powder X-ray diffraction
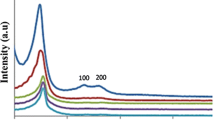
The small angle powder X-ray diffraction patterns for MCM-41, MCM-41-NH2, Schiff base@MCM-41 and Cu-complex@MCM-41 are shown in Fig. 3, which shows the presence of three peaks at 2θ ≈ 2.4°, 2θ ≈ 4.5° and 2θ ≈ 5.5° and can be assigned as (1 0 0), (1 1 0) and (2 0 0), respectively. These reflection patterns are the characteristic of long-range ordered mesoporous material. The highest peak observed for all samples (corresponding to 1 0 0 plane) indicates the order of the mesopores, as expected in MCM-41 type ordered mesoporous structure [5]. The MCM-41 sample shows (1 0 0), (1 1 0) and (2 0 0) reflections, characteristic of hexagonal channel arrays [12]. Both the diffraction peaks of (1 1 0) and (2 0 0) planes became weaker and nearly disappeared when copper complex was supported into MCM-41. This indicates that the regularity of pore structure was decreased [11]. No diffraction bands were observed at angles higher than 2θ ≈ 6°, thus demonstrating the amorphous nature of the samples.
A unit cell parameter (a0), of 44.86 Å was calculated using the following equation:
3.2.4 Fourier transform infrared spectroscopy
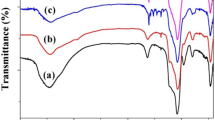
The FT-IR spectrum of catalyst shows several peaks that are characteristic of a functionalized Schiff base complex, which clearly differs from that of the unfunctionalized MCM-41 and MCM-41-NH2 nanostructure (Fig. 4). The FT-IR spectrum for all samples shows three peaks at 460, 799 and 1084 cm−1 corresponding to the symmetric and asymmetric Si–O–Si vibrations [5]. And also, the stretching vibration at 3430–3440 cm−1 which incorporates the contributions from both symmetrical and asymmetrical modes of the silanol (O–H) bonds which are attached to the MCM-41 framework. In the FT-IR spectra of MCM-41-NH2, The existence of the grafted n-propylamine group is confirmed by C–H stretching vibrations that appear at 2980–2850 cm−1 and also N–H stretching vibration modes as a broad band that appear at 3430 cm−1 [40]. Appearance of new peak at 1720 cm−1 in FTIR spectra for Schiff base@MCM-41, which may be related to the carbonyl bonds confirm the formation of Schiff base@MCM-41. Also, the bands located at around 1634 cm−1 are assigned to the C=N stretching vibrations of the Schiff base@MCM-41 which indicating that isatin has been successfully condensed with terminal amine in MCM-41-NH2. Bat in the FT-IR of catalyst, this band was shifted 12 cm−1 to a lower wavenumber which indicating the complex of copper has been successfully prepared. The FT-IR spectra of catalyst show important bands from the Cu-complex@MCM-41. These are strong evidences that Cu-complex were bonded to the surface MCM-41 mesoporous.
3.2.5 N2 adsorption–desorption isotherm
The N2 adsorption–desorption isotherms of MCM-41, MCM-41-NH2, Schiff base@MCM-41 and Cu-complex@MCM-41 samples are illustrated in Fig. 5. Based on the IUPAC classification, these materials display a typical type IV isotherm, which are the characteristics of mesoporous material [41].
The Brunauer–Emmett–Teller (BET) surface area for MCM-41, MCM-41-NH2, Schiff base@MCM-41 and Cu-complex@MCM-41 is 917, 512, 402 and 379 m2g−1, respectively, which is close to that of MCM-41 synthesized in the same way. The BET large pore volume for MCM-41, MCM-41-NH2, Schiff base@MCM-41 and Cu-complex@MCM-41 is 2.04, 1.30, 1.08 and 0.79 cm3g−1, respectively. The pore volume of Schiff base@MCM-41 and Cu-complex@MCM-41 are lower than that of MCM-41; this could be owing to the organic ligands and Cu atoms inserted into the structure.
When the isatin and Cu loaded on MCM-41, the BET specific surface areas decreased from 917 to 402 and 379 m2/g, respectively. Meanwhile, the average pore volume decreased to 1.08 and 0.79 cm3/g, respectively. The decrease in pore volume and surface area of modified MCM-41 was attributed to the immobilization of organic layers and Cu- complex onto the mesoporous wall.
As shown in Fig. 5 the BET surface and pore volume of functionalized MCM-41 decreased with the increasing substances anchored on the surface of MCM-41. These results are attributed to the occupation of organic molecules on the inner surface of the pores.
The wall thickness of MCM-41 was 2.05 nm where calculated by the following equation:
3.3 Catalytic activity of Cu-complex@MCM-41
In order to examine the catalytic activity of prepared immobilized copper Schiff base complex onto MCM-41, oxidation of sulfides and oxidative coupling of thiols were investigated.
3.3.1 Evaluation of the catalytic activity of Cu-complex@MCM-41 through the oxidation of sulfides into sulfoxides
As a first part of our organic program, herein we examined the catalytic activity of Cu-complex@MCM-41 for the oxidation of sulfides into corresponding sulfoxides. Oxidation of sulfides into sulfoxides using H2O2 in the present of this catalysts was shown in Scheme 2.
For optimization of the reaction conditions, we examined the oxidation of dibenzylsulfide using hydrogen peroxide as a model compound in the influence of different amounts of catalyst (Table 1). To choose the reaction media, different solvents such as acetone, ethylacetate, ethanol, H2O, chloroform, acetonitrile, and as well as solvent-free condition were used (Table 1, entries 4–10) and the best results were obtained in ethanol using 0.04 gr of Cu-complex@MCM-41 (Table 1, entry 1). Also, the effect of temperature were described (Table 1, entries 11, 12) and the best results were obtained at 35 °C. As shown in Table 1, dibenzylsulfide (1 mmol) in the presence of catalytic amount of Cu-complex@MCM-41 (0.04 g) in ethanol at 35 °C was found to be ideal the reaction conditions for the formation of dibenzylsulfoxide.
The generality of this approach has been demonstrated by a facile oxidation of various sulfides as shown in Table 2. The sulfoxides were obtained in high yields. As shown, a variety of sulfides bearing dialkyl sulfides (Table 2, entries 1–3), alkylaryl sulfides (Table 2, entries 4–7), cyclic sulfides (Table 2, entries 8) and other sulfide with different functional group (Table 2, entries 9–11) were successfully employed to prepare the corresponding sulfoxides in excellent yields. Therefore, the results revealed that this methodology is effective for a wide range of sulfides.
3.3.2 Evaluation of the catalytic activity of Cu-complex@MCM-41 through the oxidative coupling of thiols into disulfides
As a second part of our organic program towards the development of new methods for the coupling of thiols, herein we tested the catalytic activity of Cu-complex@MCM-41 in the oxidative coupling of thiols into corresponding disulfides. Oxidative coupling of thiols into disulfides using hydrogen peroxide in the present of this catalyst in ethanol was shown in Scheme 3.
The generality of this approach has been demonstrated by a facile oxidative coupling of various thiols as shown in Table 3. As shown, a variety of thiols bearing aromatic thiols (Table 3, entries 1–7), aliphatic thiols (Table 3, entries 8 and 9), and other thiols with different functional group were successfully employed to prepare the corresponding disulfides in excellent yields. These oxidizing systems allowed the chemoselective oxidation of 2-(phenylthio)ethanol and 2-mercaptoethanol to the corresponding sulfoxide and disulfide (Table 2, entry 9 and Table 3, entry 9). Interestingly primary hydroxyl groups in these substrates remained intact during the oxidation reactions (Scheme 4). This result indicated that the present protocol could be applicable to the chemoselective coupling of thiols and oxidation of sulfides in the presence of other functional groups. Therefore, the results revealed that this methodology is effective for a wide range of thiols.
Because these heterogeneous oxidation systems was described in mild condition, there is no overoxidation to sulfone (for oxidation of sulfides) or thiosulfinates, disulfoxides, sulfinyl sulfones or disulfones (for the oxidative coupling of thiols) was not observed (Scheme 5).
Various systems for the oxidation of methylphenylsulfide (I) and thiophenol (II), was shown in Table 4. It is obvious that Cu-complex@MCM-41/H2O2 showed good reaction time and yield, mild reaction condition and simple work-up. Moreover the most important point that should be noted is the recycling and reusability of catalyst that our work has only discussed.
3.4 Recyclability of the Cu-complex@MCM-41
The recyclability of catalyst was examined using the model reaction under identical conditions. To investigate this issue, oxidation of methylphenyl sulfide and 4-methyl thiophenol using H2O2 in the influence of Cu-complex@MCM-41 was carried out successfully and the average isolated yield for several successive runs was obtained in 96 and 95 %, respectively. After the required time, the catalyst was recovered from the reaction mixture by simple filtration, washed with ethyl acetate, and subsequently dried and reused without any significant deactivation for the oxidation of sulfides even after eight runs. After six consecutive reuses in coupling oxidative of thiols, the catalyst exhibited almost identical catalytic activity (Fig. 6). This reusability demonstrates the high stability and turnover of the catalyst under the employed conditions.
4 Conclusions
In conclusion, an efficient and environmentally friendly procedure has been developed for the oxidation of sulfides to sulfoxides and oxidative coupling of thiols into their corresponding disulfides in the presence of catalytic amounts of Cu-complex@MCM-41. This new methodology offers several advantages including excellent yields, operational simplicity, mild reaction conditions, short reaction time, simple work-up procedure and use of non-toxic catalyst. Furthermore, the catalyst could be isolated with simple filtration and the average yields achieved above 96 % after reused at eight cycles.
References
Y. Zhu, L.P. Stubbs, F. Ho, R. Liu, C.P. Ship, J.A. Maguire, N.S. Hosmane, Chemcatchem 2, 365 (2010)
A. Ghorbani-Choghamarani, M. Norouzi, J. Mol. Catal. A Chem. 395, 172 (2014)
C.W. Lim, I.S. Lee, Nano Today 5, 412 (2010)
S. Shylesh, V. Schunemann, W.R. Thiel, Angew. Chem. Int. Ed. 49, 3428 (2010)
M. Hajjami, F. Ghorbani, F. Bakhti, Appl. Catal. A Gen. 470, 303 (2014)
A. Benhamou, J.P. Basly, M. Baudu, Z. Derriche, R. Hamacha, J. Colloid Interface Sci. 404, 135 (2013)
S.A. Idris, S.R. Harvey, L.T. Gibson, J. Hazard. Mater. 193, 171 (2011)
S. Yan, L. Xiu-Wu, S. Wei, Z. Yaping, Z. Li, Appl. Surf. Sci. 253, 5650 (2007)
G. Maria, A.I. Stoica, I. Luta, D. Stirbet, G.L. Radu, Microporous Mesoporous Mater. 162, 80 (2012)
H. Yu, H. Zhang, W. Yang, J. Feng, W. Fan, S. Song, Microporous Mesoporous Mater. 170, 113 (2013)
M. Nikoorazm, A. Ghorbani-Choghamarani, F. Ghorbani, H. Mahdavi, Z. Karamshahi, J. Porous Mater. 22, 261 (2015)
M. Abdollahi-Alibeik, M. Pouriayevali, Catal. Commun. 22, 13 (2012)
Y. Huang, W. Hao, G. Ding, M.Z. Cai, J. Organomet. Chem. 715, 141 (2012)
A.R. Silva, K. Wilson, A.C. Whitwood, J.H. Clark, C. Freire, Eur. J. Inorg. Chem. 2006, 1275 (2006)
A. Corma, H. Garcia, A. Moussaif, M.J. Sabater, R. Zniber, A. Redouane, Chem. Commun. (10), 1058 (2002)
A. Fodor, Z. Hell, L. Pirault-Roy, Appl. Catal. A Gen. 484, 39 (2014)
I. Jlalia, F. Gallier, N. Brodie-linder, J. Uziel, J. Auge, N. Lubin-Germain, J. Mol. Catal. A Chem. 393, 56 (2014)
X.B. Li, Z.J. Li, Y.J. Gao, Q.Y. Meng, S. Yu, R.G. Weiss, G.H. Tung, L.Z. Wu, Angew. Chem. Int. Ed. 53, 2085 (2014)
A. Ghorbani-Choghamarani, G. Azadi, B. Tahmasbi, M. Hadizadeh-Hafshejani, Z. Abdi, Phosphorus Sulfur Silicon Relat. Elem. 189, 433 (2014)
H.B. Jeon, K.T. Kim, S.H. Kim, Tetrahedron Lett. 55, 3905 (2014)
A. Shaabani, A.H. Rezayan, Catal. Commun. 8, 1112 (2007)
B. Sreedhar, P. Radhika, B. Neelima, N. Hebalkar, A.K. Mishra, Catal. Commun. 10, 39 (2008)
G.P. Romanelli, P.I. Villabrille, C.V. Cáceres, P.G. Vázquez, P. Tundo, Catal. Commun. 12, 726 (2011)
L. Villalobos, T. Ren, Inorg. Chem. Commun. 28, 52 (2013)
B.V. Tamhankar, Int. J. Res. Org. Chem. 4, 4 (2014)
S. Thurow, V.A. Pereira, D.M. Martinez, D. Alves, G. Perin, R.G. Jacob, E.J. Lenardão, Tetrahedron Lett. 52, 640 (2011)
Y. Liu, H. Wang, C. Wang, J.P. Wan, C. Wen, RSC Adv. 3, 21369 (2013)
H. Firouzabadi, N. Iranpoor, M. Abbasi, Tetrahedron Lett. 51, 508 (2010)
M. Nikoorazm, A. Ghorbani-Choghamarani, H. Mahdavi, S.M. Esmaeili, Microporous Mesoporous Mater. 211, 174 (2015)
A. Ghorbani-Choghamarani, B. Ghasemi, Z. Safari, G. Azadi, Catal. Commun. 60, 70 (2015)
J. Feng, M.-F. Lv, G.-P. Lu, C. Cai, Org. Biomol. Chem. 13, 677 (2015)
N. Taniguchi, Eur. J. Org. Chem. 2014, 5691 (2014)
A. Ghorbani-Choghamarani, Z. Darvishnejad, M. Norouzi, Appl. Organomet. Chem. 29, 170 (2015)
H. Firouzabadi, N. Iranpoor, M.A. Zolfigol, Synth. Commun. 28, 1179 (1998)
H. Firouzabadi, N. Iranpoor, M.A. Zolfigol, Synth. Commun. 28, 377 (1998)
N. Iranpoor, H. Firouzabadi, M.A. Zolfigol, Synth. Commun. 28, 367 (1998)
G. Chehardoli, M.A. Zolfigol, Phosphorus Sulfur Silicon Relat. Elem. 185, 193 (2010)
A. Hasaninejad, M.A. Zolfigol, G. Chehardoli, M. Mokhlesi, J. Serb. Chem. Soc. 75, 307 (2010)
S. Rayati, F. Nejabat, S. Zakavi, Inorg. Chem. Commun. 40, 82 (2014)
S. Das, S. Bhunia, T. Maity, S. Koner, J. Mol. Catal. A Chem. 394, 188 (2014)
A. Mathew, S. Parambadath, S.S. Park, C.S. Ha, Microporous Mesoporous Mater. 200, 124 (2014)
Acknowledgments
This work was supported by the research facilities of Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajjami, M., Rahmani, S. Schiff base complex of Cu immobilized on MCM-41 as reusable catalyst for efficient oxidative coupling of thiols and oxidation of sulfides using hydrogen peroxide. J Porous Mater 22, 1265–1274 (2015). https://doi.org/10.1007/s10934-015-0004-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0004-z