Abstract
β-Glucosidase (β-d-glucoside glucohydrolase, EC 3.2.1.21) is a catalytic enzyme present in both prokaryotes and eukaryotes that selectively catalyzes either the linkage between two glycone residues or between glycone and aryl or alkyl aglycone residue. Growing edible mushrooms in the soil with increased cellulose content can lead to the production of glucose, which is a process dependent on β-glucosidase. In this study, β-glucosidase was isolated from Agaricus bisporus (white button mushroom) using ammonium sulfate precipitation and hydrophobic interaction chromatography, giving 10.12-fold purification. Biochemical properties of the enzyme were investigated and complete characterization was performed. The enzyme is a dimer with two subunits of approximately 46 and 62 kDa. Optimum pH for the enzyme is 4.0, while the optimum temperature is 55 °C. The enzyme was found to be exceptionally thermostable. The most suitable commercial substrate for this enzyme is p-NPGlu with Km and Vmax values of 1.751 mM and 833 U/mg, respectively. Enzyme was inhibited in a competitive manner by both glucose and δ-gluconolactone with IC50 values of 19.185 and 0.39 mM, respectively and Ki values of 9.402 mM and 7.2 µM, respectively. Heavy metal ions that were found to inhibit β-glucosidase activity are I−, Zn2+, Fe3+, Ag+, and Cu2+. This is the first study giving complete biochemical characterization of A. bisporus β-glucosidase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
β-glucosidase (β-d-glucoside glucohydrolase, EC 3.2.1.21) is a catalytic enzyme present in both prokaryotes and eukaryotes. It selectively catalyzes either the linkage between two glycone residues or between glycone and aryl or alkyl aglycone residues. In humans, it is called glucocerebrosidase and its deficiency leads to the development of Gaucher’s disease. In plants, β-glucosidase plays different roles, including chemical defense against pests, lignification, regulation of phytohormones, improving the food quality, flavor enhancement, and beverage quality improvement [1–3]. Fungal β-glucosidases are important for pathogenicity of phytopathogenic fungi since they are able to hydrolyze saponin, an anti-fungal molecule secreted by many plants as part of plant defense system, and to successfully overcome plant immunity in that way [4]. When it comes to animals, β-glucosidase was most widely studied in insects, where it is usually found in the intestinal tract [5–7].
Apart from being important for physiological processes in all classes of living organisms, β-glucosidase is an industrially and economically important enzyme, being used in food, feed, textile, detergents, pharmaceutical, and bioethanol conversion industries [1, 2, 8]. An important application of fungal β-glucosidases is the production of glucose, a food source and an important metabolite, by the edible mushrooms grown in the forest soil with increased cellulose content [9]. The process is carried out by secreting fungal enzymes from hyphal tips into the soil. There, cellulose gets digested into glucose, which is later used as a fungal metabolite and can also be used as an energy source through bioethanol conversion process [10].
Cellulose is the most abundant renewable biomaterial in the biosphere. In cellulose hydrolysis to glucose, β-glucosidase acts along with other two enzymes, namely endo-1,4-β-glucanase (EC 3.1.2.4) and cellobiohydrolase (or exoglucanase; EC 3.1.2.91). In this process, fungal β-glucosidase converts cellobiose to glucose, but also enhances glucose yield in the process, since cellobiose alone acts as an inhibitor of other two enzymes. Fungal β-glucosidases are especially interesting for industry since they have high yields, high activity, and useful enzymatic properties, such as thermal stability [11].
Agaricus bisporus, also known as the white button mushroom, is one of the most widely used edible mushrooms in human nutrition that is being cultivated for about 350 years [10, 12]. This mushroom is considered a healthy food since it contains high levels of polyphenols, vitamins, minerals, polysaccharides, and especially proteins [13]. Its natural habitat is leaf and needle litter in the forests of Western, Central, and Southern Europe, American continent, and Northern Africa, where it acts as a secondary decomposer. Through sequencing projects, it was shown that A. bisporus genome contains genes for cellulose degradation [10].
In this paper, β-glucosidase was isolated and purified from A. bisporus flesh using ammonium sulfate precipitation and hydrophobic interaction chromatography. Complete biochemical characterization of the purified enzyme was carried out in order to compare it to the previously characterized fungal and yeast β-glucosidases and to determine whether this enzyme can be utilized in industry.
2 Materials and Methods
2.1 Chemicals
All chemicals used in this research were of the highest available grade and were ordered from Sigma-Aldrich (St. Louis, MO, USA), except for glycine which was manufactured by Merck KGaA (Darmstadt, Germany) and protein molecular weight marker which was supplied by Thermo Scientific (Waltham, MA, USA).
2.2 Obtaining Crude Extract
Fresh white button mushrooms (A. bisporus) were obtained in the local market in Balıkesir, Turkey. In order to isolate the enzyme, 25 g of mushrooms was mixed with 100 ml of Tris-HCl buffer (pH 7.5) for 2 min using kitchen-type blender. Obtained solution was filtered using double sterile cheesecloth and the filtered aliquot was used as a crude extract.
2.3 Enzyme Purification
All purification steps were performed at 4 °C unless otherwise stated. Crude extract was treated with solid ammonium sulfate to obtain 40–80 % saturation fraction, as that fraction was identified as optimal for A. bisporus β-glucosidase precipitation (data not shown). After dissolving the salt, samples were centrifuged at 15,000 rpm for 30 min. The precipitated material was dissolved in 50 mM sodium phosphate buffer (pH 6.8) and final saline concentration was adjusted to 1 M with ammonium sulfate.
β-glucosidase was purified from the dissolved pellet by using hydrophobic interaction chromatography column through previously prepared Sepharose-4B-l-tyrosine-1-napthylamine gel [1]. Column was pre-equilibrated with 50 mM sodium phosphate buffer (pH 6.8) with 1.5 M ammonium sulfate. Samples were loaded and 2 ml fractions were collected in Eppendorf tubes at a flow rate of 200 ml/h. Enzyme was eluted using a linear gradient of 1.5-0 M ammonium sulfate. The tubes with the highest activity were combined together and used as a purified enzyme for subsequent characterization experiments.
2.4 Enzyme Activity Assay
Enzyme activity was measured in order to determine the activity of β-glucosidase when reacting against its substrates. Samples were assayed in a 96-well plate by mixing 70 µl of 5 mM substrate solution in 50 mM sodium acetate buffer (pH 5.5) and 70 µl of the purified enzyme. After incubation at 37 °C for 30 min, reactions were terminated by the addition of 70 µl of 0.5 M sodium carbonate solution and yellow coloration that arose as a consequence of p-/o-NP (p-/o-nitrophenol) liberation was recorded at 405 nm. Enzyme activity was expressed as the µmol of substrate converted into the reaction product per minute of time (1 U).
2.5 Protein Amount and Concentration Determination
Protein amount was determined at 280 nm by putting 200 µl of pure enzyme into a quartz 96-well plate reader or using 1 ml of pure enzyme in quartz cuvettes.
Modified Lowry protocol for protein concentration determination was also utilized [14]. Bovine serum albumin (BSA) solution was used as a protein standard for the construction of a standard curve.
2.6 Enzyme Kinetics
Enzyme kinetics of β-glucosidase was studied on two different substrates, namely 4-nitrophenyl beta-d-glucopyranoside (p-NPGlu) and 2-nitrophenyl beta-d-glucopyranoside (o-NPGlu) and Km and Vmax values were determined. Moreover, relative enzyme activity was determined against two other substrates, 4-nitrophenyl beta-d-galactopyranoside (p-NPGal) and 2-nitrophenyl beta-d-galactopyranoside (o-NPGal). For all these experiments, substrate was present in final concentrations in the range from 0.36 to 2.5 mM.
2.7 Optimum pH Determination
In order to determine optimum pH, the enzyme was incubated with 5 mM p-NPGlu solution in buffers of different pH values: sodium acetate buffer (pH 3.0–6.0), phosphate buffer (pH 6.0–8.5), and glycine-NaOH buffer (pH 8.5–12.0).
2.8 Temperature Optimum Determination and Thermal Denaturation Study
In order to determine temperature optimum, the purified enzyme was incubated with 5 mM p-NPGlu at seven different temperatures in the range from 25 to 85 °C for 30 min.
Thermal denaturation of the enzyme was investigated at six different temperatures in the range from 25 to 75 °C. Purified enzyme was incubated for 2 h at all temperatures, except for 75 °C, where incubation was terminated after 1 h.
2.9 Inhibition Studies
Inhibition studies were performed in order to find Ki and IC50 values, as well as inhibition types, of glucose and δ-gluconolactone (d-glucono-1,5-lactone). Double reciprocal Lineweaver–Burk plot was used to calculate these parameters. Also, relative activity of β-glucosidase in the presence of variable volumes of 0.005 M solutions of six heavy metal ions was determined, namely I−, Zn2+, Fe3+, Ag+, Cu2+, and Pb2+. Relative activity was determined as a %, where 100 % activity corresponds to the enzymatic activity with no inhibitor present.
2.10 Polyacrylamide Gel Electrophoresis
In order to determine molecular weight of the enzyme, SDS-PAGE and native PAGE were performed, both using the Mini-PROTEAN Tetra Cell system (Bio-Rad Laboratories, Hercules, CA, USA). SDS-PAGE was performed on 10 % separating gel and 3 % stacking gel. Gel was stained using Coomassie Brilliant Blue G-250 solution and de-stained using standard methods.
Enzyme activity was detected on 8 % resolving and 3 % separating native gels. Gel was incubated on a rotating platform in 50 mM sodium acetate buffer (pH 5.5) for 45 min in three buffer changes and in a 0.1 % 4-MUG solution for 30 min at 45 °C. Enzyme activity band was photographed under the UV light.
3 Results
In order to purify β-glucosidase from mushroom flesh, three steps were performed: obtaining crude extract, ammonium sulfate precipitation of proteins (that is, protein salting-out), and hydrophobic interaction chromatography. The efficiency of this process can be represented through the purification fold and percent yield (Table 1).
The results of hydrophobic interaction chromatography are presented in the figure below (Fig. 1). It can be observed that the tubes 20–35 gave the most active pure enzyme, as well as the highest enzyme amount, so they were used as the purified enzyme for subsequent characterization steps. Enzyme activity and amount were determined at 405 and 280 nm, respectively.
The results of hydrophobic interaction chromatography reveal the highest enzyme activity and the highest enzyme amount in the same tubes (tube numbers 20–35). Peaks in both enzyme activity and enzyme amount were observed after the gradual decrease of salt concentration in sodium phosphate buffer started. Enzyme activity was determined at 405 nm, while enzyme amount was determined at 280 nm
Enzyme kinetics was studied on two substrates, namely p-NPGlu and o-NPGlu. Km and Vmax values for p-NPGlu were 1.751 mM and 833 U/mg, respectively, while those values for o-NPGlu were 8.547 mM and 556 U/mg, respectively (Fig. 2a, b). These results imply that A. bisporus β-glucosidase has much higher affinity for p-NPGlu than it is the case with o-NPGlu.
Enzyme kinetics was studied with two β-glucosidase substrates: p-NPGlu (a) and o-NPGlu (b). Both substrates were present in concentrations from 0.36 to 2.5 mM and were incubated at 37 °C for 30 min in 50 mM sodium acetate buffer, pH 5.5. c Relative activities of β-glucosidase against four different substrates: p-NPGlu, o-NPGlu, p-NPGal, and o-NPGal. Enzyme activity against p-NPGlu was taken to be 100 % and relative activities against other substrates were calculated accordingly
Additionally, relative activities against four substrates were compared in triplicate, namely: p-NPGlu, o-NPGlu, p-NPGal, and o-NPGal (Fig. 2c). According to the results of the present study, p-NPGlu was the most suitable substrate for mushroom β-glucosidase, so enzyme activity with this substrate was taken to be 100 %. When compared to p-NPGlu, relative activities towards other three substrates were calculated to be 28.25, 81.07, and 39.84 % for o-NPGlu, p-NPGal, and o-NPGal, respectively. Therefore, it can be concluded that para isomers of these substrates are more suitable for the activity of A. bisporus β-glucosidase when compared to their ortho isomers.
Optimum pH and temperature for A. bisporus β-glucosidase were determined in the study. Optimum pH was found to be 4.0 (Fig. 3), while optimum temperature for this enzyme equals to 55 °C (Fig. 4, upper panel).
The graph for optimum pH value determination. Enzyme activity was measured spectrophotometrically at 405 nm following enzyme and p-NPGlu incubation for 30 min at 37 °C in three different buffers: sodium acetate buffer (pH 3.0–6.0), phosphate buffer (pH 6.0–8.5), and glycine-NaOH buffer (pH 8.5–12.0)
Upper panel Optimum temperature determination for A. bisporus β-glucosidase was done by incubating enzyme with 5 mM p-NPGlu solution on seven different temperatures from 25 to 85 °C. Bottom panel Thermal denaturation of mushroom β-glucosidase was done by incubating enzyme for 2 h on five temperatures from 25 to 65 °C and for 1 h at 75 °C. Enzyme activity obtained after usual activity assay was taken to be 100 % and the rest of the results are calculated accordingly
Thermal denaturation study revealed that mushroom β-glucosidase is an exceptionally stable enzyme, since the decrease in activity did not happen at the temperatures below 65 °C. It showed decline in activity at 65 °C after approximately 20 min of incubation, while it got almost instantly deactivated at 75 °C (Fig. 4, bottom panel).
Glucose and δ-gluconolactone were used for inhibition studies in the current research. Glucose inhibited enzyme activity in a competitive manner since the graph of enzyme activity is showing that Vmax value remains almost the same in different concentrations of inhibitor and in the absence of inhibitor, while Km value gets higher when the inhibitor is present (Fig. 5a). Ki and IC50 values were calculated to be 9.402 mM (Fig. 5a) and 19.185 mM (Fig. 5b), respectively.
a Glucose inhibition type against p-NPGlu as a substrate was found to be competitive. In the graph, I0 denotes enzyme activity without inhibitor, I1 with 3 mM of glucose, and I2 with 25 mM of glucose in total reaction volume. b Inhibition of A. bisporus β-glucosidase activity by glucose. While enzyme and substrate (p-NPGlu) concentrations were kept constant, inhibitor concentration was increased from 0 to 150 mM. Enzyme activity without inhibitor was taken as 100 % and other results were normalized accordingly. c Inhibition type for δ-gluconolactone was found to be competitive against p-NPGlu as a substrate. I0 represents enzyme activity with no inhibitor present, I1 with 0.05 mM of δ-gluconolactone, and I2 with 0.003 mM of δ-gluconolactone present in the reaction. d Inhibition curve for δ-gluconolactone. While enzyme and substrate concentrations were constant, inhibitor concentration varied from 0 to 3 mM. Enzyme activity at 0 mM inhibitor concentration was taken as 100 % activity
In the case of δ-gluconolactone, inhibition effects were much more dramatic, which is demonstrated by Ki and IC50 values for this inhibitor that were calculated to be 0.0072 mM (7.2 µM) and 0.39 mM, respectively (Figs. 5c, d), while enzyme activity was inhibited in a competitive way (Fig. 5c).
Relative enzyme activity was studied in the presence of six heavy metal ions which were potential inhibitors of β-glucosidase activity, namely: I−, Zn2+, Fe3+, Ag+, Cu2+, and Pb2+. Metal ion concentration in these reactions was in the range from 0 to 1.5 mM. All of them, with the exception of lead ion, showed inhibitory effects (Fig. 6) with relative enzyme activity of 57.33, 81.11, 95.65, 64.72, and 49.01 % in the presence of I−, Zn2+, Fe3+, Ag+, and Cu2+, respectively. When it comes to lead, increase in enzyme activity was observed at metal concentrations below 0.5 mM and only slight inhibition at higher concentrations. Since all other metal ions exhibited only inhibitory effects on β-glucosidase activity, lead was excluded from relative enzyme activity calculations.
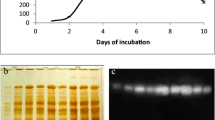
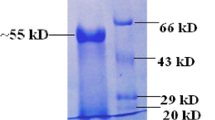
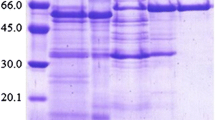
In order to determine protein molecular weight, both SDS-PAGE and native PAGE were performed. SDS-PAGE gel showed two bands that correspond to molecular weights of approximately 46 and 62 kDa. In order to confirm that A. bisporus β-glucosidase is truly a dimer, native PAGE gel was analyzed. Single band corresponding to approximately 110 kDa was observed, which confirmed that this enzyme has two subunits (Fig. 7b, c). Native PAGE coupled to incubation in 4-MUG solution showed a single band of enzymatic activity of mushroom β-glucosidase after exposure to UV light (Fig. 7d).
a Protein molecular weight marker (Thermo Scientific, Waltham, MA, USA) with the following bands: 116 kDa (β-galactosidase), 66.2 kDa (bovine serum albumin), 45 kDa (egg albumin), 35 kDa (lactate dehydrogenase), 25 kDa (REase Bsp981, E. coli), and 18.4 kDa (β-lactoglobulin). b Image of SDS-PAGE gel. Lane 1 (left) contains molecular weight marker, while lane 2 (right) contains A. bisporus β-glucosidase with two subunits of approximately 46 and 62 kDa. c Image of native gel. Lane 1 (left) contains non-denatured protein molecular weight marker, while lane 2 (right) contains non-denatured β-glucosidase of approximately 110 kDa. d Image of native gel coupled to incubation in 4-MUG solution. β-glucosidase activity was recorded under the UV light
4 Discussion
Throughout the precipitation and chromatography steps, enzyme was purified 10.12-fold. This purification fold can be considered satisfying when compared to the purification folds of fungal β-glucosidases from the previous studies. Enzyme from Penicillium piceum [15] was purified 8.7-fold, while Rhizomucor miehei [11] β-glucosidase had 18.2-fold purification. However, the majority of studies brought data about higher purification folds. For example, β-glucosidase from Pichia pastoris [3] had 99.5-fold purification, one from Daldinia eschschdzii [16] had 50.23-fold, while the enzyme from Pholiota adiposa [17] was purified 130.3-fold. These results imply that better purification fold could be obtained if enzyme of interest was purified using more than two purification steps. However, numerous steps usually cause dramatic decrease in enzyme activity. In the present study, high enzyme activity was one of the most important properties and purification was done in a way that would preserve the majority of that activity.
Km values for p-NPGlu and o-NPGlu in this study were 1.751 and 8.547 mM, respectively. Km value for p-NPGlu is comparable to the results obtained for other species. Some examples are D. eschschdzii with Km value of 1.52 mM [16], Botrytis cinerea with 1.05 mM [18], and P. adiposa with 2.23 mM [17]. On the other hand, Vmax values for p-NPGlu and o-NPGlu were 833 and 556 U/mg, respectively. Vmax values for p-NPGlu in fungal β-glucosidases were ranging from 0.077 U/mg in Termitomyces clypeatus [19] to 4390 U/mg in P. adiposa [17]. Thus, A. bisporus β-glucosidase showed high conversion rate of p-NPGlu into reaction product p-NP and, therefore, is the enzyme with relatively high activity rate.
By comparing A. bisporus β-glucosidase activity against four different substrates (p-NPGlu, o-NPGlu, p-NPGal, and o-NPGal), it was concluded that p-NPGlu gave the highest enzyme activity. Due to that, enzyme activity against p-NPGlu was taken to correspond to 100 % relative activity, while relative activities against other substrates were calculated as follows: 28.25 % against o-NPGlu, 81.07 % against p-NPGal, and 39.84 % against o-NPGal. Similar behavior has been observed with other fungal β-glucosidases. Another study of fungal enzyme from D. eschschdzii [16] offered similar results, since it was concluded that p-NPGlu corresponds to 100 % and o-NPGlu to 15.5 % relative enzyme activity. At the same time, P. adiposa β-glucosidase [17] had 100 % activity against p-NPGlu and 23.7 % against o-NPGlu. Enzyme from R. miehei was active against three substrates used in the current study in the following manner: 100 % against p-NPGlu, 20 % against o-NPGlu, and 69 % against p-NPGal [20], while Paecilomyces thermophila enzyme was active against two substrates: 100 % for p-NPGlu and 50.7 % for p-NPGal [8]. Many other fungal β-glucosidases did not show any activity against either p-NPGal or o-NPGal. The most prominent exception from all these results is the study of P. pastoris, where o-NPGlu gave 116 % relative enzyme activity when compared to p-NPGlu, which was taken as 100 %. This enzyme also did not exhibit any activity against galactopyranosides [3].
The optimum pH for A. bisporus β-glucosidase was found to be 4.0. This finding was expected since many other fungal β-glucosidases were found to prefer acidic over neutral or basic pH, many of them having pH optimum of 5.0. Some examples are β-glucosidases from the following fungal species: D. eschschdzii [16], Aspergillus niger [21], Tricholoma matsutake [22], Periconia sp. [23], P. adiposa [17], P. piceum [15], R. miehei [11, 20], and T. clypeatus [19]. Other species also exhibited pH optima in acidic environment, like B. cinerea that had pH optimum 3.0 [18] and Zygosaccharomyces bailii [24] with pH optimum of 5.5.
The optimum temperature for A. bisporus β-glucosidase is 55 °C, which is the same temperature optimum as found in the study of another edible mushroom, Volvariella volvacea [9] and in thermophilic fungus P. thermophila [8]. High temperature optima are characteristic for other fungal β-glucosidases, most prominently 60 °C optimum, which is the most suitable temperature for many fungal species, like P. piceum [15], T. matsutake [22], and B. cinerea [18]. Additionally, 50 °C was optimum for enzyme activity of some species, like D. eschschdzii [16] and R. miehei [11].
The results of thermal denaturation study revealed that β-glucosidase from the present study is a very stable enzyme, since its denaturation and loss of activity did not happen at the temperatures below 65 °C. Similar behavior was observed in V. volvacea β-glucosidase, which experienced rapid denaturation at temperatures above 60 °C, but was not affected by lower temperatures [9]. Also, A. niger β-glucosidase was another fungal thermostable enzyme [21].
In this study, both glucose and δ-gluconolactone had certain inhibitory effects on β-glucosidase activity, both of them acting as competitive inhibitors and gluconolactone being much more efficient inhibitor. This can be confirmed by comparing Ki values for glucose and gluconolactone, which were calculated to be 9.402 mM and 7.2 µM, respectively. Similar results were found in the previous studies of yeast and fungal β-glucosidases. All of them identified gluconolactone as more efficient inhibitor when compared to glucose. Many fungal species showed competitive inhibition type for glucose and had similar Ki values. For example, P. pastoris β-glucosidase had Ki value of 7.2 mM [3], R. miehei 8 mM [20], while Piromyces sp. had Ki value for glucose of 9.5 mM [25]. However, many fungal β-glucosidases were completely inhibited by glucose and, thus, had very low Ki values, implying high inhibition potential of glucose. Some of them are: Talaromyces emersonii with Ki value of 0.245 mM [26], Z. bailii with 0.137 mM for extracellular and 0.105 mM for intracellular enzyme [24], and D. eschschdzii with 0.79 mM [16]. The third group of fungal β-glucosidases was almost completely insensitive to inhibitory effects of glucose, having very high Ki values. Two examples are P. adiposa with Ki value of 40.6 mM [17] and Periconia sp. with 20 mM [23]. Therefore, it can be concluded that glucose had moderate inhibitory effect on the activity of A. bisporus β-glucosidase. On the other hand, all fungal species studied so far experienced strong inhibition by gluconolactone with very low Ki values. Many examples were found in the available literature, some of them being Piromyces sp. with Ki value of 22 µM [25] and both extracellular and intracellular β-glucosidase from Z. bailii with Ki values of 30 and 1 µM, respectively [24].
According to the results of this study, five heavy metal ions were successful inhibitors of A. bisporus β-glucosidase activity, namely I−, Zn2+, Fe3+, Ag+, and Cu2+. These ions were mentioned in literature as the inhibitors of enzyme activity in other fungal species as well. For example, Cu2+ and Zn2+ were mentioned as inhibitors of β-glucosidases from D. eschschdzii [16], T. clypeatus [19], and R. miehei [20], while Fe3+ and Cu2+ were effective inhibitors of the activity of β-glucosidase from P. adiposa [17]. β-glucosidase from T. matsutake was inhibited by Ag+ ion, while Pb2+ was activator of enzyme activity until certain metal concentration, which corresponds to the present results [22].
SDS-PAGE and native PAGE gels showed that A. bisporus β-glucosidase is a dimer, consisted of two subunits of approximately 46 and 62, or 110 kDa for non-denatured enzyme. Therefore, this enzyme is relatively small. Through the search of previously published literature, it was found that many other fungal β-glucosidases are indeed small enzymes. As an example, A. niger β-glucosidase had an enzyme of identical molecular weight of 110 kDa [21], T. clypeatus had an enzyme of 116 kDa [19], P. piceum β-glucosidase has molecular weight of 92 kDa [15], while edible mushroom V. volvacea had a 158 kDa enzyme [9].
To conclude, β-glucosidase from A. bisporus was successfully purified using ammonium sulfate precipitation and hydrophobic interaction chromatography and its biochemical characterization was done. Its high activity and substrate specificity, along with high thermal stability and relative resistance to glucose inhibition make it an excellent choice for a range of industrial applications, most prominently for glucose production by cellulose degradation. To our knowledge, this is the first detailed study of β-glucosidase from the white button mushroom.
Abbreviations
- 4-MUG:
-
4-Methylumbelliferyl β-d-glucopyranoside
- BSA:
-
Bovine serum albumin
- o-NP:
-
o-Nitrophenol
- o-NPGal:
-
2-Nitrophenyl beta-d-galactopyranoside
- o-NPGlu:
-
2-Nitrophenyl beta-d-glucopyranoside
- p-NP:
-
p-Nitrophenol
- p-NPGal:
-
4-Nitrophenyl beta-d-galactopyranoside
- p-NPGlu:
-
4-Nitrophenyl beta-d-glucopyranoside
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Kara HE, Sinan S, Turan Y (2011) Purification of beta-glucosidase from olive (Olea europaea L.) fruit tissue with specifically designed hydrophobic interaction chromatography and characterization of the purified enzyme. J Chromatogr B 879:1507–1512
Turan Y (2008) A pseudo-β-glucosidase in Arabidopsis thaliana: correction by site-directed mutagenesis, heterologous expression, purification, and characterization. Biochemistry (Moscow) 73(8):912–919
Turan Y, Zheng M (2005) Purification and characterization of an intracellular β-glucosidase from the methylotrophic yeast Pichia pastoris. Biochemistry (Moscow) 70(12):1656–1663
Collins CM, Murray PG, Denman S, Morrissey JP, Byrnes L, Teeri TT, Tuohy MG (2007) Molecular cloning and expression analysis of two distinct β-glucosidase genes, bg1 and aven1, with very different biological roles from the thermophilic, saprophytic fungus Talaromyces emersonii. Mycol Res 111:840–849
Kara HE, Turan Y, Er A, Acar M, Tumay S, Sinan S (2014) Purification and characterization of β-glucosidase from greater wax moth Galleria mellonella L. (Lepidoptera: Pyralidae). Arch. Insect Biochem 86(4):209–219
Pontoh J, Low NH (2002) Purification and characterization of β-glucosidase from honey bees (Apis mellifera). Insect Biochem Mol 32:679–690
Zhang D, Allen AB, Lax AR (2012) Functional analyses of the digestive β-glucosidase of Formosan subterranean termites (Coptotermes formosanus). J Insect Physiol 58:205–210
Yang S, Hua C, Yan Q, Li Y, Jiang Z (2013) Biochemical properties of a novel glycoside hydrolase family 1 β-glucosidase (PtBglu1) from Paecilomyces thermophila expressed in Pichia pastoris. Carbohydr Polym 92:784–791
Cai YJ, Buswell JA, Chang ST (1998) β-glucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme Microb Technol 22:122–129
Kerrigan RW, Challen MP, Burton KS (2013) Agaricus bisporus genome sequence: a commentary. Fungal Genet Biol 55:2–5
Guo Y, Yan Q, Yang Y, Yang S, Liu Y, Jiang Z (2015) Expression and characterization of a novel β-glucosidase, with transglycosylation and exo-β-1,3-glucanase activities, from Rhizomucor miehei. Food Chem 175:431–438
Jeong SC, Jeonh YT, Yang BK, Islam R, Koyyalamudi SR, Pang G, Cho KY, Song CH (2010) White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr Res 30:49–56
Liu J, Jia L, Kan J, Jin C-H (2013) In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 51:310–316
Waterborg JH (2002) In: Walker J (ed) The protein protocols handbook, 2nd edn. Humana Press Inc, Totowa, NJ
Gao L, Gao F, Zhang D, Zhang C, Wu G, Chen S (2013) Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresour Technol 147:658–661
Karnchanatat A, Petsom A, Sangvanich P, Piaphukiew J, Whalley AJS, Reynolds CD, Sihanonth P (2007) Purification and biochemical characterization of an extracellular β-glucosidase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. FEMS Microbiol Lett 270(1):162–170
Jagtap SS, Dhiman SS, Kim T-S, Li J, Kang JC, Lee J-K (2013) Characterization of a β-1,4-glucosidase from a newly isolated strain of Pholiota adiposa and its application to the hydrolysis of biomass. Biomass Bioenergy 54:181–190
Sasaki I, Nagayama H (1994) β-glucosidase from Botrytis cinerea: its relation to the pathogenicity of this fungus. Biosci Biotechnol Biochem 58(4):616–620
Pal S, Banik SP, Ghorai S, Chowdhury S, Khowala S (2010) Purification and characterization of a thermostable intra-cellular β-glucosidase with transglycosylation properties from filamentous fungus Termitomyces clypeatus. Bioresour Technol 101:2412–2420
Krisch J, Bencsik O, Papp T, Vagvolgyi C, Tako M (2012) Characterization of a β-glucosidase with transgalactosylation capacity from the zygomycete Rhizomucor miehei. Bioresour Technol 114:555–560
Xue D-S, Chen H-Y, Ren Y-R, Yao S-J (2012) Enhancing the activity and thermostability of thermostable β-glucosidase from a marine Aspergillus niger at high salinity. Process Biochem 47:606–611
Kusuda M, Ueda M, Konishi Y, Araki Y, Yamanaka K, Nakazawa M, Miyatake K, Terashita T (2006) Detection of β-glucosidase as saprotrophic ability from an ectomycorrhizal mushroom, Tricholoma matsutake. Mycoscience 47:184–189
Harnpicharnchai P, Champreda V, Sornlake W, Eurwilaichitr L (2009) A thermotolerant β-glucosidase isolated from an endophytic fungi, Periconia sp., with a possible use for biomass conversion to sugars. Protein Expr Purif 67:61–69
Gueguen Y, Chemardin P, Arnaud A, Galzy P (1995) Comparative study of extracellular and intracellular β-glucosidases of a new strain of Zygosaccharomyces bailii isolated from fermenting agave juice. J Appl Bacteriol 78:270–280
Harhangi HR, Steenbakkers PJM, Akhmanova A, Jetten MSM, Van der Drift C, Op den Camp HJM (2002) A highly expressed family 1 β-glucosidase with transglycosylation capacity from the anaerobic fungus Piromyces sp. E2. Biochim Biophys Acta 1574:293–303
Murray P, Aro N, Collins C, Grassick A, Penttila M, Saloheimo M, Tuohy M (2004) Expression in Trichoderma reesei and characterisation of a thermostable family 3 β-glucosidase from the moderately thermophilic fungus Talaromyces emersonii. Protein Expr Purif 38:248–257
Acknowledgments
We would like to thank Assist. Prof. Hatibe Kara (Veterinary Faculty, Balıkesir University) for her invaluable help during the experimental part of the research. We would also like to thank Head of Department of Genetics and Bioengineering Prof. Dr. Damir Marjanović for his endless support and useful comments during this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ašić, A., Bešić, L., Muhović, I. et al. Purification and Characterization of β-Glucosidase from Agaricus bisporus (White Button Mushroom). Protein J 34, 453–461 (2015). https://doi.org/10.1007/s10930-015-9640-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-015-9640-z