Abstract
Aquatic biodiversity is commonly linked with environmental variation in lake networks, but less is known about how local factors may influence within-lake biological heterogeneity. Using a combined ecological and multi-proxy palaeoecological approach we investigated long-term changes in the pathways and processes that underlie eutrophication and water depth effects on lake macrophyte and invertebrate communities across three basins in a shallow lake—Castle Lough, Northern Ireland, UK. Contemporary data allow us to assess how macrophyte assemblages vary in composition and heterogeneity according to basin-specific factors (e.g. variation in water depth), while palaeoecological data (macrophytes and co-occurring invertebrates) enable us to infer basin-specific impacts and susceptibilities to nutrient-enrichment. Results indicate that variability in water depth promotes assemblage variation amongst the lake basins, stimulating within-lake macrophyte assemblage heterogeneity and hence higher lake biodiversity. The palaeo-data indicate that eutrophication has acted as a strong homogenising agent of macrophyte and invertebrate diversities and abundances over time at the whole-lake scale. This novel finding strongly suggests that, as eutrophication advances, the influence of water depth on community heterogeneity is gradually eroded and that ultimately a limited set of eutrophication-tolerant species will become homogeneously distributed across the entire lake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lakes have been regarded as ideal models for studying the influence of local environmental effects on species turnover in systems that are interconnected at the landscape level (Leibold and Norberg 2004). The structuring influence of environmental factors on within-lake spatial variation in community composition has, however, received less attention although such an idea is acknowledged theoretically by the “submetacommunity concept” of Leibold and Norberg (2004). This oversight may reflect the fact that research has largely focused on populations of mobile planktonic organisms assumed to be well-mixed within lakes. Lake environmental heterogeneity may, however, be important in influencing the distributions and abundances of taxa with limited mobility. Local distributions of aquatic macrophytes, for example, may depend on competition for space and tolerance to local environmental conditions (Barrat-Segretain 1996). Moreover, different areas within lakes may vary substantially, for example, in water depth, sediment type, wind exposure, proximity to inflows/outflows and the presence of shoreline vegetation. Such within-lake variation influences the spatial distribution of aquatic vegetation (Spence 1967; Carpenter and Titus 1984) and, in turn, associated invertebrates due to local variation in habitat, structural complexity and feeding opportunities (Lauridsen et al. 1996).
Studies of biological assembly dynamics in lake systems are generally limited to snapshots in time, focusing on short-term or contemporary patterns of species turnover or on biogeographical patterns. The interplay between spatial distributions and environmental drivers may, however, shift locally over time (Korhonen et al. 2010). Indeed, increasing evidence that colonisation histories, priority effects and temporal changes in environmental variables influence both local and regional species distributions highlights the importance of studying species turnover (beta-diversity) within lakes over time (Fukami and Morin 2003). For instance, contemporary and palaeolimnological studies of Daphnia colonisation patterns revealed that assembly history initially influenced species composition, but that changes in water temperature and lake stratification subsequently drove species turnover (Allen et al. 2011). Furthermore, species-specific differences in colonisation and adaptive capacity have been shown to substantially influence temporal beta-diversity and to obscure direct relationships between Daphnia species distributions and environmental gradients (Urban and De Meester 2009). Palaeolimnological studies have also demonstrated that changes in the nature and intensity of local factors can influence distributions and abundances over time. For example, drivers of macrophyte assembly change were shown to shift from lake infilling during most of the Holocene to eutrophication around 120 years ago (Rasmussen and Anderson 2005).
By utilising a combined ecological and multi-proxy palaeoecological approach, this study aims to understand how key long-term environmental drivers (i.e. shallowing and nutrient-enrichment) influence temporal variation in the distribution of lake macrophytes and associated invertebrate assemblages across three basins of Castle Lough, a shallow lake in Northern Ireland, UK. Our study evaluates the hypothesis that variation in macrophyte and co-occurring invertebrate assemblages is reduced over time due to the homogenising influence of eutrophication.
Study site
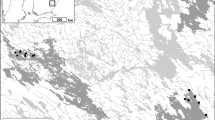
Castle Lough is a small (surface area = 13 ha), shallow (5 m maximum depth), lowland (45 m above sea level) lake located in the south of the Upper Lough Erne (ULE) system, a highly connected shallow lake network in Co. Fermanagh, Northern Ireland (54°12′N, 007°37′W). The lake has three distinct basins and moderate annual mean total phosphorus (29 μg TP L−1) and total nitrogen (1.03 mg TN L−1) concentrations. The River Finn connects the lake to the main ULE system (Fig. 1), which consists of a large “mother” lake and several linked satellite lakes.
Over the last 120 years hydrological change and eutrophication have profoundly influenced the ecology of the ULE system (Battarbee 1986; Gibson et al. 1995). Frequent flood events in the catchment caused by high rainfall led to the development of a major drainage scheme between 1880 and 1890 (Price 1890). Because of this scheme, water levels in the main lake dropped from around 46 to 44 m above sea level (Price 1890). A second attempt to regulate water levels (dredging of 30 km of channel between the ULE and Lower Lough Erne systems) was undertaken in the early 1950s under the Erne Drainage and Development Act (Northern Ireland). Water levels have subsequently been maintained between 43 and 45 m, but the system (including Castle Lough) is still prone to major flood events (Mathers et al. 2002). Diatom-based paleolimnological studies indicate a gradual acceleration of nutrient-enrichment in the ULE since the 1900s with a major phase of eutrophication after c. 1950 (Battarbee 1986; Gibson et al. 1995).
Materials and methods
Contemporary macrophyte surveys
To characterize present-day distributions and abundances of macrophytes in Castle Lough, we sampled three circular areas of 30 m radius in each of the lake’s three main basins (Fig. 1; Table 1). To ensure broad and equivalent sampling, each area was divided into three sub-areas delimited by 10 m radii (Fig. 1b). Six points were surveyed from the innermost area, and 18 and 36 points for the successively larger sub-areas, respectively (total = 60 points). We used the method of Canfield et al. (1984) to determine the percentage of lake volume filled by macrophytes (PVI) at each point. This entailed surveying macrophytes from a boat using a combination of grapnel sampling and visual observations made with a bathyscope. At each point water depth, average plant height and species percentage cover were recorded for an estimated area of 1 m2. For each sampling point, PVI was calculated as: (macrophyte % cover × average height of macrophyte)/water depth.
Palaeolimnological analyses
We retrieved three sediment cores (NCAS1, NCAS2 and NCAS3) from the midpoint of each of the sampling circular areas in each basin in June 2008 (Fig. 1b) using a wide-bore (14 cm) “Big-Ben” piston corer (Patmore et al. 2014). Cores NCAS1, NCAS2 and NCAS3 were collected from water depths of 117, 180 and 160, respectively, and were extruded in the field at 1-cm intervals. Lithostratigraphic changes in the cores were recorded in the field. Core chronologies were determined using 210Pb gamma counting (Appleby et al. 1986) at the Bloomsbury Environmental Isotope Facility (BEIF), University College London (UCL). Dates were ascribed using the Constant Rate of Supply (CRS) model (Appleby and Oldfield 1978).
Eleven 1-cm slices were analysed for macrofossils from each core at a resolution of approximately 10-year intervals, spanning the last c. 110 years. Exceptions were two 15-year intervals (1940–1955 and 1965–1980) due to differential sedimentation rates between cores. Macrofossil analyses were performed using an adaptation of standard methods (Birks 2001). We analysed approximately 70 cm3 of sediment and all samples were disaggregated in 10% potassium hydroxide (KOH) before sieving. Three sieves of mesh sizes 355, 125 and 90 μm were used to separate plant, chironomid and other invertebrate remains. Given the high fossil retent on the 125 and 90 μm sieves, we combined and mixed both samples after sieving, and analysed a 20-mL subsample. Plant macrofossils included seeds and fruits, leaf-spines, leaf fragments (including water lilies leaf tissue-sclereids), charophyte oospores and Isoetes megaspores. Invertebrate macrofossils included bryozoan statoblasts (counted as valves), daphnid ephippia, molluscs (counts of whole shells, half shells, opercula, shell fragments and glochidia larvae), and chironomid head capsules. Chironomids were prepared for analysis using standard methods (Brooks et al. 2007). Plant and animal macrofossil data were standardised as the number of fossils per 100 cm3 and identified by comparison with reference material held at the Environmental Change Research Centre (ECRC), UCL and the Natural History Museum, London, and by using relevant taxonomic keys (Aldridge and Horne 1998; Birks 2001; Wood and Okamura 2005).
Given lower sedimentation rates for core NCAS2 (ESM1) and to establish decadal comparisons amongst the cores, we combined the macrofossil data for three time periods, 1941–1950, 1966–1980 and 1981–1990 for NCAS2. We used mean macrofossil abundances between adjacent sediment samples for each given time period. To avoid overestimating abundance values for the time intervals, we took a parsimonious approach and rounded values to the lowest adjacent number. For example, if adjacent sample values were 1 and 2 we gave a score of 1 for the sample average. If it was 1 and 0 we coded with 0 and so on.
Data analysis
Contemporary environmental factors and macrophyte spatial distributions
As a measure of current lake environmental variation, we used the water depths derived from the PVI data for each macrophyte sampling point. Similarly, we used macrophyte percentage cover (for each sampling point) to characterise spatial distributions and abundances of plant species in the three basins. Relationships between macrophyte percentage frequencies and variation in water depth at the whole-lake and basin levels were analysed using generalized linear models (GLM), permutational analysis of multivariate dispersions (perMANOVA; Anderson 2001) and homogeneity multivariate dispersion analysis (HMD; Anderson 2006). Whole-lake scale analysis was assessed through a global GLM on all basin macrophyte frequencies and water depths. Adjusted goodness of fit (R2) and Akaike Information Criteria (AIC) were used as GLM quality indicators. We evaluated the dispersion parameter phi (Residual deviance (full model)/residual degrees of freedom) to assess any over-dispersion in the data and applied a negative binomial distribution if necessary (i.e. ϕ > 1). Lastly, logistic regression using presence/absence as a response (with a binomial error distribution) was applied to evaluate the probability of finding key environmentally sensitive macrophyte species that are commonly lost following eutrophication across the observed depth profiles. Those macrophyte species highly vulnerable to eutrophication-induced declines were selected according to Madgwick et al. (2011). The explained percentage of macrophyte assemblage variation was corrected following Peres-Neto et al. (2006) and expressed as R2 adjusted.
HMD and perMANOVA were applied to assess independent variation in macrophyte assemblages and water depth profiles amongst the three basins. perMANOVA compares variability of dissimilarity distances within groups versus variability between groups, while HMD comprises a distance-based test of the homogeneity of multivariate dispersions between groups to their group centroid (Anderson 2006). Macrophyte species dissimilarities were calculated using the Bray–Curtis dissimilarity index and water depth dissimilarities using Euclidean distances. Each basin was treated as independent (Anderson 2006). Using this approach, a basin having high multivariate dispersion (high values of dissimilarities and/or mean distance to group centroid) would be associated with large dissimilarities between macrophyte species or water depth and thus high heterogeneity (Anderson et al. 2006). The significance of the analyses was assessed by ANOVA (P < 0.05). A significant result indicates high variation between basins, while a lack of significance denotes no variation in macrophyte assemblage or depth variation between basins (Anderson et al. 2006).
To visualise how plant assemblage and depth variation were related across the three basins, we used NMDS on Bray–Curtis dissimilarities for the PVI data (which combines plant percentage cover and water depth into one measure). Of many potential measures of dissimilarity, Bray–Curtis has been shown to have one of the strongest relationships between site dissimilarity and ecological distance, hence providing optimum ordination results for the NMDS technique (Faith et al. 1987).
Spatial and temporal dynamics of plant and invertebrate macrofossils
To quantify change over time in the spatial distributions of plant and invertebrate macrofossils (henceforth referred to as space–time interaction), we applied an ANOVA space–time test analysis (Legendre et al. 2010). We used “Model 5” of Legendre et al. (2010), which uses principal coordinates of neighbour matrices (PCNM) variables to assess the interaction between space and time, and Helmert contrasts, also called “orthogonal dummy variables”, to reconstruct a predictive model assessing the independent effects of space and time.
To facilitate comparisons between cores, macrofossil data were expressed as fluxes. As plant macro-remains include a variety of differentially produced plant structures (e.g. spines, leaves and seeds), making realistic comparisons of taxon abundances is notoriously challenging (Birks 2001). Consequently, similar to the approach of Odgaard and Rasmussen (2001), we transformed each macrofossil flux record into a 0–5 abundance scale, where 0 is absent and 5 is highly abundant, as follows: (1) we merged macrofossil fluxes from all three cores into a single matrix and ordered each taxon flux record from highest to lowest values; (2) flux data were then transformed into percentage frequencies by assuming 100% for the highest flux value for each taxon; (3) percentage frequencies were clustered using a DAFOR (Dominant, Abundant, Frequent, Occasional, Rare) scale as follows: 5 (100–80%); 4 (79–60%); 3 (59–40%); 2 (39–20%); 1 (19–1%). Macrophyte DAFOR data were Hellinger transformed, while bryozoan, chironomid, mollusc and daphnid fluxes were first log-transformed and then Hellinger-transformed prior to ANOVA space–time analyses. Each taxon group was tested independently and we constructed a site-by-taxon response data table with three-row blocks corresponding to a spatial and temporal location (i.e. basin 1, basin 2 and basin 3 at time i). We divided the macrofossil abundance data of each lake basin into 11 time-periods (a total of 33 data points) as follow: c. pre-1900; 1901–1910; 1911–1920; 1921–1930; 1931–1940; 1941–1950; 1955–1965; 1966–1980; 1981–1990; 1991–2000 and 2001–2008. To assess the significance of each taxon group space–time interactions we used a significance of 0.05 and 999 permutations. Multidimensional scaling (NMDS) (Bray–Curtis metric) was used to visualize trends in assemblage variation in space and time and K-means partitioning analysis to detect significant changes in assemblage composition over time (“cascadeKM” function of the “vegan” Package in R). The simple structure index (ssi) was used to identify the best partition. To summarise the main temporal changes in assemblage composition in relation to environmental driving factors, we identified characteristic species for each time-period using the IndVal method (“indval” function of the “labdsv”’ Package in R) of Dufrene and Legendre (1997). For simplification purposes, we divided the palaeo-record of each biological group into three synchronous time intervals of assemblage variation detected by K-means across the five groups (see ESM4). These three time intervals were: pre-1900–1940, 1941–1980, and 1981–present. Statistical analyses were conducted in R (v.2.13; R Core Development Team 2009).
Results
Contemporary macrophyte spatial patterns
Fourteen macrophyte species were recorded among the three basins (Fig. 2a). Elodea canadensis Michx., Nuphar lutea (L.) Sm. Sagittaria sagittifolia L., and Sparganium emersum Rehmann were the most abundant species, occurring in all three basins. Filamentous algae (undifferentiated), Lemna trisulca L., Nitella flexilis L., and Utricularia vulgaris L., were also recorded in all basins but at lower percentage cover. Chara globularis J.L.Thuiller, Potamogeton obtusifolius Mert. & W.D.J. Koch, and Stratiotes aloides L. were present in basins 1 and 3 only, Potamogeton praelongus Wulfen. was absent in basin 1, Callitriche sp. and Equisetum fluviatile L. were absent in basins 1 and 3, and Myriophyllum verticillatum L. was absent in basins 2 and 3. Filamentous algae occurred in all three basins and were more abundant in basins 2 and 3.
Basin 1 was characterised by homogeneous shallow water depths (mean 116.7 ± 6.43 cm), basin 2 by more heterogeneous and deeper waters (mean 164.7 ± 28.01 cm) and basin 3 by homogenous deeper waters (mean 152.1 ± 3.5 cm) (ESM2a). Negative binomial GLM on macrophyte species percentage cover and water depth values showed that water depth explained a highly significant (P < 0.0001; R 2adj = 30%) proportion of the variation in macrophyte assemblages at the whole-lake scale (Fig. 2b). A marked decline in macrophyte percentage cover was observed above a depth of 160 cm. Logistic regressions indicated that M. verticillatum, C. globularis, and S. aloides were highly restricted (P < 0.001 in all cases) by water depth (ESM3) with probability of occurrences greatly declining above 115–120 cm. P. praelongus and P. obtusifolius occurrences were similarly limited to depths between 115 and 160 cm but with no statistically significant trend.
Multivariate analysis revealed substantial spatial variation in macrophyte assemblages and water depths between the three basins (P = 0.001 in all perMANOVA and HMD cases) (ESM2b). HMD analysis revealed that macrophyte assemblage and water depth profiles in basin 2 were significantly more heterogeneous than in the other two basins (ESMS2c). The NMDS plot of PVI values showed a separation between macrophyte Bray–Curtis dissimilarities of basin 1 (groups on the left-hand side of the plot) and the other two basins (Fig. 3a). Bray–Curtis macrophyte dissimilarities of basins 2 and 3 overlapped in some cases.
Historical spatial patterns
Plant and invertebrate macrofossils were detected throughout the cores from each basin (Figs. 4, 5, 6). 210Pb-based radiometric chronologies and sedimentation rates for cores NCAS1, NCAS2 and NCAS3 are given in ESM1.
Plant-macrofossil stratigraphy for cores NCAS1-basin 1 (black), NCAS2- basin 2 (dark grey), and NCAS3- basin 3 (light grey). Dotted lines represent a c. 10-year time-period. Solid black lines represent the zones determined by K-means analysis, corresponding to c. pre-1900–1920, 1931–1980 and 1981–present
Representative chironomid-macrofossil stratigraphy for cores NCAS1-basin 1 (black), NCAS2-basin 2 (dark grey), and NCAS3-basin 3 (light grey). Dotted lines represent a c. 10-year time-period. Solid black lines represent the zones determined by K-means analysis, corresponding to c. pre-1900–1920, 1921–1940, 1941–1955, 1956–1980 and 1981–present
a Mollusc; b Bryozoan; and c Daphnid macrofossil stratigraphies for cores NCAS1-basin 1 (black), NCAS2-basin 2 (dark grey), and NCAS3-basin 3 (light grey). Dotted lines represent a c. 10-year time-period. Solid black lines represent zones determined by K-means analysis, corresponding to c. pre-1900–1930, 1931–1955, 1955–1980 and 1981–present
NMDS plots of all five taxonomic groups revealed a greater dissimilarity between basin 1 assemblages and the other two sampling basins over time (Fig. 3b–e). The ANOVA space–time analysis of plant macrofossil abundances revealed a highly significant space–time interaction (P = 0.001) that explained 27% of assemblage variation (Table 1). The analysis also revealed a significant (P = 0.001) space–time interaction for chironomids and molluscs, accounting for 32 and 29% of total assemblage variation, respectively (Table 1).
Multivariate trajectory and K-means analyses revealed three significant time intervals (ESM4a) in which plant macrofossil composition differed significantly across the three basins (Fig. 4). These corresponded to c. pre-1900–1930, 1931–1980 and 1981–present. The initial changes are mostly attributed to early reductions in bryophytes (including Sphagnum spp. leaf remains), Najas flexilis (Willd.) Rost and Schmidt. seeds, Isoetes lacustris L. megaspores and S. aloides leaf-spines (Fig. 4; Table 2). Myriophyllum spp. leaves and seeds were present at high abundances (in particular in basin 1) along with P. praelongus/lucens (basins 2 and 3) during the 1930–1980s. After 1981 Nitella sp. oospores increased in basin 1 and remains of floating-leaved taxa such as L. trisulca, Nymphaeaceae and Sparganium sp. increased in all basins (Fig. 4; Table 2).
For chironomids, multivariate trajectory and K-means analyses revealed five main time intervals (ESM4b) in which assemblages differed significantly corresponding to c. pre-1900–1910, 1911–1940, 1941–1955, 1956–1980 and 1981–2008 (Fig. 5). At c. pre-1900–1920 differences are mostly attributed to prevalence in basin 3 of Ablabesmyia spp., Cryptochironomus spp., Cladotanytarsus mancus, Dicrotendipes nervosus, Pseudochironomus spp., Tanytarsus lugens, Tanytarsus pallidicornis, Stempellina spp., Stilocladius and the diamesine Protanypus sp. (Fig. 5; Table 2). The second-time interval (1921–1940) was associated with a reduction or disappearance of most of these taxa in basin 3, the appearance in subsequent time interval (1941–1955) of Glyptotendipes pallens and, especially in basin 1, of D. nervosus, Endochironomus albipennis, Cricotopus intersectus, Cricotopus laricomalis and Psectrocladius sordidellus. After 1956 (the fourth-time interval), Procladius spp. increased in abundance, especially in basin 2, together with a general increase in numbers of E. albipennis (basins 1 and 2), and of both G. pallens and Polypedilum sordens. From 1981 to present most of these taxa generally increased in abundance and were similarly distributed across the three basins (Fig. 5; Table 2).
Multivariate trajectory and K-means analyses identified three time intervals in which mollusc assemblages differed significantly (ESM4c): c. pre-1900–1920, 1921–1950 and 1951–present. In the two earlier time intervals, most of the current taxa were absent and gastropods and the bivalves Pisidium spp. and Anodonta cignea L. (which produces glochidia larvae) occurred in very low abundances. Mollusc abundances showed a general increase in the 1950s (Fig. 6a; Table 2). The invasive bivalve, Dreissena polymorpha Pallas, first appeared in the 1990s consistent with its known recent arrival in the ULE system (Rosell et al. 1998).
No space–time interaction was revealed in the analyses of bryozoan statoblasts and daphnid ephippia (Table 1). Independent tests on the spatial factor confirmed, however, that both bryozoan and daphnid remains were strongly spatially structured over time (P = 0.001 for both cases) (Table 1). Spatial patterns explained 64% of assemblage variation for bryozoans and 41% for daphnids. For bryozoans, Plumatella spp. were generally absent in basin 1 and Plumatella fruticosa Allman was abundant in basin 3 (Fig. 6b; Table 2). Likewise, Ceriodaphnia spp. occurred abundantly throughout basin 1, while Daphnia spp. dominated in basins 2 and 3 (Fig. 6c; Table 2). For bryozoans, K-means analysis detected four time intervals in which assemblages differed significantly (ESM4d) at c. pre-1900–1940, 1941–1955, 1956–1980 and 1981–present. These temporal changes occurred mostly in basins 2 and 3, where the first-time interval was typified by dominance of P. fruticosa in basin 3. At the second-time interval (1941–1955), P. fruticosa abundances declined while Plumatella spp., increased. The third-time period (1956–1980) was characterised by an increase in C. mucedo and Plumatella spp. as was the final post-1981 interval (Fig. 6b; Table 2). K-means analysis for daphnid ephippia resulted in three time intervals in which assemblages differed significantly (ESM4e) at c. pre-1900–1955, 1956–1990 and 1991–present. The first early time interval was typified by dominance of Ceriodaphnia spp. (basin 1), followed by a second-time period characterized by increases in Daphnia spp. and minor reductions in Ceriodaphnia spp. (Fig. 6c; Table 2). The final time period was characterised by an increase in Daphnia spp. and Ceriodaphnia spp. in basins 2 and 3.
The comparison of K-means analyses across the five biological groups revealed three relatively synchronous time intervals of assemblage variation across the five groups (ESM4) at pre-1900s–1940, 1941–1980, and 1981–1990. The first early time interval corresponded with synchronous changes in plant, chironomid and bryozoan remains, whereas synchronous changes characterised all five groups during the second and most recent time intervals.
Discussion
Contemporary distributions of macrophytes
Our analyses have revealed significant spatial heterogeneity in macrophyte assemblages across the three basins. Despite a general prevalence of the same three or four species, the results highlighted macrophyte heterogeneity across basins both in terms of species turnover and variation in species relative abundances. Furthermore, our data revealed associations between macrophyte assemblage variation and heterogeneity in water-depth (ESM1). This indicates that intra-basin variation may also create other complex, non-linear effects on macrophyte spatial patterns (e.g. greater niche availability with different depth profiles) (Anderson et al. 2006).
The detected strong relationship between water depth and spatial variation in macrophyte community structure likely reflects light limitation. This is supported by the peaty-brown colour of Castle Lough water and a general prevalence of macrophyte species with floating leaves (e.g. water lilies, S. emersum and S. sagittifolia) and high shade tolerance (e.g. E. canadensis) (Spence and Chrystal 1970; Fig. 2a). A widespread shading effect by water lilies (N. lutea and N. alba-both recently growing in the lake and greatly represented by sclereids in the paleo-data) likely also contributes to reducing the abundances of other submerged species such as M. verticillatum, U. vulgaris and C. globularis in the contemporary lake (Sculthorpe 1967). Other correlated abiotic factors may also influence macrophyte distributions. For example, basin 1 is relatively well protected by reedswamp and floating-leaved species, while basins 2 and 3 are more exposed to wind and wave action (Fig. 1). Exposure may reduce plant stands through fragmentation and uprooting (especially in soft organic-rich sediments) and prevent the establishment of M. verticillatum, broad-leaved species (e.g. P. praelongus and P. lucens; Barko and Smart 1986; Riis et al. 2001) and short and/or non-rooted species (e.g. S. aloides; Smolders et al. 2003), which require sheltered habitats, a pattern consistent with our data (Fig. 2a). Increased sediment transport with wave-movement can also influence propagule transport and bury established plant stands (Keddy and Reznicek 1986). Differences in nutrient concentrations between basins due to differential external loadings [e.g. proximity to inflow (basin 1), pine woodland (basin 2), and the outflow (basin 3)] are also potential co-associated factors influencing macrophyte spatial distributions (Carpenter and Titus 1984).
In conjunction with water depth, plant seasonality and dispersal may also contribute to macrophyte spatial distributions (Carpenter and Titus 1984; Sayer et al. 2010a). However, a strong concordance of our palaeo-data with observed macrophyte spatial patterns suggests that the latter are informative, robust and not unduly influenced by seasonality (Figs. 2a, 5). In contrast to the restricted and patchy distributions of C. globularis, M. verticillatum, and P. praelongus in the present-day, the palaeo-data indicate that these species were present across the whole lake in the past. It can be inferred, therefore, that dispersal is probably sufficient to enable all species to reach all lake basins, but species sorting has occurred over time linked to between-basin variation in environmental forcing (Leibold et al. 2004).
The above considerations demonstrate that there may well be other drivers of macrophyte assemblage structure in Castle Lough besides water depth that we did not specifically measure. These drivers may act at similar or dissimilar spatial scales and may also vary over time. In general, the detection of various drivers of assemblage structure will be dependent on experimental design, the measurement of relevant conditions at appropriate scales and times, the ability to conduct statistical analyses focusing on measured drivers, and identifying or discounting other potential drivers by evidence-based argument.
Drivers of temporal changes in community assembly
The palaeo-record suggests that the basins have retained similar depth profiles over time. Temporal patterns in distributions of daphnid ephippia support this inference. For example, Ceriodaphnia species are commonly reported to prefer macrophyte-covered shallow waters (Lauridsen et al. 1996) and were mostly found in basin 1, the shallowest basin (Fig. 6c; Table 2). On the other hand, some Daphnia species prefer non-macrophyte dominated open water (Lauridsen and Lodge 1996; Davidson et al. 2010) and occurred throughout time in greater abundances in the less vegetated deeper waters offered by basins 2 and 3 (Fig. 6c; Table 2). Similarly, the profundal-associated chironomid taxa Microchironomous spp. and C. anthracinus exhibited greatest abundances in basins 2 and 3 (Fig. 5; Table 2). These strong inter-basin differences suggest that as in the current day, water depth variation has been an important long-term driver of spatial ecology in Castle Lough.
Significant space–time interactions for macrophyte, chironomid and mollusc assemblages and differing temporal trends in bryozoan and daphnid assemblages between basins, suggest that the distributions of these groups have been modified across basins over time in response to conditions unrelated to water depth. The synchronous temporal changes in assemblages of all five groups (ESM4) and species characteristic of each time-interval (detected by the IndVal analysis; Table 2), suggest compositional changes reflecting a previously inferred acceleration of eutrophication after around 1900 (Battarbee 1986). Before 1930, the lake was characterised by taxa associated with low to intermediate nutrient conditions including the macrophytes N. flexilis, I. lacustris, and bryophytes (Carpenter and Titus 1984; Sand-Jensen et al. 2008), the chironomids Stempellina spp., Pseudochironomus spp., Orthocladius consobrinus and Protanypus spp. (Pinder and Reiss 1983; Brodersen and Lindegaard 1999) and the bryozoan P. fruticosa (Økland and Økland 2002) (Table 2). Post-1930 macrophytes converged spatially towards communities associated with mesotrophic–eutrophic conditions, exemplified by increased abundances of Myriophyllum spp. and P. praelongus/lucens (Sand-Jensen et al. 2008; Table 2). Subsequent dominance of floating-leaved taxa (L. trisulca, water-lilies and Sparganium sp.), declines in the macrophytes I. lacustris and N. flexilis, increases in Plumatella spp. (Hartikainen et al. 2009) and concomitant reductions in chironomids intolerant of nutrient-rich conditions (e.g. Stempellina spp., Pseudochironomus spp., O. consobrinus and Protanypus spp.) in recent times (post 1981) collectively suggest further development of eutrophication and its effects (Table 2).
Our data indicate that spatial and temporal dynamics of invertebrate assemblages since 1931 are to a large extent linked to those of macrophytes (Table 2). Indeed, many chironomids depend on macrophytes for food, with some (e.g. Microtendipes and Polypedilum species) feeding on epiphytic algae (Moller Pillot 2009), and others relying on living (e.g. Cricotopus species) or decomposing (e.g. Stenochironomus species) plants as a source of food or substratum (Vallenduuk and Moller Pillot 2007; Moller Pillot 2013). Direct associations between macrophyte and chironomid abundances have been demonstrated previously in both contemporary (Langdon et al. 2010) and palaeolimnological studies (Brodersen et al. 2001). Our analysis suggests a particularly close association between Myriophyllum spp. and the majority of Cricotopus morphotypes in basin 1 (Figs. 4, 5), perhaps reflecting the large surface area provided by finely dissected Myriophyllum leaves that can in turn support dense epiphytic algal communities (Sculthorpe 1967). Similarly, post 1981 increases abundances of chironomids (E. albipennis, G. barbipes and P. nubeculosum) and molluscs (Pisidium spp. and snails) coincident with the expansion of floating-leaved plant taxa (e.g. water lilies) could reflect increased availability of epiphytic food (Sculthorpe 1967) (Table 2).
It should be noted that K-means analysis did not detect the apparently close links between macrophyte and invertebrate abundances after the early stages of eutrophication in the 1930s as described above. Instead, K-means analysis indicated that macrophyte assemblage variation remained stable until the 1980s, while invertebrate assemblages varied in keeping with a proposed acceleration of nutrient-enrichment in ULE after 1955 (Battarbee 1986). This apparent temporal disparity between macrophyte and invertebrate dynamics could be attributed to a lack of statistical power in the macrophyte data (Legendre et al. 2010). Between 1955 and 1980, there were indeed strong increases in abundances of Myriophyllum spp. and of the chironomid Cricotopus spp. but mainly in core NCAS1 (basin 1) (Figs. 4, 5). This suggests that an important phase of change probably occurred earlier and was undetected in the study.
Subsequent synchronous assemblage changes detected by K-means analysis across all biological groups post-1981 suggest a distinctive phase in the ecology of the ULE system. One possible explanation is the introduction of zebra mussels after the mid-1990s (Fig. 6b). Zebra mussels are well known to alter lake environments and food webs by reducing phytoplankton and hence grazer abundances and by stimulating macrophyte growth due to increases in water transparency (Higgins and Vander Zanden 2010). Our data provide little support for such zebra mussel effects, however. For example, grazer abundances (e.g. Daphnia spp.) increased during the same period, as did abundances of taxa tolerant of eutrophic conditions (e.g. the macrophytes L. trisulca, N. lutea, P. berchtoldii and P. pusillus) (Table 2). Similarly, ordination plots reveal convergence of macrophyte and chironomid assemblages to associations of eutrophication-tolerant taxa (Fig. 3). Glochidia larvae of Anodonta also increased during this time period. Anodonta competes directly with zebra mussels for food, and populations commonly diminish after the establishment of zebra mussels (Higgins and Vander Zanden 2010). Thus, all evidence points to negligible zebra mussel impacts in Castle Lough so far.
As a caveat, we note that constraints in palaeo-data and radiometric analyses should be considered when conducting plant macrofossil studies (Birks 2014). For example, some species (e.g. E. canadensis and U. vulgaris) are poorly preserved in sediments (Davis 1985; Davidson et al. 2005). However, surface sediment samples have also been shown to faithfully record the main spatial patterns in plant assemblages (Zhao et al. 2006; Clarke et al. 2014; Levi et al. 2014). Furthermore, the macrofossil record can over- or under-represent certain macrophyte taxa (Birks 2001; Davidson et al. 2005). For example, C. globularis, Nitella spp, and N. flexilis, produce large numbers of oospores/seeds, while Potamogeton species produce low numbers of seeds. Such disparity in propagule production can lead to misinterpretations of true plant abundances (Zhao et al. 2006). Our use of a semi-quantitative abundance scale (Odgaard and Rasmussen 2001) for the plant macrofossil data helps to reduce such effects. Moreover, similar to previous plant macrofossil studies in lakes (Davidson et al. 2005; Zhao et al. 2006; Salgado et al. 2010; Clarke et al. 2014; Levi et al. 2014), our palaeo-data capture most of the contemporary macrophyte community and faithfully reflect current spatial distributions and differences between basin 1 and basins 2 and 3 (Figs. 2a, 3; Table 2). Finally, our study is based on characterising relative abundances over space and time within the same localities. Constraints therefore are not expected to substantially influence our inferences.
Implications for long-term changes in ecological processes
Our data suggest a trend of spatial convergence of macrophytes and co-occurring invertebrate communities post-1981 (Fig. 3; Table 2). This suggests that, as eutrophication advances, the influence of water depth variation on assemblage heterogeneity is gradually eroded, and that ultimately a limited set of eutrophication-tolerant species will become homogeneously distributed across the entire lake. Previous evidence for eutrophication effects on macrophytes includes reductions in diversity and changes in seasonality (Ayres et al. 2008; Sayer et al. 2010a), which ultimately result in loss of resilience (Sayer et al. 2010a, b). However, prior to our study little was known regarding changes in macrophyte spatial distributions in response to long-term nutrient-enrichment processes, nor of associated invertebrate taxa. Our data revealed minimal macrophyte species turnover over time, but substantial changes in macrophyte relative abundances across sites. This suggests that reduced spatial variation in macrophyte and invertebrate relative abundances may reflect an ecological phase that precedes major changes in species richness and turnover (Arts 2002; Anderson et al. 2006). Such spatial homogenisation of relative abundances may contribute to the loss of resilience associated with eutrophication (Donohue et al. 2009) and warrants examination in future studies.
Conclusions
Our study provides novel insights into how environmental influences have varied over time to structure within-lake assemblages. We have analysed contemporary ecological and palaeoecological data to collectively infer long-term changes in the pathways and processes that underlie eutrophication effects in shallow lakes. The contemporary data allow us to assess how macrophyte assemblages vary in composition and heterogeneity according to basin-specific factors (e.g. variation in water depth). In turn, the palaeoecological data enable us to infer basin-specific impacts of and susceptibilities to eutrophication exhibited by macrophytes and invertebrates.
Our results indicate that variability in water depth promotes contemporary assemblage variation amongst Castle Lough’s basins, thus stimulating within-lake macrophyte and invertebrate assemblage heterogeneity and thus higher lake biodiversity (Anderson et al. 2006). These insights are in keeping with growing evidence for the importance of spatial heterogeneity in structuring local populations and assemblages and the concomitant implications of scaling up from small-scale studies (Ford et al. 2016). Our study also strongly suggests that eutrophication has acted as a homogenising agent of macrophyte and co-occurring invertebrate diversities and abundances over time at the whole-lake scale. Such homogenisation of communities may have profound implications for shallow lake ecosystem functioning including reductions in community resistance and resilience due to alterations in e.g. productivity and biomass production, variations in intra- and interspecific competition and increased vulnerability to species invasions (Hillebrand et al. 2008).
Currently, Castle Lough is in a mesotrophic–eutrophic condition, presenting high variation in assemblages between basins and relatively high species richness. Recently it has been inhabited by species regarded as sensitive to eutrophication and rare in Northern Ireland (e.g. N. flexilis and broad-leaved Potamogeton taxa). Unfortunately, hypertrophic states now characterise many water bodies of the ULE system because of nutrient loading deriving from increasing dairy farming and urban development (Gibson et al. 1995). If nutrient inputs continue, it is likely that Castle Lough will soon be characterised by spatially homogenous assemblages comprising a few tolerant taxa and the conservation value of the lake will be greatly diminished.
References
Aldridge DC, Horne DC (1998) Fossil glocchidia (Bivalvia, Unionidae): identification and value in palaeoenvironmental reconstructions. J Micropalaeontol 17:179–182
Allen MR, Vandyke JN, Caceres CE (2011) Metacommunity assembly and sorting in newly formed lake communities. Ecology 92:269–275
Anderson M (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson M (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson M, Ellingsen K, McArdle B (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210 Pb to the sediment. Catena 5:1–8
Appleby PG, Nolan PJ, Gifford DW, Godfrey MJ, Oldfield F, Anderson NJ, Battarbee RW (1986) 210Pb dating by low background gamma counting. Hydrobiologia 141:21–27
Arts GH (2002) Deterioration of Atlantic soft water macrophyte communities by acidification, eutrophication and alkalinisation. Aquat Bot 31:373–393
Ayres KR, Sayer CD, Skeate ER, Perrow MR (2008) Palaeolimnology as a tool to inform shallow lake management: an example from Upton Great Broad, Norfolk, UK. Biodivers Conserv 17:2153–2168
Barko JW, Smart RM (1986) Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology 67:1328–1340
Barrat-Segretain MH (1996) Strategies of reproduction, dispersion, and competition in river plants: a review. Vegetatio 123:13–37
Battarbee R (1986) The Eutrophication of Lough Erne inferred from changes in the diatom assemblages of 210Pb- and 37Cs dated sediment cores. Proc R Ir Acad 86B:141–168
Birks HH (2001) Plant macrofossils. In: Smol JP, Birks HJB, Lasts WM (eds) Tracking environmental change using lake sediments, Terrestrial, algal and siliceous indicators, vol 3. Kluwer, Dordecht, pp 49–74
Birks HJ (2014) Challenges in the presentation and analysis of plant-macrofossil stratigraphical data. Veg Hist Archaeobot 23:309–330
Brodersen K, Lindegaard C (1999) Classification, assessment and trophic reconstruction of Danish lakes using chironomids. Freshw Biol 42:143–157
Brodersen KP, Odgaard BV, Vestergaard O, Anderson NJ (2001) Chironomid stratigraphy in the shallow and eutrophic Lake Søbygaard, Denmark: chironomid-macrophyte co-occurrence. Freshw Biol 46:253–267
Brooks SJ, Heiri O, Langdon PG (2007) The identification and use of palaearctic chironomidae larvae in palaeoecology. Technical guide No. 10. Quaternary Research Association, London
Canfield DE Jr, Shireman J, Colle DE, Haller WT, Watkins CE II, Maceina MJ (1984) Prediction of chlorophyll a concentrations in Florida lakes: importance of aquatic macrophytes. Can J Fish Aquat Sci 41:497–501
Carpenter S, Titus JE (1984) Composition and spatial heterogeneity of submersed vegetation in a softwater lake in Wisconsin. Plant Ecol 57:153–165
Clarke GH, Sayer CD, Turner S, Salgado J, Meis S, Patmore IR, Zhao Y (2014) Representation of aquatic vegetation change by plant macrofossils in a small and shallow freshwater lake. Veg Hist Archaeobot 23:265–276
Cummins RH (1994) Taphonomic processes in modern freshwater molluscan death assemblages: implications for the freshwater fossil record. Palaeogeogr Palaeoclimatol Palaeoecol 108:55–73
Davidson TA, Sayer CD, Bennion H, David C, Rose N, Wade M (2005) A 250 year comparison of historical, macrofossil and pollen records of aquatic plants in a shallow lake. Freshw Biol 50:1671–1686
Davidson TA, Sayer CD, Langdon PG, Burgess A, Jackson M (2010) Inferring past zooplanktivorous fish and macrophyte density in a shallow lake: application of a new regression tree model. Freshw Biol 55:584–599
Davis FW (1985) Historical changes in submerged macrophyte communities of upper Chesapeake Bay. Ecology 66:981–993
Donohue I, Jackson AL, Pusch MT, Irvine K (2009) Nutrient-enrichment homogenizes lake benthic assemblages at local and regional scales. Ecology 90:3470–3477
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69:57–68
Ford JR, Shima JS, Swearer SE (2016) Interactive effects of shelter and conspecific density shape mortality, growth, and condition in juvenile reef fish. Ecology 97:1373–1380
Fukami T, Morin P (2003) Productivity-biodiversity relationships depend on the history of community assembly. Nature 424:423–426
Gibson C, Wu Y, Smith S, Wolfe-Murphy S (1995) Synoptic limnology of a diverse geological region: catchment and water chemistry. Hydrobiologia 306:213–227
Hartikainen H, Johnes P, Moncrieff C, Okamura B (2009) Bryozoan populations reflect nutrient-enrichment and productivity gradients in rivers. Freshw Biol 54:2320–2334
Higgins SN, Vander Zanden MJ (2010) What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr 80:179–196
Hillebrand H, Bennett DM, Cadotte MW (2008) Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89:1510–1520
Jeppesen E, Sondergaard M, Sondergaard M, Christofferson K (eds) (2012) The structuring role of submerged macrophytes in lakes. Springer Science and Business Media, Dordrecht
Keddy PA, Reznicek AA (1986) Great Lakes vegetation dynamics: the role of fluctuating water levels and buried seeds. J Great Lakes Res 12:25–36
Korhonen JJ, Soininen J, Hillebrand H (2010) A quantitative analysis of temporal turnover in aquatic species assemblages across ecosystems. Ecology 91:508–517
Langdon PG, Ruiz Z, Wynne S, Sayer CD, Davidson TA (2010) Ecological influences on larval chironomid communities in shallow lakes: implications for palaeolimnological interpretations. Freshw Biol 55:531–545
Lauridsen T, Lodge D (1996) Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnol Oceanogr 41:794–798
Lauridsen T, Pedersen LJ, Jeppesen E, Sønergaard M (1996) The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. J Plankton Res 18:2283–2294
Legendre P, Cáceres MD, Borcard D (2010) Community surveys through space and time: testing the space–time interaction in the absence of replication. Ecology 91:262–272
Leibold M, Norberg J (2004) Biodiversity in metacommunities: Plankton as complex adaptive systems? Limnol Oceanogr 49:1278–1289
Leibold M, Holyoak M, Mouquet N, Amarasekare P, Chase J, Hoopes M, Holt R, Shurin J, Law R, Tilman D (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Levi EE, Çakıroğlu Aİ, Bucak T, Odgaard B-V, Davidson TA, Jeppesen E, Beklioğlu M (2014) Similarity between contemporary vegetation and plant remains in the sediment surface in Mediterranean lakes. Freshw Biol 59:724–736
Madgwick G, Emson D, Sayer CD, Willby N, Rose NL, Jackson MJ, Kelly A (2011) Centennial-scale changes to the aquatic vegetation structure of a shallow eutrophic lake and implications for restoration. Freshw Biol 56:2620–2636
Mathers R, De Carlos M, Crowley K, Teangana DÓ (2002) A review of the potential effect of Irish hydroelectric installations on Atlantic salmon (Salmo salar L.) populations, with Particular Reference to the River Erne. Proc R Ir Acad 102B:69–79
Moller Pillot HKM (2009) Chironomidae larvae: biology and ecology of the chironomini. KNNV Publishing, Zeist
Moller Pillot HKM (2013) Chironomidae larvae: biology and ecology of the aquatic orthocladiinae. KNNV Publishing, Zeist
Odgaard B, Rasmussen P (2001) The occurrence of egg-cocoons of the leech Piscicola geometra (L.) in recent sediments and their relationship with the remains of submerged macrophytes. Arch Hydrobiol 52:671–686
Økland KA, Økland J (2002) Freshwater bryozoans (Bryozoa) of Norway III: distribution and ecology of Plumatella fruticosa. Hydrobiologia 479:11–22
Patmore IR, Sayer CD, Goldsmith B, Davidson TA, Rawcliffe R, Salgado J (2014) Big Ben: a new wide-bore piston corer for multi-proxy palaeolimnology. J Paleolimnol 51:79–86
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pinder LCV, Reiss F (1983) The larvae of Chironominae (Diptera: Chironomidae) of the Holartic region. Keys and diagnoses. Entomol Scand Suppl 19:293–435
Price J (1890) Lough Erne drainage. P I Civil Eng C1:73–94
R Core Development Team (2009) R 2.9.2. R project for statistical computing. Vienna, Austria. www.r-project.com
Rasmussen P, Anderson NJ (2005) Natural and anthropogenic forcing of aquatic macrophyte development in a shallow Danish lake during the last 7000 years. J Biogeogr 32:1993–2005
Riis T, Sand-Jensen K, Larsen SE (2001) Plant distribution and abundance in relation to physical conditions and location within Danish stream systems. Hydrobiologia 448:217–228
Rosell RS, Maguire CM, McCarthy TK (1998) First reported settlement of zebra mussels Dreissena polymorpha in the Erne System, Co. Fermanagh, Northern Ireland. Biol Environ 98:191–193
Salgado J, Sayer CD, Carvalho L, Davidson TA, Gunn I (2010) Assessing aquatic macrophyte community change through the integration of palaeolimnological and historical data at Loch Leven, Scotland. J Paleolimnol 43:191–204
Sand-Jensen K, Pedersen NL, Thorsgaard I, Moeslund B, Borum J, Brodersen KP (2008) 100 years of vegetation decline and recovery in Lake Fure, Denmark. J Ecol 96:260–271
Sayer CD, Davidson TA, Jones JI (2010a) Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: a eutrophication-driven pathway from plants to plankton? Freshw Biol 55:500–513
Sayer CD, Burgess AM, Kari K, Davidson TA, Peglar S, Yang H, Rose N (2010b) Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: implications for the stability of macrophyte-dominance. Freshw Biol 55:565–583
Sculthorpe CD (1967) The biology of aquatic plants. Edward Arnold Ltd., London
Smolders A, Lamers L, Hartog C, Roelofs J (2003) Mechanisms involved in the decline of Stratiotes aloides L. in the Netherlands: sulphate as a key variable. Hydrobiologia 506:603–610
Spence D (1967) Factors controlling the distribution of freshwater macrophytes with particular reference to the lochs of Scotland. J Ecol 55:147–170
Spence DHN, Chrystal J (1970) Photosynthesis and zonation of freshwater macrophytes. New Phytol 69:217–227
Urban MC, De Meester L (2009) Community monopolization: local adaptation enhances priority effects in an evolving metacommunity. Proc R Soc Biol Sci Ser B 276:4129–4138
Vallenduuk HJ, Moller Pillot HKM (2007) Chironomidae larvae: general ecology and tanypodinae. KNNV Publishing, Zeist
Wood TS, Okamura B (2005) A new key to the freshwater bryozoans of Britain, Ireland and continental Europe, with notes on their ecology. The Freshwater Biological Association, Ambleside
Zhao Y, Sayer CD, Birks HH, Hughes M, Peglar S (2006) Spatial representation of aquatic vegetation by macrofossils and pollen in a small and shallow lake. J Paleolimnol 35:335–350
Acknowledgements
We thank the Department of Zoology of The Natural History Museum, London for funding this work as part of J. Salgado’s Ph.D. A Hugh Cary Gilson Memorial Award from the Freshwater Biological Association provided support for fieldwork. We also thank the Departamento Administrativo de Ciencia, Tecnología e Innovación-COLCINECIAS for supporting J. Salgado under the postdoctoral program “Es tiempo de volver”. T.A. Davidson’s contribution was supported by CIRCE funding under the AU ideas programme. We especially thank the Castle Lough landowners for site access and hospitality, G. Simpson for statistical analysis advice, I. Jones and N. Willby for constructive suggestions and P. Bexell and L. Petetti for fieldwork assistance. We also thank the EU FP7 Project Biofresh (Biodiversity of Freshwater Ecosystems: Status, Trends, Pressures, and Conservation Priorities) Contract No. 226874 for financial support for sediment core dating analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10933_2017_9950_MOESM1_ESM.eps
Figure ESM1. Radiometric chronologies and sedimentation rates for cores (a) NCAS1; (b) NCAS2; and (c) NCAS3 (EPS 723 kb)
10933_2017_9950_MOESM2_ESM.eps
Figure ESM2. Boxplot of (a) depth variation between basins; (b) Macrophyte average distance to centroid group and perMANOVA (F = 13.414, P = 0.001) and HMD (F = 7.87, P = 0.001) results; (c) Depth distance to centroid group and perMANOVA (F = 137.84, P = 0.001) and HMD (F = 93.155, P < 0.001) results (EPS 329 kb)
10933_2017_9950_MOESM3_ESM.eps
Figure ESM3. Logistic regressions on presence/absence data of macrophyte species sensitive to eutrophication across the observed depth profiles. (a) Chara globularis; (b) Myriophyllum verticillatum; (c) Stratiotes aloides (EPS 1072 kb)
10933_2017_9950_MOESM4_ESM.eps
Figure ESM4. Spatiotemporal maps showing K-means partition of (a) Plant macrofossils, (b) Chironomids; (c) Molluscs; (d) Bryozoans; and (e) Daphnid assemblages in the cores NCAS1, NCAS2 and NCAS3. Simple structure index (ssi) is indicated on the right-hand side of each map. Selected number of groups by ssi is indicated with a bold black circle (EPS 5041 kb)
Rights and permissions
About this article
Cite this article
Salgado, J., Sayer, C.D., Brooks, S.J. et al. Eutrophication erodes inter-basin variation in macrophytes and co-occurring invertebrates in a shallow lake: combining ecology and palaeoecology. J Paleolimnol 60, 311–328 (2018). https://doi.org/10.1007/s10933-017-9950-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-017-9950-6