Abstract
Sedimentary postabdominal claws, among other remains, have been used successfully in paleolimnological studies to reconstruct past environmental conditions and the distribution pattern of certain Daphnia species. However, morphological analysis of postabdominal claws has not proven adequate for the clear taxonomic differentiation among species within a complex, such as the Daphnia pulex complex. The presence of the invasive North American (NA) D. pulex lineage was recently detected in an alpine lake in the Sierra Nevada mountain range (Southeast Spain). This lineage has spread throughout Africa and the Mediterranean basin, suggesting a trend towards its increased presence in Europe. The aim of this study was to examine whether this invasive lineage could be differentiated from the native European (Eu) Daphnia pulicaria lineage based on morphological differences in postabdominal claws recovered from three alpine lakes in the Sierra Nevada. The most useful differential variables were the postabdominal claw length (PCL), ratio of distal comb length to PCL (Cldist/PCL), and number of stout spines. Thus, NA D. pulex may be identifiable by a short PCL, low Cldist/PCL value and the presence of < 5 stout spines. Because of a wide variability in PCL within the Eu D. pulicaria species and an overlap in stout spine number between the species, morphological analysis results cannot unequivocally differentiate these lineages. However, they make a useful contribution to recognition of the possible presence of this invasive lineage. The present findings assist identification of the invasive NA D. pulex lineage in potentially affected regions, facilitating reconstruction of its historic dispersion and colonization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daphnia is a keystone species in many freshwater ecosystems due to its intermediate trophic position, providing a key link in the energy flow between primary producers and secondary consumers in the pelagic habitat (Persson et al. 2007). Nevertheless, Daphnia species differ considerably in size, filtration rate, and vulnerability to predators, among other features (Gliwicz 1990; Rudstam et al. 1993; Walsh et al. 2016). The identification of Daphnia species is therefore important to establish its functional role in the plankton community. Daphnia is a relatively easily dispersed taxon (Havel et al. 2000; Van Damme 2016), and species identification is crucial for understanding the dispersion and colonization of Daphnia species over time (Hairston et al. 1999; Brede et al. 2009). However, the identification of Daphnia species is challenging because of morphological changes triggered by environment factors, cyclomorphosis, and/or hybridization (Alonso 1996), which hamper the attribution of local phenotypes to distinct species (Hebert and Finston 1996). The taxonomic challenges posed by the Daphnia genus have led to the use of genetic techniques (Hebert and Finston 1997) for differentiation within species complexes such the D. pulex complex, in which morphology-based species identification is notoriously difficult to do (Vergilino et al. 2011; Crease et al. 2012). This complex is composed of at least 12 lineages that belong to three major groups (1 in the pulex group, 9 in the pulicaria group and 2 in the tenebrosa group) (Crease et al. 2012), and despite of the molecular divergence in D. pulex lineages, the lack of morphological innovation has hampered taxonomic labor. Furthermore, resolution of taxonomic boundaries in D. pulex complex has been arduous due to hybridization, introgression, asexuality and polyploidy (Colbourne et al. 1998).
In paleolimnological studies, subfossil cladocera remains have been used to examine changes in species composition as indicators of past environmental conditions (Labaj et al. 2017; Jiménez et al. 2018). Thus, Daphnia remains have been used as bioindicators of phosphorus, organic carbon, pH, and calcium levels, among others (Paterson 1994; Jeppesen et al. 2001; Korosi et al. 2008; Shapiera et al. 2012). Morphological analyses of subfossil Daphnia remains also have allowed the reconstruction of distribution patterns of certain Daphnia species (Korhola 1999).
Subfossil Daphnia remains are usually identified by their postabdominal claws (Korhola and Rautio 2001; Szeroczyńska and Zawisza 2005), the morphology of which permits differentiation among species complexes. For example, the presence/absence of stout spines in the middle comb has been used to distinguish satisfactorily between D. longispina (without stout spines) and D. pulex (with stout spines) complexes (Szeroczyńska and Sarmaja-Korjonen 2007; Korosi et al. 2008; Szeroczyńska and Zawisza 2005). Nevertheless, morphological discrimination among species within species complexes such as D. pulex complex becomes arduous due to the presence of hybrids, introgression, asexuality, polyploidy and the clonal variation in morphology (Colbourne et al. 1998; Hebert and Finston 2001). Korosi et al. (2011) have been the only authors to attempt differentiation among species within Daphnia species complexes (D. pulex and D. longispina species complexes) based on postabdominal claw morphology; however, they concluded that the taxonomic differences in postabdominal claws were insufficient to unambiguously differentiate among species within each complex.
Difficulties in species identification based solely on the morphology of subfossil remains have led to the use of subfossil ephippia in paleogenetic analyses for reconstruction of the historical dynamics of invasive Daphnia species (Ortells et al. 2014; Möst et al. 2015; Van Damme 2016). Thus, it proved possible to reconstruct the invasion history of the asexual clone of North American (NA) D. pulex (hybrid of American D. pulex with American D. pulicaria) that arrived at Naivasha Lake (Kenya) in 1927–1929 and displaced the native D. pulex within around 60 years. The asexual clone spread more than 5500 km from the lake into a wide range of habitats between Ethiopia and South Africa. This lineage also spread to several points in the Mediterranean basin, being detected in Eastern Spain (Amadorio reservoir) (Mergeay et al. 2006), Sardinia (Sos Canales reservoir) (Fadda et al. 2011), and Northern Italy (Avigliana Lake) (Vergilino et al. 2011; Crease et al. 2012; Marková et al. 2013).
Paleogenetic analyses of resting eggs revealed that the NA D. pulex arrived in one lake in the Sierra Nevada mountain range in Southern Spain around 60 years ago (Jiménez et al. 2018). This species has not been detected in other lakes in this mountain range, most of which have contained the native European (Eu) D. pulicaria for around 180 years (Jiménez et al. 2018). The broad ecological tolerance described for NA D. pulex (Kurek et al. 2011; Mergeay et al. 2006) could result in the displacement of and/or hybridization with the native species. Although the clone that invaded Africa has been described as an asexual clone, Duggan et al. (2012) reported the presence of males of NA. D. pulex in laboratory cultures, as also observed by our group, suggesting that this species could reproduce by sexual reproduction. Therefore, hybridization with native species is a future possibility, and it is essential to monitor the distribution, dispersion and population dynamics of the exotic NA D. pulex to adequately managing invaded ecosystems and/or regions. One approach to species differentiation is through the analysis of subfossil remains in paleolimnological studies, yielding data on the possible dispersion of NA D. pulex to other lakes over time and on its invasion dynamics. Biomonitoring of invasive species is essential to counter the threat that they pose to ecosystems (Tilman et al. 2017), including freshwater ecosystems (Strayer 2010).
The objective of this study was to develop a reliable taxonomic tool for the differentiation of Eu D. pulicaria and NA D. pulex lineages in Sierra Nevada lakes, based on the morphological characters of postabdominal claws recovered from sediments throughout the twentieth century in three alpine lakes. Although Korosi et al. (2011) were not able to differentiate unambiguously between species from D. pulex complex based on the morphology of postabdominal claws, the possibility of finding clear morphological differences in claws from species different from those analyzed by Korosi et al. (2011) and the slight differences demonstrated by Eu D. pulicaria and NA D. pulex in rostral cuticular region, maximum body size and type of neckteeth formation (Petrusek et al. 2005; Juračka et al. 2011) encouraged us to analyze the morphology of postabdominal claws to distinguish between these species. As other paleolimnological studies have proven successful in determining the historical occurrence of certain species (Lavery et al. 2014), a high taxonomic resolution based on subfossil remains would facilitate the biomonitoring of NA D. pulex across the Mediterranean basin, where it appears to have spread (Mergeay et al. 2006; Vergilino et al. 2011; Marková et al. 2013), allowing reconstruction of its historic dispersion and colonization. The presence of this species poses a threat to Daphnia populations elsewhere in Europe (Fadda et al. 2011), where the results obtained in our study may also be applicable.
Study area
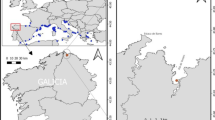
In the Sierra Nevada mountains of SE Spain (36°55′–37°15′N, 2°31′–3°40′W), there are ~ 50 small lakes of glacial origin at an elevation of ~ 2800–3100 m asl (Fig. 1; Castillo-Martín 2009). Sierra Nevada lakes are situated above the tree line, and the geologic substrate of catchment basins is metamorphosed siliceous rocks. The lakes are typically shallow and small and usually remain ice-covered from November through June. These lakes do not thermally stratify during the summer and are fishless, with clear, well-mixed water and low primary production (Table 1).
a Map of the Iberian Peninsula with inset showing the location of the study area. b Map of the Sierra Nevada mountain range showing the location of the three study lakes. Circles: Cuadrada (CD); Río Seco (RS); Borreguil (BG). Boundaries of the Natural and National Park are indicated by white and black continuous lines, respectively
Methods
Field measurements
Three lakes, Borreguil, Cuadrada and Río Seco were selected for analysis of the morphological characteristics of Daphnia postabdominal claws (Fig. 1, Table 1). Their selection was based on data available on subfossil cladoceran assemblages from high-resolution paleolimnological sedimentary records (Jiménez et al. 2018) and on a phylogenetic tree and network analyses of Daphnia pulex group using the mitochondrial ND5 sequence of ephippia and individuals of Sierra Nevada lakes (Veiga 2014). From these analyses we concluded the native Eu D. pulicaria, recorded for at least ~ 200 years, is the only Daphnia species that inhabits both Cuadrada and Río Seco, while the exotic NA D. pulex, recorded for the past ~ 50 years, is the only Daphnia species that inhabits Borreguil. Sediment cores were collected from the deepest area of the three lakes during the summer of 2008 (Río Seco) and 2011 (Borreguil and Cuadrada), using a slide-hammer gravity corer (Aquatic Research Instruments, Hope, Idaho, USA) with inner core-tube diameter of 6.8 cm. Sedimentary cores from Borreguil and Cuadrada were extruded on-site at 0.25-cm intervals for the upper 5-10 cm and then at 0.5-cm intervals to the base of the core. The sediment core retrieved from Río Seco was sectioned at 0.5-cm intervals throughout. All cores were immediately sealed in sterile Whirlpak® bags, wrapped in a dark bag, and placed in a cooler before transport to the University of Granada (Spain), where they were stored at ~ 4 °C until analysis. Tube samplers (6.7-cm diameter) of different lengths were used to collect an integrated sample of the whole water column from the deepest point of each lake and were analyzed for a suite of physico-chemical variables (Table 1) following the techniques detailed in Barea-Arco et al. (2001) and Morales-Baquero et al. (2006). Specific conductivity and pH were measured on site with a Waterproof PC 300 m.
Chronology
Sediment cores were dated using gamma spectroscopy to measure radio isotope activities and establish a chronology for the past ~ 150 years as reported by Jiménez et al. (2015, 2018). Sedimentary intervals for each core were examined for 210Pb activity following the techniques outlined in Schelske et al. (1994). Chronologies were estimated using the constant rate of supply (CRS) model (Appleby and Oldfield 1978) and were corroborated by using the 137Cs chronological marker (Appleby 2001).
Laboratory methods
Subfossil cladoceran remains from different sedimentary intervals were analyzed and treated using the methods described by Szeroczyńska and Sarmaja-Korjonen (2007) (Table 2). The low relative abundance of Daphnia in our lakes hamper us from obtaining a representative number of individuals in every interval. Therefore, remains from different intervals were grouped together to obtain representative samples from the first and second half of the twentieth century. Thus, 5 cm3 samples of fresh sediment from “modern” (second half of the twentieth century) and “old” (first half of twentieth century) core intervals in the three study lakes were heated for 20 min in 10% potassium hydroxide (KOH) to deflocculate the sediment and dissolve humic acids (Table 2; modern sediment samples: 0–2 cm for Borreguil and Río Seco and 0–4 cm for Cuadrada; and old sediment intervals: 6–9 cm for Río Seco and 7–8 cm for Cuadrada). No Daphnia remains exist in old samples from Borreguil because Daphnia was absent from this lake until the 1950s (Jiménez et al. 2018). We concentrate our analysis effort on the 2000–2010 decade because Daphnia presence was continuous and with a slightly higher relative abundance in Borreguil lake since then. Samples were then washed and sieved through a 38 µm-mesh size with tap water, transferring retained residues to a beaker for concentration of the subfossil cladocera remains. Next, these remains were dyed with safranin, facilitating their examination. Postabdominal claws were mounted on microscope slides using safranin-dyed glycerol gelatin for their preservation. Postabdominal claws were analyzed at 400 × magnification under a light microscope (Leica DM 2500) using LAS Image Analysis software.
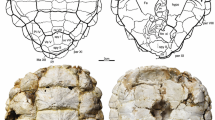
Following previous paleolimnological investigations (Korosi et al. 2011), the following postabdominal claw measurements were recorded: postabdominal claw length (PCL), postabdominal claw width (PCW), proximal comb length (Clprox), middle comb length (Clmid), distal comb length (Cldist), proximal spinule length (Slprox), distal spinule length (Sldist), stout spines length (SSL), and the number of stout spines (Fig. 2). Furthermore, the ratio between the distal comb length and postabdominal claw length was measured (Cldist/PCL). Like other similar studies (Brahney et al. 2010; Labaj et al. 2017), a minimum of 40 Daphnia claws were measured per lake. It was not possible to record all measurements in some incompletely preserved claws.
Photomicrograph of NA D. pulex claw showing the different measurements performed on postabdominal claws of both NA D. pulex and Eu D. pulicaria species. aPCL, postabdominal claw length; PCW, postabdominal claw width; Clprox, proximal comb length; Clmid, middle comb length; Cldist, distal comb length. bSlprox, proximal spinal length; SSL1–SSL5, Stout spine length 1–5; Sldist, distal spinule length
Statistical analyses
The Student’s t test was used to analyze morphological differences between modern claws from NA D. pulex and Eu D. pulicaria and to determine morphological differences between Eu D. pulicaria remains from the first and second half of the twentieth century in a single lake. A power analysis was performed to evaluate the reliability of the t-test results because of the relatively low number of individuals sampled (Table 2). One-way ANOVA was carried out to evaluate morphological differences in twentieth century claws among the three study lakes, followed by the post hoc Tukey Honestly Significant Difference (HSD) test. The non-parametric Mann–Whitney U test and Kruskal–Wallis ANOVA test followed by the Mann–Whitney post hoc test were used for analyses of the number of stout spines because it was the only analyzed variable showing a non-normal distribution (Shapiro–Wilk’s test, P < 0.05) and heteroscedasticity (Levene’s test, P < 0.05).
Following Korosi et al. (2011), non-parametric classification and regression tree (CART) analyses were conducted to assess the usefulness of morphological characters to differentiate between NA D. pulex and Eu D. pulicaria claws and to distinguish between Eu D. pulicaria claws from Río Seco and Cuadrada lakes, using the rpart package (Therneau et al. 2015) from R software (R Development Core Team 2016). Classification trees are constructed by repeatedly splitting the data, which each split is defined by a single rule on a single explanatory variable. CART model resulting in a decision tree which terminal nodes provide an explicit probability of class membership (De’ath and Fabricius 2000; Lindbladh et al. 2002). Misclassifying rates of classification trees generated were estimated through the cross-validation process.
Results
D. pulex–D. pulicaria
Eu D. pulicaria claws from Río Seco and Cuadrada were compared with NA D. pulex claws from Borreguil, analyzing claws from the second half of twentieth century. The PCL was significantly longer in Eu D. pulicaria than in NA D. pulex (t-test, P < 0.01) (Fig. 3), and the ratio between Cldist and PCL (Cldist/PCL) was significant greater (t test, P < 0.05) in NA D. pulex claws. Although the overall claw length was greater in Eu D. pulicaria, only marginally significant between-species differences (t test, P = 0.06) were observed in Cldist (Table 3). Table 3 also reports the significant difference (Mann–Whitney U test, P < 0.001) found in the number of stout spines on claws between Eu D. pulicaria (4–7) and NA D. pulex (4–5).
Boxplots comparing postabdominal claw length (PCL) between modern claws (i.e. from 2nd half of twentieth century) of NAD. pulex recovered from Borreguil and modern claws of Eu D. pulicaria recovered from both Río Seco and Cuadrada (t-test, P < 0.01). Boxes represent the interquartile range; whiskers represent minimum and maximum observations
CART analysis separated NA D. pulex claws from Eu D. pulicaria claws using PCL and Cldist/PCL values. Based on Cldist/PCL ratios, the base tree separated 39 claws of NA D. pulex from 34 claws of Eu D. pulicaria, with this ratio being < 0.68 in 6 claws of NA D. pulex and 19 of Eu D. pulicaria and ≥ 0.68 in 33 claws of NA D. pulex and 15 of Eu D. pulicaria. Claws with Cldist/PCL < 0.68 were further subdivided, finding Cldist/PCL ≥ 0.65 in 15 claws of Eu D. pulicaria and 1 of NA D. pulex and Cldist/PCL < 0.65 in 4 claws of Eu D. pulicaria and 5 of NA D. pulex. Claws with Cldist/PCL > 0.68 were subdivided, finding a PCL < 234.2 µm in 4 claws of Eu D. pulicaria and 20 of NA D. pulex and a PCL > 234.2 µm in 13 claws of NA D. pulex and 11 of Eu D. pulicaria. Claws with PCL ≥ 234.2 µm were subdivided by Cldist/PCL ratio, observing Cldist/PCL ≥ 0.70 in 10 claws of Eu D. pulicaria and 7 of NA D. pulex and Cldist/PCL < 0.70 in 1 claw of Eu D. pulicaria and 6 of NA D. pulex (Fig. 4). 23.25% was the misclassification rate of this classification tree.
Classification tree produced by CART analysis performed with modern NA D. pulex claws recovered from Borreguil and modern Eu D. pulicaria claws from both Río Seco and Cuadrada. The number of claws correctly classified (bold) and incorrectly classified (not bold) were displayed below species name. In each base node individuals are classified following the condition defined by an explanatory variable. Those individuals that fulfilled that condition were classified on the left branch of each base node otherwise individuals were classified on the right branch of each base node
One-way ANOVA results for morphological differences in “modern” Daphnia specimens among the study lakes were based on NA D. pulex claws from Borreguil and Eu D. pulicaria claws from Cuadrada and Río Seco. Significant differences were found in Cldist (P < 0.05), PCL (P < 0.001), Cldist/PCL (P < 0.05), and PCW (P < 0.05), and the post hoc TUKEY’s HSD test confirmed that the postabdominal claw length was greater in specimens from Río Seco than from Borreguil (P < 0.001) or Cuadrada (P < 0.01). The mean Cldist value was significantly higher in claws from Río Seco than in those from Borreguil (P < 0.05), and the Cldist/PCL ratio was significantly higher in claws from Borreguil than in those from Río Seco (P < 0.01).
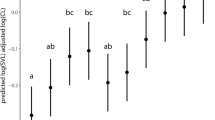
Kruskal–Wallis ANOVA results revealed significant differences in the number of stout spines (P < 0.01) among specimens from the three lakes, finding more stout spines in claws from Río Seco or Cuadrada (≥ 5) than in those from Borreguil (≤ 5) (Tables 3, 4; Fig. 5). Mann–Whitney post hoc analysis showed that the number of stout spines of claws from both Río Seco and Cuadrada were significantly greater than in those from Borreguil (P < 0.01) while no significance differences were found between claws from Río Seco and Cuadrada (P > 0.05).
D. pulicaria
Morphological features were compared between Eu D. pulicaria claws from Río Seco and Cuadrada, based on specimens recovered from the twentieth century as a whole. No significant between-lake difference (t test, P > 0.05) was observed in PCL values, whereas the Clmid was significantly higher (t test, P < 0.05) in claws from Cuadrada than in those from Río Seco, and the Cldist was significantly longer (t test, P < 0.05) in claws from Río Seco than in those from Cuadrada (Table 4). The Cldist/PCL ratio was significantly greater (t test, P < 0.05) in claws from Cuadrada than in those from Río Seco (Table 4), but no significant difference was found in the number of stout spines (Mann–Whitney U test, P > 0.05), after observing 4-7 in claws from Cuadrada and 4-6 in those from Río Seco (Fig. 5, Table 4).
CART analysis differentiated between Eu D. pulicaria claws from Río Seco and those from Cuadrada, based on Clmid and Cldist values. Using Clmid values, the base tree separated 32 claws of Cuadrada specimens from 29 claws of Río Seco specimens. Out of the claws with Clmid ≥ 48.7 µm, 17 were from Cuadrada and 3 from Río Seco; out of the claws with Clmid < 48.7 µm, 15 were from Cuadrada and 26 from Río Seco. Claws with Clmid < 48.7 µm were again subdivided, finding Cldist < 142.3 µm in 7 claws from Cuadrada and claws from Río Seco and Cldist > 142.3 µm in 8 claws from Cuadrada and 26 from Río Seco. Among the claws with Cldist > 142.3 µm, this value was > 176.9 in 13 claws from Río Seco whereas no claws from Cuadrada showed > 176.9 µm and was < 176.9 µm in 8 claws from Cuadrada and 13 from Río Seco. Among claws with Cldist < 176.9 µm, the Clmid value was ≥ 37.8 µm in 5 claws from Cuadrada and 3 from Río Seco and was < 37.8 µm in 3 claws from Cuadrada and 10 from Río Seco (Fig. 6). The misclassification rate of this classification tree was 33.3%.
Classification tree produced by CART analysis performed with Eu. pulicaria from Río Seco (RS) and Eu D. pulicaria from Cuadrada (CD). Claws belonged to both first and the second halves of the twentieth century. The number of claws correctly classified (bold) and incorrectly classified (not bold) were displayed below lake name. In each base node individuals are classified following the condition defined by an explanatory variable. Those individuals that fulfilled on the left branch of each base node otherwise individuals were classified on the right branch of each base node
Comparison between claws from the first and second halves of the twentieth century was conducted separately in Eu D. pulicaria specimens from Río Seco and Cuadrada. In specimens from Río Seco, Eu D. pulicaria claws from the first half of the twentieth century were significantly longer than those from the second half (t test, P < 0.001; power analysis value = 0.97) (Fig. 7), and the degree of difference was similar to that observed between modern Eu D. pulicaria claws from Río Seco and NA D. pulex claws from Borreguil (t test, P < 0.001) (Fig. 7). In addition, the Cldist/PCL ratio was significantly higher in the specimens from the first versus second half of the century (t test, P < 0.001; power analysis value = 0.99).
Among specimens from Cuadrada, claw lengths did not show significant differences between claws from the first and the second half of the twentieth century (Fig. 7). A significantly higher Cldist/PCL ratio was also observed in claws from the first versus second half of the twentieth century (t test P < 0.01; power analysis value = 0.60).
Discussion
In this study of alpine lakes in Southern Spain, CART analysis identified the PCL and Cldist/PCL ratio as useful variables to differentiate between Eu D. pulicaria and NA D. pulex fossil remains (Fig. 4). Although significant differences were also observed between lineages in the number of stout spines, this variable was not identified by CART analysis as a useful differential feature. The number of stout spines is most easily measured character of postabdominal claws, and their presence/absence on the middle comb has been used to distinguish satisfactorily between D. longispina (without stout spines) and D. pulex species complex (with stout spines) (Szeroczyńska and Sarmaja-Korjonen 2007). However, considerable difficulties are encountered in using the number of stout spines to distinguish species within the D. pulex complex.
In the present study, we observed 4–5 stout spines in NA D. pulex and 4–7 in Eu D. pulicaria (Tables 3, 4; Fig. 5). In North America, Schwartz et al. (1985) characterized D. pulex as having 4–9 stout spines, while Hebert and Finston (1997) recorded 5 or more spines in both D. pulex and D. pulicaria. More recently, D. pulicaria was described as having 3–7 stout spines by Korosi et al. (2011) and 5–7 stout spines by Boehler et al. (2012). Eu D. pulicaria has high morphological similarity to North American D. pulicaria, and the frequent confusion in taxonomic affiliation means that a formal description or re-description is required (Petrusek et al. 2005). For example, Eu D. pulicaria from four lakes located in Western Italian Alps were previously assigned to D. middendorffiana (Bellati et al. 2014) and were reported by Tiberti (2011) to have 5–11 stout spines. Hence, the literature evidences a wide variability in the number of stout spines in the D. pulex complex, as supported by the present findings.
This overlap in the number of stout spines with Eu D. pulicaria means that the ready differentiation between this lineage and NA D. pulex is not possible in the study lakes based on this morphological character of subfossil remains. Korosi et al. (2011) also concluded that the number of stout spines within D. pulex complex species can be used as additional indication for species identification but cannot be used as a reliable diagnostic character. This differentiation is made even more challenging by the frequent presence of hybrids between D. pulex and D. pulicaria (Hebert and Finston 1996; Marková et al. 2013) and by changes in the number of stout spines with photoperiod and temperature (Dodson 1981). Nevertheless, it can be concluded that claws with more than 5 spines belong to Eu D. pulicaria (Table 3; Fig. 5), at least in Sierra Nevada alpine lakes.
The PCL is also easy to measure and it may be useful feature for taxonomic identification of Eu D. pulicaria and NA D. pulex (Fig. 3). However, post hoc analyses revealed that the difference in PCL between Eu D. pulicaria and NA D. pulex derived from the significantly greater length of claws from Río Seco in comparison to NA D. pulex claws from Borreguil and Eu D. pulicaria claws from Cuadrada. Wide differences within the Eu D. pulicaria species cast doubt on the role of PCL in the identification of these species. In the present study, differences in the PCL of specimens from Río Seco between the first and second halves of the twentieth century (Fig. 7; Table 4) were significantly greater than the differences observed between Eu D. pulicaria and NA D. pulex. As in Korosi et al. (2011), the size overlap in PCL hampers its use as a reliable morphological feature for taxonomic differentiation.
Size differences in sedimentary claws imply size differences in the total length of the organism, given the high correlation between claw length and total body length in Daphnia (Dodson 1970; Manca and Comoli 1996). Thus, our results on PCL likely reflects the overlap in body length described for D. pulicaria and D. pulex species (Alonso 1996). Hence, PCL would only be useful to detect NA D. pulex individuals in paleolimnological studies of Sierra Nevada lakes if it is below the minimum length recorded for Eu D. pulicaria, i.e. PCL < 171.9 µm (Table 4).
CART analysis (Fig. 4) did not allow a definitive separation between Eu D. pulicaria from NA D. pulex. Nevertheless, this analysis can be considered useful as a guide to species identification if combined with other proxies. Thus, remains with fewer than five stout spines (Table 3; Fig. 5), short PCL (Table 3; Fig. 3), and high Cldist/PCL ratio (Table 3) might suggest the presence of NA D. pulex and prompt the consideration of further measurements of additional remains, examination of the whole body and, when possible, genetic analysis.
The present results apply not only to Sierra Nevada lakes, which this invasive species may have colonized, being identified in one of the three study lakes, but also to other Mediterranean aquatic systems. It has been recorded in only a few systems in Mediterranean Europe to date (Fadda et al. 2011; Vergilino et al. 2011; Crease et al. 2012), but there appears to be a high likelihood of its rapid and wide expansion, as already observed in Africa (Mergeay et al. 2006).
Paleolimnological analyses allow the timing of its arrival in different systems to be determined and the history of its expansion to be recorded (Lavery et al. 2014). Although genetic analysis of ephippial eggs can fully elucidate taxonomic affiliation (Ortells et al. 2014; Möst et al. 2015; Van Damme 2016), this approach is not widely adopted and it is not always possible to find viable ephippial eggs in sediment. Moreover, paleolimnological analysis of cladocera remains indicates changes in the density of different taxa and allows them to be related to environmental changes, whereas the use of genetic analysis to examine changes in relative species abundance is more costly and laborious.
Colonization of Sierra Nevada lakes by NA D. pulex has coincided with and may have been favored by the accelerated warming and increased Saharan winds over the past ~ 50 years (Jiménez et al. 2018), and these conditions are predicted to strengthen over the next few decades (IPCC 2014). In this case, this exotic Daphnia may expand to other lakes and hybridize with or displace local species, as reported in Africa and Italy (Mergeay et al. 2006; Fadda et al. 2011; Marková et al. 2013). Given that Daphnia is a key species in the trophic web of aquatic ecosystems, between-species differences in reproduction and algal consumption rate may introduce major changes in the ecological conditions of lakes.
Comparison between claws of Eu D. pulicaria from Río Seco versus Cuadrada revealed only small differences in Cldist/PCL ratio or Clmid and no differences in PCL, and tree CART analysis did not yield a clear separation between these populations (Fig. 6). However, the morphological uniformity of postabdominal claws within Eu D. pulicaria suggested by these findings is contradicted by the major increase over time in the claw length of specimens from Río Seco (Fig. 7; Table 4). This lengthening may be linked to the upsurge in Daphnia relative abundance in Río Seco associated with the rise in warming and atmospheric Saharan Ca deposition since the 1970s–80s (Jiménez et al. 2018). However, the effects of warming on body size are contradictory, with warmer water temperatures leading to a smaller individual size (Perrin 1988; McKee and Ebert 1996) but longer growing seasons has been reported to lead to older and larger individuals in Sierra Nevada lakes (Pérez-Martínez et al. 2007). The observation by Hessen et al. (2000) of the positive impact of Ca availability on body size may also explain the observations in Daphnia from Río Seco, where Daphnia development is calcium limited (Jiménez et al. 2018). However, no size differences were found over time in Daphnia from Cuadrada, exposed to the same environmental conditions and also calcium limited, which may be related to the markedly smaller increase in Daphnia abundance in Cuadrada. Moreover, potential invertebrate predators existing in Sierra Nevada lakes (mainly aquatic coleopteran), may also be responsible for changes in Daphnia body size. Consequently, although analysis of postabdominal claw size can be useful in the reconstruction of past environmental changes, this also requires in-depth knowledge of the multiple factors involved.
Biological invasions pose one of the most important environmental challenges of the twenty-first century (Mack et al. 2000; Pimentel et al. 2000). The considerable efforts directed towards the detection and early management of invasive species in numerous countries (Bogich et al. 2008) depend on reliable identification methods. According to the present morphologic findings in Sierra Nevada lakes, a combination of several easy-to-measure variables, such as the number of stout spines, PCL and Cldist/PCL, may allow sufficient differentiation between native Eu D. pulicaria and the exotic NA D. pulex to warn of a possible invasion by the latter species in any aquatic system.
Conclusions
Our objective was to analyze morphological differences in postabdominal claws recovered from the sediment of alpine lakes in Sierra Nevada (SE Spain) in order to differentiate the North American (NA) D. pulex, an invasive lineage in the area, from the native European (Eu) D. pulicaria. The postabdominal claw length (PCL), ratio of distal comb length to PCL (Cldist/PCL), and number of stout spines are the most useful morphological features for differentiating both species. However, a definitive separation between both species based in postabdominal claws features is unreliable because of a wide variability in PCL within the Eu D. pulicaria species and an overlap in stout spine number between species. Nevertheless, this analysis can be useful as a guide to species identification if combined with other proxies. Thus, remains with fewer than five stout spines, short PCL, and high Cldist/PCL ratio might suggest the presence of NA D. pulex and incite to further analyses to verify it.
The present study results can be applied to other Mediterranean aquatic systems where this invasive species lineage has been recorded or can potentially colonize, given its apparently rapid and wide expansion ability. Unlike genetic analyses, paleolimnological analyses allow investigators to record the history of invasive species expansion and to indicate changes in the density of different taxa allowing them to be related to environmental changes. Thus, any advance in these analyses would help ecologists to understand colonization processes.
References
Alonso M (1996) Fauna Ibérica. In: Crustacea: Branchiopoda, vol 07. Consejo Superior de Investigaciones Científicas, Madrid
Appleby PG (2001) Chronostratigraphic techniques in recent sediments. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments, vol 1. Basin analysis, coring, chronological techniques. Kluwer Academic Publishers, Dordrecht, pp 171–203
Appleby PG, Oldfield F (1978) The calculation of 210Pb dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 5:1–8
Barea-Arco J, Pérez-Martínez C, Morales-Baquero R (2001) Evidence of a mutualistic relationship between an algal epibiont and its host, Daphnia pulicaria. Limnol Oceanogr 46:871–881
Bellati A, Tiberti R, Cocca W, Galimberti A, Casiraghi M, Bogliani G, Caleotti P (2014) A dark shell hiding great variability: a molecular insight into the evolution and conservation of melanic Daphnia populations in the Alps. Zool J Linn Soc 171:697–715
Boehler JA, Keller TS, Krieger KA (2012) Taxonomic atlas of the water Fleas, “Cladocera” (class Crustacea) recorded at the Old Woman Creek national estuarine research reserve and state nature preserve, Ohio. Final report to Ohio Department of Natural Resources, Division of Wildlife, Columbus
Bogich TL, Liebhold AM, Shea K (2008) To sample or eradicate? A cost minimization model for monitoring and managing an invasive species. J Appl Ecol 45:1134–1142
Brahney J, Routledge R, Bos DG, Pellatt MG (2010) Changes to the productivity and trophic structure of a sockeye salmon rearing lake in British Columbia. N Am J Fish Manag 30:433–444
Brede N, Sandrock C, Straile D, Spaak P, Jankowski T, Streit B, Schwenk K (2009) The impact of human-made ecological changes on the genetic architecture of Daphnia species. Proc Natl Acad Sci USA 106:4758–4763
Castillo-Martín A (2009) Las lagunas de Sierra Nevada. Universidad de Granada, Granada
Colbourne JK, Crease TJ, Weider LJ, Hebert PDN, Dufresne F, Hobaek A (1998) Phylogenetics and evolution of a circumarctic species complex (Cladocera: Daphnia pulex). Biol J Linn Soc 65:347–365
Crease TJ, Omilian AR, Costanzo KS, Taylor DJ (2012) Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 10:e46620
De’ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Dodson SI (1970) Complementary feeding niches sustained by size-selective predation. Limnol Oceanogr 15:131–137
Dodson SI (1981) Morphological variation of Daphnia pulex Leydig (Crustacea: Cladocera) and related species from North America. Hydrobiologia 83:101–114
Duggan IC, Robinson KV, Burns CW, Banks JC, Hogg ID (2012) Identifying invertebrate invasions using morphological and molecular analyses: North American Daphnia ‘pulex’ in New Zealand fresh waters. Aquat Invasions 7:585–590
Fadda A, Marková S, Kotlík P, Lugliè A, Padedda B, Buscarinu P, Sechi N, Manca M (2011) First record of planktonic crustaceans in Sardinian reservoirs. Biologia 66:856–865
Gliwicz ZM (1990) Food thresholds and body size in Cladocerans. Nature 343:638–640
Hairston NG, Perry LJ, Bohonak AJ, Fellows MQ, Kearns CM, Engstrom DR (1999) Population biology of a failed Invasion: paleolimnology of Daphnia exilis in upstate New York. Limnol Oceanogr 44:477–486
Havel JE, Colbourne JK, Hebert PDN (2000) Reconstructing the history of intercontinental dispersal in Daphnia lumholtzi by use of genetic markers. Limnol Oceanogr 45:1414–1419
Hebert PDN, Finston TL (1996) A taxonomic reevaluation of North American Daphnia (Crustacea: Cladocera). II. New Species in the Dapnia pulex group from the south-central United States and Mexico. Can J Zool 74:632–653
Hebert PDN, Finston TL (1997) A taxonomic reevaluation of North American Daphnia (Crustacea: Cladocera). III. The D. catawba complex. Can J Zool 75:1254–1261
Hebert PDN, Finston TL (2001) Macrogeographic patterns of breeding system diversity in the Daphnia pulex group from the United States and Mexico. Heredity 87:153–161
Hessen DO, Alstad NEW, Skardal L (2000) Calcium limitation in Daphnia magna. J Plankton Res 22:553–568
IPCC (2014) Summary for policymakers. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) Climate change 2014: Impacts, adaptation, and vulnerability. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 1–32
Jeppesen E, Leavitt P, De Meester L, Jensen JP (2001) Functional ecology and palaeolimnology: using cladoceran remains to reconstruct anthropogenic impact. Trends Ecol Evol 16:191–198
Jiménez L, Romero-Viana L, Conde-Porcuna JM, Pérez-Martínez C (2015) Sedimentary photosynthetic pigments as indicators of climate and watershed perturbations in an alpine lake in southern Spain. Limnetica 34:439–454
Jiménez L, Rühland KM, Jeziorski A, Smol JP, Pérez-Martínez C (2018) Climate change and Saharan dust drive recent cladoceran and primary production changes in remote alpine lakes of Sierra Nevada, Spain. Glob Change Biol 24:139–158
Juračka PT, Laforsch C, Petrusek A (2011) Neckteeth formation in two species of the Daphnia curvirostris complex (Crustacea: Cladocera). J Limnol 70:359–368
Korhola A (1999) Distribution patterns of cladocera in subarctic Fennoscandian lakes and their potential in environmental reconstruction. Ecography 22:357–373
Korhola A, Rautio M (2001) Cladocera and other branquipod crustaceans. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments: zoological indicators, vol 4. Kluwer Academic Publishers, Dordrecht
Korosi JB, Paterson AM, Desellas AM, Smol JP (2008) Linking mean body size of pelagic Cladocera to environmental variables in Precambrian Shield lakes: a paleolimnological approach. J Limnol 67:22–34
Korosi JB, Jeziorski A, Smol JP (2011) Using morphological characters of subfossil daphniid postabdominal claws to improve taxonomic resolution within species complexes. Hydrobiologia 676:117–128
Kurek J, Weeber RC, Smol J (2011) Environment trumps predation and spatial factors in structuring cladoceran communities from Boreal Shield lakes. Can J Fish Aquat Sci 68:1408–1419
Labaj AL, Michelutti N, Smol JP (2017) Changes in cladoceran assemblages from tropical high mountain lakes during periods of recent climate change. J Plankton Res 39:211–219
Lavery JM, Kurek J, Rühland KM, Gillis CA, Pisaric MFJ, Smol JP (2014) Exploring the environmental context of recent Didymosphenia geminata proliferation in Gaspésie, Québec, using paleolimnology. Can J Fish Aquat Sci 71:616–662
Lindbladh MS, O’Connor R, Jacobson GL (2002) Morphometric analysis of pollen grains for paleoecological studies: classification of Picea from eastern North America. Am J Bot 89:1459–1467
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences and control. Ecol Appl 10:689–710
Manca M, Comoli P (1996) Reconstructing population size structure in Cladocera by measuring their body remains. Mem Ist Ital Idrobiol 54:61–68
Marková S, Dufresne F, Manca M, Kotlík P (2013) Mitochondrial capture misleads about ecological speciation in the Daphnia pulex complex. PLoS ONE 8(7):e69497
McKee D, Ebert D (1996) The effect of temperature on maturation threshold body-length in Daphnia magna. Oecologia 108:627–630
Mergeay J, Verschuren D, De Meester L (2006) Invasion of an asexual American water flea clone throughout Africa and rapid displacement of a native sibling species. Proc R Soc Biol Sci Ser B 273:2839–2844
Morales-Baquero R, Carrillo P, Reche I, Sánchez-Castillo P (1999) Nitrogen-phosphorus relationship in high mountain lakes: effects of the size of catchment basins. Can J Fish Aquat Sci 56:1809–1817
Morales-Baquero R, Carrillo P, Barea-Arco J, Pérez-Martínez C, Villar-Argaiz M (2006) Climate-driven changes on phytoplankton–zooplankton coupling and nutrient availability in high mountain lakes of Southern Europe. Freshw Biol 51:989–998
Möst M, Oexle S, Marková S, Aidukaite D, Baumgartner L, Stich HB, Wessels M, Martin-Creuzburg D, Spaak P (2015) Population genetic dynamics of an invasion reconstructed from the sediment egg bank. Mol Ecol 24:4074–4093
Ortells R, Vanoverbeke J, Louette G, De Meester L (2014) Colonization of Daphnia magna in a newly created pond: founder effects and secondary immigrants. Hydrobiologia 723:167–179
Paterson MJ (1994) Paleolimnological reconstruction of recent changes in assemblages of Cladocera from acidified lakes in the Adirondack Mountains (New York). J Paleolimnol 11:189–200
Pérez-Martínez C, Barea-Arco J, Conde-Porcuna JM, Morales-Baquero R (2007) Reproduction strategies of Daphnia pulicaria population in a high mountain lake of southern Spain. Hydrobiologia 594:75–82
Perrin N (1988) Why are offspring born larger when it is colder? Phenotypic plasticity for offspring size in the cladoceran Simocephalus vetulus (Müller). Funct Ecol 2:283–288
Persson J, Brett MT, Vrede T, Ravet JL (2007) Food quantity and quality regulation of trophic transfer between primary producers and a keystone grazer (Daphnia) in pelagic freshwater food webs. Oikos 116:1152–1163
Petrusek A, Bastiansen F, Schwenk K (2005) European Daphnia species. Taxonomic and genetic keys. [Build 2006-01-12 beta]. CD-ROM, distributed by the authors. Department of Ecology and Evolution, J.W. Goethe-University, Frankfurt am Main, Germany & Department of Ecology, Charles University, Prague, Czechia
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reche I, Pulido-Villena E, Morales-Baquero R, Casamayor EO (2005) Does ecosystem size determine aquatic bacterial richness? Ecol Soc Am 86:1715–1722
Rudstam LG, Lathrop RC, Carpenter SR (1993) The rise and fall of a dominant planktivore: direct and indirect effects on zooplankton. Ecol Soc Am 74:303–319
Sánchez-Castillo P, Cruz-Pizarro L, Carrillo P (1989) Caracterización del fitoplancton de las lagunas de alta montaña de Sierra Nevada (Granada, España) en relación con las características físico-químicas del medio. Limnetica 5:37–50
Schelske CL, Peplow A, Brenner M, Spencer CN (1994) Low-background gamma counting: applications for 210Pb dating of sediments. J Paleolimnol 10:115–128
Schwartz SS, Innes DJ, Hebert PDN (1985) Morphological separation of Daphnia pulex and Daphnia obtusa in North America. Limnol Oceanogr 30:189–197
Shapiera M, Jeziorski A, Paterson AM, Smol JP (2012) Cladoceran response to calcium decline and the subsequent inadvertent liming of a softwater Canadian lake. Water Air Soil Pollut 223:2437–2446
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Szeroczyńska K, Sarmaja-Korjonen K (2007) Atlas of Subfossil Cladocera from Central and Northern Europe Atlas of Subfossil Cladocera from Central and Northern Europe. Friends of the Lower Vistula Society, Świecie
Szeroczyńska K, Zawisza E (2005) Daphnia remains from the sediment of lake Somaslampi (NW Finnish Lapland) and lake Wigry (NE Poland). Stud Quat 22:55–57
Therneau T, Atkinson B, Ripley B (2015) Rpart: recursive partitioning and regression trees. R package version 4.1-10. https://cran.r-project.org/rpart/. Accessed 1 Apr 2017
Tiberti R (2011) Morphology and ecology of Daphnia middendorffiana, Fisher 1851 (Crustacea, Daphniidae) from four new populations in the Alps. J Limnol 70:239–247
Tilman D, Clark M, Williams DR, Kimmel K, Polasky S, Packer C (2017) Future threats to biodiversity and pathways to their prevention. Nature 546:73–81
Van Damme K (2016) Endemism and longdistance dispersal in the waterfleas of Easter Island. Zootaxa 4154:251–272
Veiga J (2014) Diversidad genética del complejo de Daphnia pulex-pulicaria en Sierra Nevada: diferencias espacio-temporales. Master’s thesis, University of Granada, Granada, Spain
Vergilino R, Marková S, Ventura M, Manca M, Dufresne F (2011) Reticulate evolution of the Daphnia pulex complex as revealed by nuclear markers. Mol Ecol 20:1191–1207
Walsh JR, Carpenter SR, Vander Zanden MJ (2016) Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc Natl Acad Sci USA 113:4081–4085
Acknowledgements
The authors are grateful to their colleagues for assistance in the core collection. Financial support was provided by MMA Project 87/2007, MICINN Project CGL2011-23483 and Programa Nacional de Movilidad de Recursos Humanos de Investigación Grant (MICINN) for C. Pérez-Martínez and a FPU fellowship (AP2007-00352) for L. Jiménez from the Spanish Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burillo, J.P., Jiménez, L. & Pérez-Martínez, C. Identifying invasive Daphnia species by morphological analysis of postabdominal claws in Sierra Nevada alpine lakes. J Paleolimnol 62, 121–135 (2019). https://doi.org/10.1007/s10933-019-00078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-019-00078-0