Abstract
Lignin with a phenolic structure can act as a fire retardant, but different origins of lignin and extraction methods impact the fire behavior capabilities. The thermal characteristics of lignin, such as glass transition (Tg) temperature, thermal degradation, molecular weight, lignin purity, and phenolic content, influence the capability of lignin as a flame retardant (FR) by causing significant char residue. However, lignin faced significant constraints in the final polymer to meet industry requirements, with the main issue being a lack of homogeneity when blending lignin with polymeric matrices. To improve the FR performance of lignin, various advances have been taken such as altering lignin with nitrogen and/or phosphorus chemicals, as well as shrinking lignin to the nanoscale to reduce lignin aggregation with matrices and obtaining considerable FR behavior in which FR system, lignin can present as a single component, lignin-based composite, modified lignin, and nano lignin. The present review addresses the manufacture of lignin as FR and the attributes of the product with lignin added to the system. It also covers the structure, source, extraction technique, physical-chemical properties, and chemical modification of lignin as an FR source, as well as the basic principle of flame retardancy, influencing factors (current development and application till the industrial analysis need of FR). Throughout this review, it aids in the discovery of a better strategy to introduce lignin as a source of bio-based FR for achieving the low cost in the fabrication method of FR.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chemical, physical, biological, and material sciences made tremendous progress, particularly in polymer chemistry commodities, over the twentieth century. Rising the trend of polymer chemistry resulted in the ubiquitous application of synthetic materials such as fiber and plastic in daily life. However, these materials have high levels of flammability that gave a consequence of daily use. For instance, when a building fire occurred, building equipment mostly made of high flammability-material created severe combustion due to the high caloric capacity of the polymer [1]. This problem is an intriguing point and pushes researchers to develop and study stronger flame-retardant polymers especially natural-based polymers due to environmental concerns.
Lignin, as the second most abundant biopolymer, preceded by cellulose only, is readily available in adequate quantities [2] and does not compete directly with food security. Lignin is mostly obtained from biomass approximately 150 billion tons [3] while technical lignin was greatly generated from pulp mills as a waste, called black liquor. The global production of lignin from these sources is accounted for about 50 to 70 million tons annually [3]. The availability of lignin is predicted to increase by 225 million tons per year in 2030 [4]. Besides that, it can also be obtained from the by-product of bioethanol production. However, less than 5% lignin is utilized as a green chemical while the rest is usually burned for boiler heating [2, 5]. A complex structure of lignin consists of three basic units: p-coumaryl alcohol (H), coniferyl alcohol (G), and sinapyl alcohol (S) which are connected by interunit linkages [6], with the phenolic hydroxyl, methoxyl groups, and carboxyl as functional groups [7]. The decomposition of lignin is mainly divided into three stages. The first stage indicated volatile product evaporation from room temperature until about 150 °C, the next stage (150–270 °C) corresponded to the aromatic decomposition of lignin, while the rest (270–750 °C) with the prominent peak around 350–400 °C expressed as fragmentation of inter-unit linkage in lignin structure. This result is corresponding with the previous thermo gravimetry analysis (TGA) result on Acacia mangium lignin as reported by Solihat et al.[8]. At high temperatures (750 °C), char is about 40% formed as a result of aromatic structure condensation and rearrangement [8]. Another important lignin thermal property is the glass transition temperature (Tg). Tg which is correlated to the degree of polymerization is usually analyzed by differential scanning calorimetry (DSC) or dynamic mechanical analysis (DMA) [9, 10]. The thermal properties of lignin may vary depending on the lignin source, extraction method, moisture content, low molecular weight species, and chemical modifications [11,12,13]. However, fundamentally, the huge aromatic backbone in lignin of any type created a high charring ability and hence it potentially can be used as a carbon source for flame-retardant (FR) material [14].
The development of lignin-based FR has received much interest in the last twenty years [15]. Furthermore, the toxicity issue and global prohibition of some conventional halogenated FR such as hexabromocyclododecane (HBCD), polybrominated dibenzofuran (PBDF), tetrabromobisphenol A (TBBA), decabromodiphenyl ether (DBDPO) have accelerated this development.[16]. Generally, FR can be derived from four types such as pristine lignin, composites lignin, chemical-modified lignin, and nanolignin [17]. Lignin pristine can offer certain flame retardancy polymers yet the end product of polymer/lignin composites frequently fail to fulfill the industrial requirement test such as UL-94 analysis due to failure to achieve the V-0 rating and to achieve limiting oxygen index (LOI) > 28%. Cone calorimetry and pyrolysis combustion flow calorimeter (PCFC) analysis are two other main flame retardancy requirement tests. One of the scientist’s endeavors to circumvent the pristine lignin problem is a chemical modification of lignin. According to Yang et al.’s report, there are four types of chemical modification for lignin to increase its properties for FR application: (i) nitrogen or phosphorus modified lignin, (ii) phosphorus-nitrogen modified lignin, (iii) phosphorus-nitrogen modified lignin-containing metal ions, and (iv) combination of lignin with silica-containing FR [16]. To create an FR polymer, two ways methods are usually applied: additive method and reactive method. In the additive method, FR is melt-mixed with polymer using normal processing methods (e.g., extrusion) while in the reactive method, chemical alteration of the polymer chain to integrate with FR directly during polymerization is conducted. An additive method has been reported by some researchers who blended lignin with polylactic acid (PLA)/ammonium polyphosphate (APP) [18] and polypropylene (PP) [19]. Meanwhile, a reactive method has been reported by Handika et al. who reacted lignin with polyurethane (PU) for textile fire-resistant application [20].
Overall, lignin has a bright future as a polymeric FR solution, especially as the insulating material in the construction and building industry, due to its high charging capacity [21]. Despite FR has been reported in some review chapters to date [1, 14, 16, 22], no study reported comprehensively on lignin-based FR including the effect of lignin structure, source, the extraction process, physical-chemical properties, and chemical modification of lignin extraction and properties, basic principle of flame retardancy and current development, and application until industrial analysis requirement of FR. Therefore, this study will contribute to enrich the update on lignin-based FR development. Eventually, challenges and envisages are also suggested.
Lignin structure, extraction, and its properties
Lignin structure
Lignin is a natural binder found in woody plants that gives the plant’s cell walls strength, stiffness, and structure. It provides some rigidity to the wood substance, reducing microbial breakdown and protecting it from stress [23, 24]. Lignin fills the space between hemicellulose and cellulose in plants and maintains the lignocellulose matrix together [25]. In addition, due to these properties exhibited by lignin as a natural binder, lignin can be used as a natural flame retardant to slow down the flame progress when subjected to fire [22, 26, 27]. Thus, the extracted lignin as a natural FR mixed with other material could withstand or act as a barrier to fire[28]. The lignin contents are between 24 − 33% in softwoods, 19 − 28% in hardwoods, and 20 − 30% in another biomass [29, 30]. The average molecular weight (Mw) for isolated lignin differs based on wood and lignocellulosic types. Some studies reported Mw 17,200 g mol− 1, 29,600 g mol− 1, and 53,850 g mol− 1 from eucalyptus (Eucalyptus globulus), Southern pine (Pinus palustris), and Norway spruce (Picea abies), respectively where these differences in Mw may be due to different isolation methods [31]. Lignin is formed within cell walls in plants that are composed of aromatic units. Lignin is insoluble in most solvents and has an amorphous form [32].
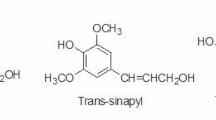
Lignin is a polyphenolic substance that, along with hemicellulose and cellulose, is one of the most important components in plant cell walls. The lignin chemical structure is composed of phenyl propane units, which are derived from three aromatic precursors known as monolignols or basic units (Fig. 1). These monolignols are G, H, and S [33] which are involved in the creation of lignin that was linked by the inter-unit linkage of monolignols’ phenolic substructures (Fig. 2) [34, 35]. There are no methoxy groups in the H unit, one methoxy group in the G unit, and two methoxy groups in the S unit [30]. The composition of lignin is determined by the lignocellulosic and wood species. Lignin is deposited in the middle lamella of hardwood cell walls, followed by the main wall and the S1 layer in the secondary wall. Following that, lignin is found in the S2 and S3 layers. Because hardwood lignin comprises primarily G and S units with traces of H units, it differs from softwood lignin (Fig. 3) [36]. Softwood lignin, on the other hand, is largely made up of G units, with small quantities of H units (about 90%) (Fig. 4) [33, 37]. Furthermore, the additional methoxyl groups on the aromatic rings hinder the development of 5–5 (dibenzodioxin linkages), allowing the lignin polymer in hardwood to form a linear structure rather than a branched structure like softwood. Hardwood has a larger lignin variability composition than softwood. Herbaceous plant lignin, for example, has all three monolignols units, as well as p-coumarate and ferulate, which are integrated with G and S units [33].
Lignin monolignols structures. Reproduced with permission from Doherty et al. [9] Copyright 2022, Elsevier B.V
Phenolic substructures from monolignols. Reproduced with permission from Doherty et al. [9]Copyright 2022, Elsevier B.V.
Softwood lignin chemical structure. Reproduced with permission fromZakzeski et al. [38]. Copyright 2022, American Chemical Society.
Hardwood lignin chemical structure. Reproduced with permission from Zakzeski et al. [38] Copyright 2022, American Chemical Society.
A representative of lignin general structure. Reproduced with permission from Vásquez-Garay et al. [30]. Copyright 2022, MDPI.
The lignin structure is made up of branching heteropolymers with no specific structure [33]. Because lignin comprises irregular functional groups, the most frequent of which are phenolic hydroxyls and methoxyl, as well as primary and secondary aliphatic hydroxyls and a few carbonyl groups. Figure 5 depicts the general structure of lignin. The common interunit linkages found for lignin such as an aryglycerol-β-ether dimer or β-O-4 ether type comprise about 45–50% of lignin. Other inter-unit linkages are aryglycerol-α-ether dimer or α-O-4 type (6–8%), resinol (β-β´) (9–12%), phenylcoumaran (β-5) (0–3%), biphenyl/dibenzodioxocin 5–5 × (18–25%), siaryl ether 4-O-5 moieties (4–8%) and a small amount of spirodienone[29]. Different lignins have different numbers of these linkages’ structures. This also includes different isolation methods and species of biomass[40].
Lignin extraction methods
Lignin can be extracted from lignocellulosic components in a variety of ways, including physical, chemical, and biological processes. Pulping techniques subjected to the dissociation of ester and ether bonds were used to remove lignin [41]. There are two categories of lignin extraction that are sulfur-bearing and sulfur-free processes [42] as shown in Fig. 6.
Lignin extraction process. Reproduced with permission from Mandlekar et al. [14]. Copyright 2018, IntechOpen.
Sulfur bearing process
Sulfur-bearing processors known as sulfur-containing lignin include sulfur as inorganic compounds after the extraction process [30]. The paper and pulp sector produces the majority of sulfur-containing lignin. This lignin process includes kraft and lignosulfonate lignin [30]. Both are produced commonly from pulp and paper industries due to the removal of lignin leaving only cellulose. Two lignin kinds can be used in similar applications even though they have different solubility mediums [2]. The availability of kraft lignin was estimated at 90 kilotons per year [43, 44], while lignosulfonates have bigger production annually about 1.8 million tons [45, 46]. Table 1 depicts the different properties of kraft lignin and lignosulfonate.
Kraft lignin
The kraft lignin method involves sodium sulfide (Na2S) and sodium hydroxide (NaOH) that first convert to white liquor [54]. This process converts both non-wood and wood materials into pulp and final form to produce black liquor that is acidified to recover lignin. This process is known and called kraft lignin. The kraft process has been used extensively and commercially to remove lignin in large quantities [55]. During the cooking process, the lignin depolymerized by cleavage of aryl ether bonds then yields kraft lignin (soluble fraction) that is high in phenolic hydroxyl groups. The condensation reaction forms a carbon-carbon bond [54]. White liquor is used to dissolve the connections that bind cellulose and lignin together. The non-woody/woody elements are then turned into black liquor and pulp [56]. Together combined with an acidic solution to collect lignin. The black liquor consists of removal lignin and considers waste or by-product [57]. This process was applied in a study by Marangon Jardim et al.[55] using two different types of wood species in a kraft lignin process that is Southern sweetgum (Liquidambar styraciflua), a species from hardwood, and Southern pine (Pinus taeda), one of the species from a softwood. First, the woods were chipped and screened. Then, it was dried. A 10 L laboratory pulping digester was used for cooking. The parameters for cooking conditions were set as for liquor to wood ratio was 4:1, Na2O percentages were 16% for hardwood and 19% for softwood, sulfidity percentage was 25% for both species, the temperature was set to 160 °C for hardwood and 170 °C for softwood, respectively and time for pulping were running at 30, 60, 90, 120 and 150 min. After cooking the pulped was washed with water and centrifuge, the black liquor as lignin precipitation was collected and filtered to remove any dirt. Then, the black liquor was evaporated to solid content.
The residual sulfur content for this kraft lignin usually is between 1.0 and 3.0% and 0.5–3.0% ash [10]. The sulfur contains can give significant drawbacks for further chemical functionalization especially when catalysis is needed [58]. The kraft lignin has between 1000 and 3000 g mol− 1 in average molar mass number (Mn). The kraft lignin contains high phenolic hydroxyl groups and condensed structures [59].
Sulfite lignin
In the market, the most ubiquitous commercially available lignin is sulfite lignin. This process was commonly used as an additional way for the pulping process by employing sulfite or bisulfite in digesting biomass[60]. Sulfite lignin was produced with a mixture of sulfur dioxide and base calcium (Ca2+), ammonium (NH4+), Sodium (Na+), or magnesium (Mg2+) [61]. Because precipitation occurs only at pH 3, in bisulfite pulping calcium can be utilized. Magnesium is utilized when the pH is below 5. It is possible to employ ammonium and sodium at a higher pH. The processes at pH 1–2 known as acid sulfite, pH 5–7 as neutral sulfite, and alkaline sulfite are the three main methods of sulfite pulping (pH 9-3.5)[57]. Delignification can be produced to varying degrees by adjusting the cooking liquor, chemical-to-biomass ratio, and physical conditions [61]. Sulfite lignin has 3.5-8% sulfur and 4.0–8.0% ash contents [62]. When cooking, lignosulfonates are recovered from the liquor stream. Sulfur is found in Lignosulfonate in the form of sulfonate groups on aliphatic side chains. As a result, lignosulfonate is regarded as water-soluble [63].
Sulfur free process
Sulfur-free process lignin is a different type of class from the lignin process that no sulfur content is developed during lignin removal or extraction. There are two types of processes in this category: lignin from alkaline pulping (soda pulping) and solvent pulping (organosolv lignin) [64]. Both categories of lignin processes have low macromolecular sizes and low molar mass phenol after the process [30, 65]. This process is of particular interest because it has characteristics that differ from kraft–lignin, allowing for applications which sensitive to the presence of sulfur. Besides, the lack of thiol groups odor during heating is a significant benefit [66].
Soda process
Soda lignin is based on extraction pulping from soda or soda-anthraquinone. This method is commonly used for non-woody species such as straw, bagasse, flax, and some hardwoods [67] The process began with hydrolytic cleavage of native lignin with sodium hydroxide (NaOH) as a cooking chemical [68, 69]. The addition of anthraquinone in cooking liquor increase the rate of delignification [68]. This results in unmodified lignin becoming relatively chemically treated. The soda process procedures adapted some processes from pulp and paper factories to produce black liquors. Furthermore, in the Granit process, where acidification with mineral acids was added to lower the pH value of black liquors. The recovery of liquor is come with some problems because of the high amount of silica precipitate in lignin which makes the lignin low in quality [70].
Rice straw was employed in lignin extraction by Shao et al. [71]. In this study, soda pulping was used to cook rice straw in a 15 L autoclave using soda anthraquinone (AQ), NaOH was added with 14% concentration-based oven-dried rice straw, AQ amount 0.1%, liquor to straw ratio was 1:4, cooking time was 50 min, and the maximum temperature was 148 °C. The pulp was screened after heating, then the pulp slurry was evaporated until a solid content was achieved. Tutus et al. [70] found that increasing the cooking duration from 30, 40, and 60 min increased the silica precipitated on pulps by 77.89%, 82.89%, and 84.59%, respectively, in their investigation for rice straw pulping with AQ added. Increased cooking time was also linked to lower kappa number, screening yield, and viscosity of pulps.
Organosolv process
Lignin produced from the organosolv process was considered the one that has the highest purity compared to another process. It has higher solubility in organic solvents and is insoluble in water [63]. Due to hydrophobic properties obtained by this type of lignin. The lignin is recovered from solvent by precipitation and most organosolv process is obtained through the pulping process using low boiling organic solvents with sulfur-free contain chemicals. This method is based on ethanol-water pulping with an acetic acid mineral acid in small amounts, such as sulfuric or hydrochloric acid. The ash concentration of lignin produced by the Organosolv process is roughly 1.75% [62]. A study by Rossberg et al.[72] extracted lignin by using an organosolv process, in this research a digester 400 L was used within an integrated pilot plant. Different process parameters for pulping were used with three wood species were beech, spruce, and wheat straw, the concentration of ethanol used was 50, 65, 50% with temperatures 170, 180, 190 °C, and treatment times were 80, 80, 40 min also H2SO4 was added with concentration 0.5 to 2.9%. After treatment, black liquor was formed and lignin was precipitated with water dilution two times then evaporated to form solid lignin. Figure 7 shows the organosolv lignin process for this study.
Organosolv lignin process. Reproduced with permission from Rossberg et al.[72]. Copyright 2022 NC State University.
Lavri et al. [63] extracted lignin from the sawdust from Japanese knotweed (Reynoutria japonica) and beech trees (Fagus sylvatica). At a Parr high-pressure reactor, 140 g of sawdust was combined with water/ethanol (1:1) as much as 980 mL. To stimulate the extraction of lignin, 8.58 mL of 2 M H2SO4 was added. The reaction occurred for 1 h at 180 °C with agitation at 200 rpm while nitrogen gas was used to pressurize the reactor to a pressure of 1.7 MPa. Filtration was used to separate the mixture was then filtered, and the solid was washed with a water/ethanol solution before being oven-dried to obtain precipitated lignin.
Physical properties of lignin
Lignin is a pale-yellow chemical found in wood. Because of the development of quinoid structures by phenol oxidation, lignin separation under alkaline circumstances causes significant discoloration. With modest modifications to remove the phenolic and quinoid functions, this discoloration can be reversed [32]. The properties of lignin determine the final application in various manufacturing products. The extraction method and lignin source have a great influence on lignin properties. As a high crosslinking material, lignin contains five hydroxyl and five methoxyl groups per building unit [73]. It is easily oxidized and very soluble in hot alkali solution but not hydrolyzed in acids and condensed chemical structure with phenol [42]. Important properties in lignin are the glass transition (Tg) which measures the degree of crosslinking, crystallinity, rigid phenyl groups, molecular mass, interchain hydrogen bonding, and rubbery region [9, 10]. It also is closely related to the degree of polymerization of lignin [9]. On top of that, the Tg can be defined as the characteristics of the viscoelastic behavior of amorphous polymer which change from a hard to a soft state. This can be manifested in many ways such as a change in tensile or shear modulus (or stiffness), the coefficient of thermal expansion, and heat capacity. At temperature below transition, the material becomes stiff and brittle with high modulus and glassy. In the transition region the stiffness decrease and at high temperature, the material will exhibit rubber-like elasticity as a result of entanglement. If material is not crosslinked, and thermal degradation not occur, further increase in heat temperature will eventually result in rubbery flow as the entanglement begin to slip [74, 75].
The Tg of lignin is different from different species of wood or biomasses. Usually, differential scanning calorimetry or DSC has been used to determine the DSC of materials including lignin. Another technique to investigate the Tg is DMA. Table 2 shows the Tg in different lignin types.
Another property of lignin that has been considered essential is its molecular weight. Lignin is a three-dimensional amorphous polymer with a high molecular weight [76]. The molecular weight data of lignin provide some important information in defining the end products by understanding the reactivity and physicochemical properties of lignin. In kraft pulping, for example, hydroxide and hydrosulfide anions break down aromatic ether linkages, resulting in smaller water/alkali-soluble lignin particles. Because of their reduced molecular mass, these pieces diffuse quickly in black liquor [77]. This affects the properties of lignin. Different lignin isolation processes give different numbers of molecular weight data. Table 3 show the polydispersity indices and average molecular weights of lignin from different type of biomasses.
The number of average molecular weight is defined in Eq. 1
Where \(\text{N}\text{i}\) stands for polymer chain numbers, \(\text{M}\text{i}\)is chain molecular weight
The weight average molecular weight (Mw) is defined in Eq. 2
Polydispersity (PD) is defined in Eq. 3
Flame retardancy principle and characterization
The fire behavior of polymers is nowadays an important issue in the design and production of polymer objects. The widespread utilization of polymer materials in various sectors has increased the risks related to the development of fires [87]. There are two main methods to make a polymer FR. In the first method, an FR is a melt blended with polymer via the conventional processing methods (e.g., extrusion), namely additive way. The second method consists of chemical modification of polymer chains to integrate the FR directly on polymer during polymerization, namely reactive way. Also, it should be mentioned that the FR can be also applied on the surface of an object in some limited cases, namely coating. The additive way is generally used in industry since it is cost-effective compared with the reactive way.
Once FR is incorporated into the polymer, there are two possibilities of action in gas or condensed phases to increase the flame resistance behavior. In the gas phase, it can liberate inert gases like CO2 and NH3 to dilute the gas phase or in some cases, the emission of active radicals like halogenated or phosphorus radicals helps to trap the reactive radicals like H● and OH● which aids in the propagation of flame. In the condensed phase, the main action of FR is the formation of a residue/char layer which plays a protective role, and also by transformation of polymer to carbon/char-based material; the flammability of polymer is decreased. The protective role has a significant impact on the deceleration of combustible gases to the flame and it also acts as a physical protective barrier against external heat flux and prevents the diffusion of heat into the material. There are different families of FRs acting by one or several actions in the condensed and/or gas phases including halogenated, phosphorus, nitrogen, mineral, and intumescent FRs. In recent years, the concerns related to environmental and health risks related to some halogenated FR push academic and industrial researchers to develop new FRs from biobased materials. Also, the development of biobased polymers necessitates the use of biobased FR in these polymers to assure they are fully biobased materials. For now, there are two sources to produce the biobased FR including animals (like chitosan and DNA), and biomass (lignin, phytic acid, tannins, cellulose, starch, and proteins) (Fig. 8) [88].
The origin of biobased flame retardants. Reproduced with permission from Vahabi et al. [87]. Copyright 2021 Elsevier Ltd.
The characterization techniques of flame retardancy behavior are generally very specific apparatus. Therefore, a brief description of different techniques is given herein. The most famous techniques for flame retardancy evaluation of materials are Underwriters Laboratories (UL94), LOI, PCFC analysis, and cone calorimetry.
LOI (European standard EN ISO 4589-2: 2017) [87, 89] value indicates the lowest amount of oxygen in a mixture of oxygen and nitrogen for which a flame appears when the sample is ignited. For that, the sample should resist at least 3 min against flame or a length of sample 5 cm is burnt. The dimensions of the sample are 80 × 10 × 4 mm3. The higher value means better flame retardancy behavior [90].
A small amount of material (between 2 and 4 mg) is pyrolyzed in a chamber in the presence of nitrogen, then the gases generated from the pyrolysis are transferred to another chamber, where the combustion occurs in the presence of oxygen (ASTM D7309-21) [87, 91]. The energy released can be calculated using Huggett’s relation (1 kg of oxygen used in burning equals 13.1 MJ) [92]. The most important parameters obtained are the total heat release (THR) and the peak of the heat release rate (pHRR).
Cone calorimetry is the most useful laboratory test for flame retardancy behavior evaluation of polymers. Like in PCFC, the consumption of oxygen is monitored in a cone calorimeter for heat release rate calculation. The most important parameters obtained from the cone are the peak of pHRR (kW/m2), THR (kJ/m2), and total smoke rate (m²/m²) time to ignition (TTI) (s).
UL94 is a simple test that is well known in the industry. There are two configurations for testing materials in horizontal (H) or vertical (V) positions. The ignitability and spread of flame are evaluated by UL94 V testing and let to classify samples with V-0, V-1, or V-2 classes representing high, medium, and low flammability, respectively.
Lignin based flame retardant
According to a review done by Xiao et al. [17], lignin-based FR could be classified into four groups, namely, (i) single-component lignin-based FR, (ii) lignin-based composite FR, (iii) chemically modified lignin FR, and (iv) nano-lignin FR, as shown in Fig. 9.
Types of lignin-based flame retardant [17].
Lignin FRs are single-component products that contain only lignin as a flame retardant. During the heating process of lignin, a carbon layer is formed that acts as a condensed phase, isolating oxygen and inhibiting the diffusion of combustible gases, thereby inhibiting thermal cracking and combustion. A char former (carbonizing substance), a dehydrating agent (which, upon decomposition, releases an acid capable of esterifying hydroxyl groups), a foam-forming substance (which, upon decomposition, releases large quantities of non-flammable gases), and optional modifiers are typically combined to produce an intumescent FR finish (stabilizers, synergists). Because lignin can only be used as a source of carbon in a system, its FR efficiency is low when compared to other natural materials. Lignin can be combined with other acid and gas sources to create an FR system that is intumescent as a result of its inflammability.
Lignin structure can be modified to obtain lignin FR with high FR efficiency and durability [93]. Typically, this is accomplished by chemically introducing phosphorus, nitrogen, and metal elements [94]. Several different functional groups are present in it [94], including methoxyl, alcoholic hydroxyl, and polyphenolic hydroxyl. These functional groups can serve as a large number of active sites for subsequent chemical modification. Chemical modification can also be used to increase the lignin content of the material at the same time. Dispersion and interfacial interaction are two issues that have been raised. Unmodified lignin frequently formed agglomerations in the polymer matrix, resulting in reduced flame retardancy. Chemical modification could only partially solve the problem because only a small amount of flame retardant moieties can be added to lignin. As a result, when high loading levels of lignin-based FR were used, inferior mechanical strength, flexibility, and ductility were observed.
Recent development, characterization, and application of lignin-FR biocomposites
Single-component lignin flame retardants
Direct lignin application as FRs in polymer composite and coatings is the most straightforward method to enhance the fire retardancy abilities of a material [95]. Table 4 summarizes the peak heat release rate (pHRR), the time to ignition (Tign), pHRR reduction, and total heat release (THR) of several lignin-based composites added with single-component lignin FRs. Lignin generally increased the time to ignition of polymer blends. Reduced pHRR is also a frequent observation following lignin incorporation. Both the amount of lignin and the type of additives used have a strong influence on the observed trend. Song et al. investigated the effects of dispersion and lignin concentration on the thermal characteristics and flame retardancy of the styrene-acrylonitrile–butadiene (ABS) copolymer [96]. Lignin was added to ABS at concentrations of 5, 10, and 20% by weight. TGA revealed that the char residue of the ABS and its blends increased as the lignin content increased under nitrogen conditions. At 650 °C, pure ABS without lignin has a char residue of 1.9%. Meanwhile, ABS containing 5, 10, or 20 wt% lignin contains 4.9, 7.0, or 14.5% char residues, respectively. Additionally, adding 20 wt% lignin could reduce the pHRR of ABS/lignin blends by 32%.
Canetti et al. [97] found that the incorporation of 5 wt% and 15 wt% lignin to PP resulted in higher char residue of the composites under both inert and oxidative conditions, respectively. Lignin present in the blend is capable of producing a significant amount of char and is responsible for the increase in the degradation of blend temperature over time. The char is a carbohydrate-based residue that degrades slowly due to the action of oxygen and water. Specifically, the increase is greater for experiments conducted in an atmosphere containing air, as the interactions between PP and charring lignin form a protective surface shield that inhibits oxygen diffusion into the polymer bulk during the experiments. Apart from this, Fernandes et al. [98] also confirmed that poly(vinyl alcohol) (PVA) films with improved thermal and photochemical stability could be attained by the addition of kraft lignin derivatives. A study by De Chirico et al. [19] also confirmed the role of lignin as FR for PP. However, synergist effects with some phosphate compounds such as melamine phosphate (MeP), PVA, aluminum hydroxide (Al (OH)3), ammonium polyphosphate (APP), and monoammonium phosphate (AHP) have further improved the fire properties of PP composite as indicated by the reduction of 64 to 78% in HRR.
Lignin-based composite flame retardant
Lignin has been extensively studied in combination with conventional FRs such as magnesium hydroxide (MDH), ammonium dihydrogen phosphate (ADP), boric acid, and ammonium polyphosphate (APP) to establish a more efficient FR system. Among these, APP is the polyphosphate that has received the most research attention. As reported by Xiao et al. [17], lignin can be used together with FRs to form a synergistic effect, in addition to being compounded with polyphosphates as described above. In addition, lignin can be combined with other biomass materials such as chitosan, phytic acid, and so on to form composites. Chitosan is a naturally occurring carbon source that, when burned, can cause pores to form on the surface of polymers. The carbon layer prevents the matrix from burning and decomposing further, and it also serves as an FR. As an acid, phytic acid can aid in the dehydration of materials and the formation of charcoal, as well as play a role in the production of condensed phase FR [17].
When lignin and polyphosphate are combined, polyphosphate can act as an acid source and a gas source [99]. A reaction between the lignin used as a carbon source and the inorganic acid released from polyphosphate causes the system to crosslink, expand, and foam. The non-combustible gas is responsible for the system’s expansion and foaming, while the lignin is dehydrated and carbonized, which further causes the system to expand and foam. The two chemicals are combined to form an intumescent FR, which has the potential to have a synergistic effect. Verdolotti et al. [100] observed the effect of APP and alkaline lignin (AL) on the FR properties of thermoplastic zein-based composites using a laboratory setting. It was found that the addition of both APP and AL improved the fire behavior of the composite. Poly (ethylene glycol) (PEG) volatilization and concurrent lignin degradation after AL addition result in a severe shrinkage of the zein structure, causing the film to be withdrawn from the flame. Meanwhile, APP with its superior charring effect enhanced the fire-retardant behavior of the composites. In addition, when the mass fraction of APP was 10% and AL was 10%, stiffness and tensile strength were improved. At the same time, satisfactory toughness properties could also be obtained.
Similarly, Cayla et al. [101] investigated the FR properties of polyamide 11(PA11) using kraft lignin and APP. The pHRR of PA11 after blending was reduced by 66% when compared to the original PA11. It was necessary to prepare the alkali lignin/APP intumescent FR polyurethane foam (PUF/IFR) by adding an intumescent flame retardant (IFR) to the polyurethane foam before adding it to the polyurethane foam. The limit of PUF/IFR is set at 30%. The LOI reached 26.3%, which is a high value. The synergistic effect of lignin and APP in the composite has a good FR effect. Physical blending is the most common method of preparation, and the process is straightforward. Physical blending, on the other hand, is frequently insufficient to achieve uniform interfacial interaction and dispersion of lignin in the polymer matrix in many cases. It can be resolved by blending, and the amount of lignin present and the ratio in which it is present have a significant impact on the flame retardancy of the material. Coates et al. [102]studied blending phytic acid and lignin effect on the FR properties of PLA. Comparing pure PLA to the combination of lignin and phytic acid, the combination reduced the pHRR of PLA by 44%, and the FR grade test resulted in a V-2 rating. Phytic acid containing a high concentration of phosphorus not only has good flame retardancy but also can better disperse lignin particles into the matrix, thereby resolving the problem of uneven dispersion of lignin particles in the matrix material.
Chemically-modified lignin flame retardant
Lignin contains a variety of chemically reactive sites, including carboxyl, carbonyl, hydroxyl, and methoxyl groups, among other things. Owing to their high abundance and reactivity, these hydroxyl groups (aliphatic hydroxyl or phenolic hydroxyl) could be chemically modified by the compounds they interact with. A review by Yang et al. [16] has identified four major types of chemically modified lignin that have been used as FR, namely, (i) nitrogen or phosphorus modified lignin, (ii) phosphorus-nitrogen modified lignin, (iii) phosphorus-nitrogen modified lignin-containing metal ions, and (iv) combination of lignin with silica-containing flame retardants. Table 5 displayed the types of chemically modified FR and their main functions. Chemical structures of lignin modified chemically with phosphorus, nitrogen, and metal ions are shown in Fig. 10.
Phosphorus modified lignin
Following the findings of the study by Dorez et al. [103], the acid phosphonic moiety could react with lignin hydroxyl groups, resulting in the formation of covalent P-O-C bonds and the modification of lignin’s surface by octadecyl phosphonic acid. In this regard, Ferry et al. [104] modified alkali lignin with a variety of phosphorous compounds containing P-OH moieties, including dihydrogen ammonium phosphate (DHAP), a homopolymer of (methacryloyloxy) methyl phosphonic acid (HomoP), and a copolymer of (methacryloyloxy) methyl phosphonic acid and methyl methacrylate (Hom (CopoP). They concluded that DHAP is a superior grafting material because it produces a more thermally stable residue than the other materials.
Afterward, both unmodified and modified lignin were blended into polybutylene succinate (PBS), and the combustion properties of the polymer composite were investigated using pyrolysis combustion flow calorimetry (PCFC) and thermogravimetric analysis. The findings suggested that the pHRR decreased as the lignin content increased, but that the effects of phosphorus grafting are not as noticeable when compared to unmodified lignin. The results of cone calorimeter tests, on the other hand, suggested that pHRR decreased as the lignin content of the samples increased. According to the results of the study, PBS-grafted lignin has significantly lower pHRR than PBS-unmodified lignin. It is noteworthy that the ignitability of PBS increased after the addition of lignin, as evidenced by the reduction in the time required for ignition. While pure PBS required 72 s to ignite when 20 wt% unmodified and modified lignin was added, and the time was cut in half to 37 to 42 s. Overall, the research conducted by Ferry et al. [104] revealed that grafting phosphorous compounds onto lignin did not result in a significant improvement in the fire behavior of the PBS composite when compared to the control group. As a result, the modified lignin caused a more rapid decrease in HRR after ignition, which demonstrated that the phosphorus promoted char formation on the sample surface. As the authors pointed out, the most noticeable difference between unmodified lignin and modified lignin is found in the residues of the two substances. When compared to unmodified lignin, the residue of grafted lignin is more homogeneous and compact, which may have contributed to a greater barrier effect against fire.
A study conducted by Prieur et al. [105] evaluated the fire retardant properties of ABS polymer after incorporation of phosphorylated lignin (P-lignin) that had been modified with phosphorus pentoxide (P2O5). The results proved that the ABS/P-lignin composite has the highest char residue, with 3.3 wt% and 17.1 wt%, respectively, when exposed to a thermo-oxidative and an inert atmosphere. In terms of fire performance, ABS composites containing P-lignin as well as unmodified lignin have a shorter time to ignition when compared to ABS composites that are pure ABS. However, both ABS composites incorporating P-lignin and ABS composites incorporating unmodified lignin had significantly lower pHRR and THR than the control samples. Comparing P-lignin with unmodified lignin, we found that it reduced both pHRR (58% vs. 43%) and THR (20% vs. 13%) significantly more effectively. Mendis and colleagues [106] used pyridine to catalyze the reactions of lignin and diphenylphosphine. Phosphorylation of lignin occurred as a result of the esterification of acid chlorides, and phosphorus was introduced into lignin to improve its carbon-forming ability. When 10 wt% of modified were incorporated into epoxy composites, a 40% reduction in pHRR and a 20% reduction in THR were achieved.
Nitrogen modified lignin
Among the nitrogen sources used in lignin modification, melamine and urea are significant contributors. A novel intumescent flame retardant (IFR) was developed by Fu and Cheng [107] by using melamine modified enzymatic hydrolysis lignin (MEHL) as the starting material. To create an IFR system, 12 phr of microencapsulated red phosphorus (MRP) and 0–60 phr of methacrylic acid (MEHL) were incorporated into ethylene propylene diene monomer (EDPM) rubber. Evaluations were conducted on the thermal stability and flammability of IFR and EPDM composites. In the study, it was discovered that the addition of 12 phr of MRP to 10 and 20 phr MEHL did not result in a significant improvement in inflammability, except for a slight improvement in the LOI index. FV-2 could be achieved with the addition of 30 phr MEHL, and FV-1 could be achieved with the addition of 40 phr MEHL. In the case of 50 phr being added, the best results were obtained, with FV-0 being achieved and the LOI reaching 35%. An increase in the number of hours worked, for example, 60 per hour, would only slightly improve the LOI to 36%. Starting with a 40 phr MEHL addition, no dripping can be observed, indicating that the EDPM rubber has excellent FR and anti-dripping properties at this level of incorporation.
Zhang et al.[108] conducted a study in which the lignin was modified by urea, which was similar to the one described above. Poly (lactic acid) (PLA) was treated with polyphosphate (APP) and urea-modified lignin to improve its FR properties. This resulted in the development of a novel intumescent flame retardant (IFR) system. It was found that using a blend of 77 wt% PLA, 4.6 wt% urea modified lignin, and 18 wt% APP (urea modified lignin ratio: APP of 1:4) yielded the highest LOI value of 34.5% and the highest V-0 rating possible. Furthermore, data from cone calorimeters indicated that PLA fabricated using this formulation had a prolonged time to ignition, as well as the lowest pHRR and THR values possible. Furthermore, the highest thermal stability was observed, as evidenced by the presence of the greatest amount of char residue. In general, urea-modified lignin outperformed unmodified lignin in terms of flammability and thermal stability enhancement, with the latter being significantly better.
Phosphorus-nitrogen modified lignin
Owing to the synergistic effect between the elements of phosphorus and nitrogen, phosphorus-nitrogen-modified lignin provides even greater thermal stability and flame retardancy to the material than natural lignin alone. Costes et al. [109] grafted phosphorus and nitrogen elements onto the lignin structure of PLA to enhance the FR properties of the material. It has been demonstrated that modified lignin has a significant impact on the flammability of FR composites and PLA/modified lignin composites, with this effect being especially noticeable. Incorporation of phosphorus-nitrogen modified lignin was also reported on several other polymer matrices such as epoxy, polypropylene (PP), polyurethane (PU), etc.
Increased char residue is one of the most prominent observations for lignin grafted with phosphorus and lignin (PN-lignin). PN-lignin showed an initial degradation at 240 °C and a char residue of 61.4 wt% at 600 °C, while unmodified lignin has initial degradation at 250 °C and char residue of 40.7 wt% [110]. Another study by Costes et al. (2016) reported that kraft lignin and organosolv lignin has 50 and 48 wt% residues at 800 °C, respectively, while PN-kraft lignin and PN-organosolv lignin respectively recorded residue of 58 and 60 wt% [102]. Dehydration action of phosphoric acid derivatives is one of the probable reasons that contribute to the high char residue of PN-lignin. The high content of char could confer better thermal stability and flame retardancy to the polymers [110].
The incorporation of PN-lignin improved the flame retardancy of polymer composites. Zhu et al. (2014) reported that pure PU has LOI value of 18.9%, which indicates the PU sample was quickly ignited, with rapid flame propagation, and a large amount of thick smoke was produced during the combustion process [111]. The higher the LOI value, the better the flame retardancy of a material. In addition, melt-dripping with fire was also observed during burning. However, increased lignin-based phosphate melamine compound (LPMC) content certainly improved the LOI values of the PU composites. The highest LOI value of 28.3% was observed when 20% LPMC was added. It was found that V-1 grade could be achieved when 15% and higher LMPC were employed. A similar observation was also reported by Zhou et al. (2020) [112] where the addition of 2 wt% PN-lignin could improve the LOI value of epoxy composite from 22.3 to 28.3%. At the same time, V-1 grade could be achieved [112]. When 7 wt% PN-lignin was added, the epoxy composite could reach a V-0 grade in the vertical burning test. Generally, the addition of PN-lignin reduced the time to ignition (Tign), peak heat release rate (PHRR), and total heat release (THR) of the composites (Table 6). Polypropylene (PP) composite incorporated with 30 wt% PN-lignin has higher Tign, lower PHRR, and THR than PP/lignin composite. The average mass loss rate (AMLR) of PP/PN-lignin composite is also lower than PP/lignin composite [110]. Meanwhile, the addition of 7 wt% PN-lignin into epoxy has reduced the Tign, pHRR, and THR of the composite. In addition, the total smoke production rate (TSP) was also significantly reduced [112].
PN-lignin conferred superior flame retardancy to the polymer composite compared to that of unmodified lignin. The flame retardancy mechanism is therefore investigated through the analysis of char residue using digital photos and SEM. Yu et al. (2012) observed that pure PP did not leave behind any char residue after the cone calorimeter test. Meanwhile, PP/lignin composite leaves a thin and damaged char layer after combustion is done. PP/PN-lignin composite, however, produced a thick and compact char layer which is primarily responsible for the improved flame retardancy. SEM photos showed that a loosely spheroidal structure presents in PP/lignin composite, probably caused by the volatile gases generated during the burning process. As for the PN-lignin composite, a continuous and compact structure was formed as a result of the strong dehydration effect of polyphosphoric acid generated during combustion. Therefore, Yu et al. (2012) concluded that the flame retardancy mechanism in the condensed phase plays a vital role while the compact char layer mainly contributes to the superior flame retardancy of the PP composites [110]. Lignin normally acts as a carbon source in the flame retardant system but the effects of the addition of lignin alone are rather limited. Owing to that, the gas source and the acid source are required at the same time to achieve the best flame retardancy. PN-lignin serves as an ideal candidate as it can reduce combustibles in the gas phase as well as catalyze carbon fixation in the condensed phase. The amino group, on the other hand, increases the nitrogen compounds in the gas phase products and subsequently brings down the oxygen concentration, which dilutes the combustible gas and prevents the fire from spreading [112]
piperazine-modified lignin and aluminum phosphate as capsule materials, Zhong et al. [114] demonstrated that piperazine could be attached to the lignin molecules and then microencapsulated into red phosphorus FRs with multi-level FR properties, resulting in the development of halogen-free red phosphorus FR. Using an FR grade of ABS composite containing 25% FR, it is possible to achieve the V-0 level of performance. The LOI value reaches 26.1%. By contrast, when compared to ABS resin that does not contain an FR, the pHRR and the total smoke produced during the combustion process of ABS are both reduced by 25.8% and 63.1%, respectively. First, the dense carbon layer catalyzed by the phosphorus element and the ammonia gas released by the nitrogen element must be formed to achieve halogen-free environmental protection as well as high-efficiency FRs
Phosphorus-nitrogen modified lignin-containing metal ions
Although phosphorus-nitrogen-modified lignin has proved to be able to enhance the flame retardancy of polymer composite, it is, however, required in high loading levels to meet the flame-resistance standards. Therefore, the addition of synergists or catalysts, for examples metal ions such as zinc (II), copper (II), and nickel acetates, has been practiced to improve the efficiency of phosphorus-nitrogen modified lignin [110, 115, 116]. The addition of metal ions could enhance the smoke suppression ability and fire retardancy of polymer composites. When metal compounds are added to polymer composites, they work with phosphorus and nitrogen elements to make them more fire-resistant. Even at a low loading level, this has been shown to work [117]. The possible mechanism is that it promotes polymer dehydrogenation and catalyzes the char formation in the condensed phase.
Table 7 shows the cone calorimeter measurement of lignin-based composites. Three types of salt acetates, namely nickel (Ni2+), cobalt (Co2+), and zinc (Zn2+) into intumescent FR based on modified lignin (PN-lignin) with functionalized by grafting phosphorus − nitrogen elements, to further improve the thermal and flammability of polypropylene (PP) composite[110]. In comparison to PP/PN-lignin composite, introducing metal ions reduced pHRR and THR of the composite. However, it noted that both cobalt and zinc acetates did not yield obvious effects on flame retardancy. On the other hand, nickel acetates have contributed significantly to flame retardancy and shown increased char residue and LOI.
To improve the flame retardancy of polypropylene/wood composites (PP/WP), Liu et al. [116] grafted lignin with phosphorus and nitrogen and coordinated it with cupric (Cu2+) ions, a metal element. After that, the functionalized lignin (F-lignin) was mixed into PP/WP composites at various loading levels. The addition of unaltered lignin (O-lignin) to the flammability of the PP/WP composites tested with UL-94 demonstrated no improvement in UL-94 categories. When 15 wt% O-lignin was added, and the V-2 rating was obtained. Meanwhile, when 15 wt% F-lignin was added, a V-1 rating was obtained, suggesting better flame retardancy than O-lignin counterparts at the same loading level. PP/WP composites are highly flammable, as evidenced by their quick ignition time and high peak heat release rate (pHRR) of 595 kW/m2 and THR of 93.9 MJ/m2, as indicated in Table 7. The lignin addition, whether modified or unmodified, often increased the Tign time by a few seconds, with F-lignin having a stronger effect. As a result, F-lignin can give PP/WP composites superior FR characteristics than O-lignin. Alkali lignin treated with phosphorus, nitrogen, and zinc (II) ions yielded comparable results (PNZn-lignin) [115]. Polybutylene succinate (PBS) composites added with PNZn-lignin had reduced pHRR, THR, AMLR, and TSR and the extent of reduction increased along with increasing PNZn-lignin loading levels. Moreover, a significant increment in char residue was observed in the PBS composite added with 10 wt% PNZn-lignin (54.6 wt%) compared to that of pure PBS (9.3 wt%). The strong dehydration action of phosphoric acids, the strong smoke suppression impact of zinc (II) ions, and the high char-forming capacity of lignin all combine to create a dense, thick, and powerful layer of char on the composites surface, significantly reducing the flammability and smoke emission of PBS.
Nanolignin
Nanoscale FR has seen rapid development in the past few decades as filler in polymeric composites. Commonly, these nanofillers do not possess good fire retardancy inherently [118] but could improve the thermal stability and fire properties of the composite even at a very low incorporation level [119]. According to a review by Vahidi et al. [120], common nano FR used in polymer composites includes metallic particles, bio-based fillers, and those from the carbon family. Common metallic particles include zinc oxide and aluminum tri-hydroxide (ATH). Nanolignin (LNP) falls into the class of bio-based fillers, together with starch, protein, and cellulose nanocrystals. Meanwhile, examples from the carbon family include carbon nanotube, graphene, and fullerene. Thanks to their incombustible properties, nanoclays are also a widely used FR in polymer composites. As a focus of this paper, LNP can be applied as a source of carbon in the system of polymer-based FR, and it has an effect in the condensed phase by forming char residue. Nanolignin’s main way of working is the same as that of lignin because their chemical structures are very close to each other. Because of its small size, LNP has more potential than lignin. This is because LNP can be dispersed at a nanometer level in polymers, which makes it more useful [121].
The effectiveness of LNP in improving flame retardancy and the thermal stability of polymer composites has been proven by several researchers. Through the incorporation of LNPs, Wang et al. [122] created a boron nitride (BN)-OH/polyvinyl alcohol (PVA) composite film with improved thermal conductivity, thermal stability, and flame retardancy. In the experiment, 3 mL of a 10% PVA solution and various volumes (1, 3, 6, and 9 mL) of a 1% LNP suspension were mixed (PVA/LNP). The mixture of PVA/LNP was then gently applied to the BN-OH specimen, dried at room temperature for two days, and crosslinked in glutaraldehyde at 25 °C. After crosslinking, the thermal stability of the BN-OH/PVA/LNP composite film improved even more, with a breakdown temperature of 310 °C compared to 282 °C before crosslinking. The authors concluded that the LNPs could act as a filler to fill the air voids in the polymers and hence enhance their thermal conductivity. On the other hand, Yu et al. [123] prepared a melamine-formaldehyde (MF) sponge with the addition of LNP and acetylated LNP. LNP was dissolved in a 100 mL Tetrahydrofuran (THF)/H2O (80:20, vol%) mixture, and the MF sponge was immersed in the solution. Next, the THF was evaporated and the sponge was dried in the oven. Polyurethane foam (PUF) sponge, as control, resulted in bright and vigorous flame when being ignited by an alcohol lamp. At the end of burning, PUF left only a little amount of residue. Both lignin MF sponge and acetylated MF sponge experienced a weak flame which extinguished within 3 s, and around 70% residues were recorded.
Chollet et al. [124] made LNP from microparticles of kraft lignin (LMP). Diethyl chlorophosphate (diet) and diethyl (2-(triethoxysilyl)ethyl) phosphonate (SiP) were utilized to functionalize LNP and LMP, which were subsequently employed to prepare PLA composites. Using the cone calorimeter test at 35 kW/m2, showed that the pHRR did not show a significant reduction when unmodified lignin was used, regardless of lignin particle size. It was reported that a reduction of 10% in pHRR was only observed when 20 wt% LMP was added. The findings suggested that the incorporation of unmodified lignin particles did not lead to any significant improvement in thermal stability and fire properties of polylactic acid. Nevertheless, the application of phosphorylated lignin seems to provide promising improvement in terms of fire behavior, particularly lignin functionalized with SiP. When comparing LMP and LNP, nano-scaled LNP remains effective in terms of FR effect even at a relatively modest loading level of 5 wt%. Prolonged ignition time of 84 s and 11% reduction in pHRR was recorded.
Improvement of mechanical strength of lignin-based FR composites
One of the drawbacks of incorporating modified in the polymer composite is their reduced mechanical strength, probably due to poor interfacial adhesion between lignin and the polymer matrix. Zhou et al. (2020) reported that the tensile strength of epoxy composites declined when modified lignin was added [112]. After the addition of 7 wt% PN-lignin, the tensile strength, tensile-elasticity modulus, and elongation at the break of the epoxy reduced by 56.8%, 33.8%, and 4.7% compared to that of the pure epoxy. A similar observation was also reported by Zhang et al. (2012) where PLA composite added with urea-modified lignin displayed reduced tensile strength and elongation at break [108]. Uniform dispersion of lignin-based FR in the polymer matrix is often an issue to be solved [125]. Unmodified lignin often formed an agglomeration in the polymer matrix and led to reduced flame retardancy. Chemical modification could only solve the issue partially as only a small quantity of flame retarding moieties can be added to lignin [16]. As a result, inferior mechanical strength, flexibility, and ductility was observed when a high loading level of lignin-based FR was used. On that account, several works as shown in Table 8 have emphasized improving the flame retardancy of the composite without compromising much on the mechanical properties.
Comparison between commercial lignin and modified lignin
Different technical lignins as FR were compared by Widsten et al. [129]. Commercial softwood kraft lignin (KL), CatLignin (CL), and hydrolysis lignin (HL) were chemically modified using different ways. CatLignin is lignin prepared by heat treatment of kraft black liquor. This technical lignin was chemically modified by Mannich reaction, acetylation, and phosphoric acid (H3PO4) + urea. The flame retardancy performance of the melt-blended composite containing 70% PP and 30% lignin was evaluated. The results revealed that PP composites with unmodified KL showed no significantly lower PHR and THR compared to pure PP. Meanwhile, unmodified CL and HL composites had 18–25% lower PHR and THR compared to that of pure PP. In addition, no char was observed for KL composite while some chars were observed for CL and HL composites. More interestingly, APP/CL and APP/HL composites exhibited lower PHR and more char residue compared to APP/pentaerythritol (PER) composites, indicating CL and HL could be a potential substitution for PER in the APP/PER intumescent system.
As for lignin modified with Mannich modification, PP/KL-M and PP/CL-M had 21–24% lower PHR and THR compared to unmodified PP/KL and PP/CL composites. PP/CL-M composites performed the best as they had 41% lower PHR and 36% lower THR compared to that pure PP. Acetylation, on the other hand, provided very limited effects on HL and CL, but showed a rather significant effect on KL. PP/KL-A composites have better flame retardancy than KL and APP/KL composites. Hydroxyl groups of the lignin before and after acetylation showed a high degree of acetylation. The only high number of aliphatic hydroxyls remained in HL. Lignin functional groups such as hydroxyl and carboxyl content were determined based on the lignin phosphitylation procedures specified in Granata and Argyropoulos [130]. Next, the solution was then subjected to 31P NMR on a Bruker 500 MHz NMR spectrometer at room temperature. Treatment with phosphoric acid + urea led to unsatisfactory improvements in PHR and THR in comparison with lignin modified with Mannich modification and acetylation.
Challenges and future perspective
Overall, because of its high charging capacity, lignin has a promising future as an intumescent FR system, particularly in the construction and building industry. The global lignin market, which is expected to reach USD 770 million in 2020 and grow at a compound annual growth rate (CAGR) of over 5.5% between 2021 and 2027 [21], indicates that there is increasing demand for an abundance of lignin throughout the world. Aside from the applications covered in this review, a small number of researchers throughout the world have expanded the use of lignin in the production of FR textiles to include other applications [131]. This will undoubtedly broaden the range of lignin’s potential applications as an FR.
The current lignin-based FRs and their polymer composites face some challenges, despite their many advantages. One of the most difficult challenges in achieving uniform dispersion of lignin in the polymer through physical blending, is frequently difficult to achieve. Nonetheless, lignin modifications to improve its compatibility with polymer matrixes may be able to resolve this issue in the future [96]. Meanwhile, Xiao et al. [132] have identified some additional challenges as well as future research that needs to be carried out. According to the authors, when lignin is combined with other substances, it is difficult to maintain control over the ratio and amount of each ingredient added. Considering that a small amount of addition will have an impact on the FR effect and a large amount of addition will have an impact on the physical properties of the material, determining the optimal ratio and dosage will be an important part of future research in this area. Second, although the method of chemically modifying lignin can produce a more effective FR effect, it is more expensive. However, because the process is relatively complex, it is recommended that the process flow be simplified and that environmental improvements be implemented. Thirdly, the structure of lignin should be rationally designed to improve the flame retardancy of lignin while also endowing it with multiple functions such as antibacterial and dyeing properties, among others.
Concluding remarks
Functionalization of lignin as a bio-based FR for the fabrication of long-lasting and polymeric materials with high performance has become a prominent and promising topic in the last decade. The proclivity of lignin to produce substantial char residues during thermal breakdown improves the flammability of finished polymer composites when it operates in the condensed phase. Lignin structures that correlated with thermal properties such as Tg may be nonuniform because of different extraction methods and lignin sources. In general, the extraction method of lignin can be distinguished into two types: sulfur-free and sulfur-bearing processes. The sulfur-free process collected kraft and lignosulfonates while sulfur-bearing obtained organosolv and soda lignin. Typically, organosolv lignin has low Tg content than kraft lignin. However, the utilization of pristine lignin faced some limitations in the final polymer specially to meet industrial requirements tests. Besides, simple physical blending generally fails to address the uniform interfacial interaction and dispersion of pristine lignin inside the polymer matrix. Therefore, chemical modification of lignin by introducing nitrogen and/or phosphorous is required to improve the utilization of lignin as FR. Moreover, nano-scale of lignin can reduce large agglomerates in the polymer matrix as one obstacle of pristine lignin that can interfere with the efficiency of flame retardancy. A substantial improvement in the FR behavior is achieved with the use of modified lignin or lignin on the nanoscale. Lignin can be applied as FR in two different ways: reactive (chemical alteration) and additive (melt-blending process) while the additive process is more popular in industrial processes due to cost efficiency. Eventually, FR must analyze according to standard tests for flame retardancy such as UL94, LOI, PCFC analysis, and cone calorimetry. In general, industrial standard requirements of FR such as V-0 rating in UL94 and LOI > 28% can be achieved after lignin modification. Among these, lignin has the potential to be utilized as an FR in industrial processes while being cost-effective. However, the modification process is a challenge that the researcher must investigate.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hobbs CE (2019) Polymers (Basel) 11(2). https://doi.org/10.3390/polym11020224
Ridho MR, Agustiany E, Rahmi MD, Madyaratri E, Ghozali M, Restu WK, Falah F, Lubis MAR, Syamani FA, Nurhamiyah Y, Hidayati S, Sohail A, Karungamye P, Nawawi DS, Iswanto AH, Othman N, Aini N, Hussin MH, Sahakaro K, Hayeemasae N, Ali M, Fatriasari W (2022) Advances in Materials Science and Engineering 2022:1-33.https://doi.org/10.1155/2022/1363481
Hussin MH, Appaturi JN, Poh NE, Latif NHA, Brosse N, Ziegler-Devin I, Vahabi H, Syamani FA, Fatriasari W, Solihat NN, Karimah A, Iswanto AH, Sekeri SH, Ibrahim MNM (2022) Int J Biol Macromol 200:303–326. https://doi.org/10.1016/j.ijbiomac.2022.01.007
Epa U (2018) The Air Pollution Consultant. United States Environmental Protection Energy, New York. US
Melro E, Filipe A, Sousa D, Medronho B, Romano A (2021) New J Chem 45(16):6986–7013. https://doi.org/10.1039/d0nj06234k
Solihat NN, Sari FP, Falah F, Ismayati M, Lubis MAR, Fatriasari W, Santoso EB, Syafii W (2021) Jurnal Sylva Lestari 9(1). https://doi.org/10.23960/jsl191-22
Li Y, Li F, Yang Y, Ge B, Meng F (2021) J Polym Eng 41(4):245–258. https://doi.org/10.1515/polyeng-2020-0268
Solihat NN, Santoso EB, Karimah A, Madyaratri EW, Sari FP, Falah F, Iswanto AH, Ismayati M, Lubis MAR, Fatriasari W, Antov P, Savov V, Gajtanska M, Syafii W (2022) Polymers 14(3). https://doi.org/10.3390/polym14030491
Doherty WOS, Mousavioun P, Fellows CM (2011) Ind Crops Prod 33(2):259–276. https://doi.org/10.1016/j.indcrop.2010.10.022
Sameni J, Krigstin S, Rosa DS, Leao A, Sain M (2014) BioResources 9(1). https://doi.org/10.15376/biores.9.1.725-737
Sen S, Patil S, Argyropoulos DS (2015) Green Chem 17(11):4862–4887. https://doi.org/10.1039/c5gc01066g
Ramezani N, Sain M (2018) J Polym Environ 26(7):3109–3116
Nadda A, Sharma S (2020)Springer Nature, London
Mandlekar N, Cayla A, Rault F, Giraud S, Salaün F, Malucelli G, Guan J-P (2018) An Overview on the Use of Lignin and Its Derivatives in Fire Retardant Polymer Systems. Lignin - Trends and Applications. https://doi.org/10.5772/intechopen.72963
Lia B, Zhanga X, Su R (2002) Polym Degrad Stab 75:35–44
Yang H, Yu B, Xu X, Bourbigot S, Wang H, Song P (2020) Green Chem 22(7):2129–2161. https://doi.org/10.1039/d0gc00449a
Xiao M, Zhou X, Zhang J, Ren Y (2020) J Text Res 41:182–188
Réti C, Casetta M, Duquesne S, Bourbigot S, Delobel R (2008) Polym Adv Technol 19(6):628–635. https://doi.org/10.1002/pat.1130
De Chirico A, Armanini M, Chini P, Cioccolo G, Provasoli F, Audisio G (2003) Polym Degrad Stab 79(1):139–145. https://doi.org/10.1016/S0141-3910(02)00266-5
Handika SO, Lubis MAR, Sari RK, Laksana RPB, Antov P, Savov V, Gajtanska M, Iswanto AH (2021) Mater (Basel) 14(22). https://doi.org/10.3390/ma14226850
Insights GM (2020) (Lignin Market Size, By Product (Kraft Lignin, Lignosulphonates, Low Purity Lignin), By Application (Aromatics, Macromolecules), Industry Analysis Report, Regional Outlook, Lignin Downstream Potential (Vanillin, Carbon Fiber, Phenol, BTX), Application Potential, Price Trends, Competitive Market Share & Forecast, 2021–2027) https://www.gminsights.com/industry-analysis/lignin-market Accessed 3 March 2022
Madyaratri EW, Ridho MR, Aristri MA, Lubis MAR, Iswanto AH, Nawawi DS, Antov P, Kristak L, Majlingová A, Fatriasari W (2022) Polymers 14(3):362
Voelker SL, Lachenbruch B, Meinzer FC, Strauss SH (2011) New Phytol 189(4):1096–1109. https://doi.org/10.1111/j.1469-8137.2010.03572.x
Youssefian S, Rahbar N (2015) Sci Rep 5:11116. https://doi.org/10.1038/srep11116
Zeng Y, Himmel ME, Ding S-Y (2017) Biotechnol Biofuels 10(1):263. https://doi.org/10.1186/s13068-017-0953-3
Chen S, Lin S, Hu Y, Ma M, Shi Y, Liu J, Zhu F, Wang X (2018) Polym Adv Technol 29(12):3142–3150. https://doi.org/10.1002/pat.4436
Zhang S, Li S-N, Wu Q, Li Q, Huang J, Li W, Zhang W, Wang S (2021) Compos Part B: Eng 212:108699. https://doi.org/10.1016/j.compositesb.2021.108699
Zhang YM, Zhao Q, Li L, Yan R, Zhang J, Duan JC, Liu BJ, Sun ZY, Zhang MY, Hu W, Zhang NN (2018) RSC Adv 8(56):32252–32261. https://doi.org/10.1039/C8RA05598J
Lu Y, Lu Y-C, Hu H-Q, Xie F-J, Wei X-Y, Fan X (2017) J Spectrosc 2017:1–15. https://doi.org/10.1155/2017/8951658
Vásquez-Garay F, Carrillo-Varela I, Vidal C, Reyes-Contreras P, Faccini M, Teixeira Mendonça R (2021) Sustainability 13(5). https://doi.org/10.3390/su13052697
Tolbert A, Akinosho H, Khunsupat R, Naskar AK, Ragauskas AJ (2014) Biofuels Bioprod Biorefin 8(6):836–856. https://doi.org/10.1002/bbb.1500
Glasser WG (2019) Front Chem 7:565. https://doi.org/10.3389/fchem.2019.00565
Katahira R, Elder T, Beckham GT (2018) A Brief Introduction to Lignin Structure, in Lignin Valorization: Emerging Approaches. Energy and Environment Series. Royal Society of Chemistry. p. 1–20. https://doi.org/10.1039/9781788010351-00001
Harman-Ware AE, Happs RM, Davison BH, Davis MF (2017) Biotechnol Biofuels 10(1):281. https://doi.org/10.1186/s13068-017-0962-2
Garedew M, Lin F, Song B, DeWinter TM, Jackson JE, Saffron CM, Lam CH, Anastas PT (2020) Chemsuschem 13(17):4214–4237. https://doi.org/10.1002/cssc.202000987
Börcsök Z, Pásztory Z (2021) Eur J Wood Wood Product 79(3):511–526. https://doi.org/10.1007/s00107-020-01637-3
Zhang L, Chen Z, Dong H, Fu S, Ma L, Yang X (2021) J Bioresources Bioprod 6(1):65–74. https://doi.org/10.1016/j.jobab.2021.01.005
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) Chem Rev 110(6):3552–3599. https://doi.org/10.1021/cr900354u
Watkins D, Nuruddin M, Hosur M, Tcherbi-Narteh A, Jeelani S (2015) J Mater Res Technol 4(1):26–32. https://doi.org/10.1016/j.jmrt.2014.10.009
Oriez V, Peydecastaing J, Pontalier P-Y (2020) Clean Technol 2(1):91–115
Chung H, Washburn N (2016) Extraction and Types of Lignin. In: Faruk O, Sain M (eds) Lignin in Polymer Composites. William Andrew Publishing, pp 13–25
Strassberger Z, Tanase S, Rothenberg G (2014) RSC Adv 4:25310
Ľudmila H, Michal J, Andrea Å, Aleš H (2015) Wood Res 60(6):973–986
Aro T, Fatehi P (2017) ChemSusChem 10(9):1861-77. https://doi.org/10.1002/cssc.201700082
Tribot A, Amer G, Abdou Alio M, de Baynast H, Delattre C, Pons A, Mathias J-D, Callois J-M, Vial C, Michaud P, Dussap C-G (2019) Eur Polymer J 112:228–240. https://doi.org/10.1016/j.eurpolymj.2019.01.007
Stenius P (2000)Fapet Oy
Jablonsky M, Kočiš J, Haz A, Surina I, Sládková A (2014) 5th International Scientific Conference Renewable Energy Sources 2014 Tatranské Matliare, High Tatras, Slovak Republic
Laurichesse S, Avérous L (2014) Prog Polym Sci 39(7):1266–1290. https://doi.org/10.1016/j.progpolymsci.2013.11.004
Kumar A, Anushree, Kumar J, Bhaskar T (2020) J Energy Inst 93(1):235–271. https://doi.org/10.1016/j.joei.2019.03.005
Galkin MV, Samec JSM (2016) ChemSusChem 9(13):1544-58. https://doi.org/10.1002/cssc.201600237
Sjòstròm E (1993)Academic Press, Inc., California
Saito T, Perkins JH, Vautard F, Meyer HM, Messman JM, Tolnai B, Naskar AK (2014) ChemSusChem 7(1):221-8. https://doi.org/10.1002/cssc.201300509
Chagas B, Wolski T, Vieira O (2019) Int J Adv Eng Res Sci 6. https://doi.org/10.22161/ijaers.6.4.1
Marangon Jardim J, Hart P, Lucia L, Jameel H, Chang H (2020) Bioresources 15:5464 = 80. https://doi.org/10.15376/biores.15.3.5464-5480
Abd El-Sayed E, El-Sakhawy M, Sakhawy M (2020) Nordic Pulp. Paper Res J 35. https://doi.org/10.1515/npprj-2019-0064. :215 – 30
Al-Kaabi Z, Pradhan R, Thevathasan N, Arku P, Gordon A, Dutta A (2018) AIMS Energy 6:880–907. https://doi.org/10.3934/energy.2018.5.880
Vishtal A, Kraslawski A (2011) BioResources 6:3547–3568. https://doi.org/10.15376/biores.6.3.3547-3568
Ribeiro RA, Júnior SV, Jameel H, Chang H-M, Narron R, Jiang X, Colodette JL (2019) ACS Sustain Chem Eng 7(12):10274–10282. https://doi.org/10.1021/acssuschemeng.8b06635
Deshpande R, Sundvall L, Grundberg H, Germgård U (2016) Nordic Pulp and Paper Research Journal 31:379 – 85. https://doi.org/10.3183/NPPRJ-2016-31-03-p379-385
Li T, Takkellapati S (2018) Biofuels, Bioproducts and Biorefining 12(5):756 – 87. https://doi.org/10.1002/bbb.1913
Luo H, Abu-Omar M (2017) Chemicals From Lignin. In: Abraham M (ed) Encyclopedia of Sustainable Technologies. Elsevier, Oxford, pp 573–585
Lavrič G, Zamljen A, Juhant Grkman J, Jasiukaitytė-Grojzdek E, Grilc M, Likozar B, Gregor-Svetec D, Vrabič-Brodnjak U (2021) Polym (Basel) 13(24). https://doi.org/10.3390/polym13244443
Nasrullah A, Bhat AH, Sada Khan A, Ajab H (2017) 9 - Comprehensive approach on the structure, production, processing, and application of lignin. In: Jawaid M, Md Tahir, P., Saba, N., Lignocellulosic Fibre and Biomass-Based Composite Materials. Woodhead Publishing. p. 165 – 78
Pang T, Wang G, Sun H, Sui W, Si C (2021) Industrial Crops and Products 165. https://doi.org/10.1016/j.indcrop.2021.113442
Rossberg C, Bremer M, Machill S, Koenig S, Kerns G, Boeriu C, Windeisen E, Fischer S (2015) Industrial Crops and Products 73:81 – 9. https://doi.org/10.1016/j.indcrop.2015.04.001
Bhardwaj N, Goyal SK, Gupta A, Upadhyaya JS, Ray AK (2005) Appita J 58:180–185
Omer SH, Khider TO, Elzaki OT, Mohieldin SD, Shomeina SK (2019) BMC Chem Eng 1(1):6. https://doi.org/10.1186/s42480-019-0005-9
Windeisen E, Wegener G (2012) 10.15 - Lignin as Building Unit for Polymers. In: Matyjaszewski KMM (ed) Polymer Science: A Comprehensive Reference. Elsevier, Amsterdam, pp 255–265
Tutus A, Deniz I, Hudaverdi E (2004) Pak J Biol Sci. https://doi.org/10.3923/pjbs.2004.1350.1354
Shao S, Wu C, Chen K (2017) BioResources 12:4867–4880
Rossberg C, Janzon R, Saake B, Leschinsky M (2019)BioResourcesVol 14(2)
Patel J, Parsania P (2018) Characterization, testing, and reinforcing materials of biodegradable composites. In: Shimpi N, Biodegradable and Biocompatible Polymer Composites. Woodhead Publishing. p. 55–79
Irvine GM (1985) Wood Sci Technol 19(2):139–149. https://doi.org/10.1007/BF00353074
Wang R-M, Zheng S-R, Zheng Y-P (2011) 3 - Matrix materials. In: Wang R-M, Zheng S-R, Zheng Y-P, Polymer Matrix Composites and Technology. Woodhead Publishing. p. 101–548. https://doi.org/10.1533/9780857092229.1.101
Abu Ghalia M, Dahman Y (2017) Synthesis and utilization of natural fiber-reinforced poly (lactic acid) bionanocomposites. In: Jawaid MMTPSN, Lignocellulosic Fibre and Biomass-Based Composite Materials. Woodhead Publishing. p. 313 – 45
Abdelaziz OY, Brink DP, Prothmann J, Ravi K, Sun M, García-Hidalgo J, Sandahl M, Hulteberg CP, Turner C, Lidén G, Gorwa-Grauslund MF (2016) Biotechnol Adv 34(8):1318–1346. https://doi.org/10.1016/j.biotechadv.2016.10.001
Garguiak JD, Lebo SE (1999) Commercial Use of Lignin-Based Materials. Lignin: Historical, Biological, and Materials Perspectives. ACS Symposium Series, vol 742: American Chemical Society. p. 304 – 20. https://doi.org/10.1021/bk-2000-0742.ch015
Hatakeyama H, Hatakeyama T (2010) Lignin Structure, Properties, and Applications. In: Abe ADKKS (ed) Biopolymers: Lignin, Proteins, Bioactive Nanocomposites. Springer, Berlin, Heidelberg, pp 1–63
Baumberger S, Abaecherli A, Fasching M, Gellerstedt G, Gosselink R, Hortling B, Li J, Saake B, Jong Ed (2007) 61(4):459–68. https://doi.org/10.1515/HF.2007.074
Asikkala J, Tamminen T, Argyropoulos DS (2012) J Agric Food Chem 60(36):8968–8973. https://doi.org/10.1021/jf303003d
Sarkanen S, Teller DC, Abramowski E, McCarthy JL (1982) Macromolecules 15(4):1098–1104
Fredheim GE, Braaten SM, Christensen BE (2002) J Chromatogr A 942(1–2):191–199. https://doi.org/10.1016/s0021-9673(01)01377-2
Brodin I, Sjöholm E, Gellerstedt G (2009) 63(3):290–7. https://doi.org/10.1515/HF.2009.049
Nascimento EA, Morais SA, Machado AE, Veloso D (1992) J Braz Chem Soc 3(3):61–64
Morais SA, Nascimento EA, Pilo-Veloso D (1991) J Braz Chem Soc 2:129–132
Vahabi H, Laoutid F, Mehrpouya M, Saeb MR, Dubois P (2021) Mater Sci Engineering: R: Rep 144:100604. https://doi.org/10.1016/j.mser.2020.100604
Costes L, Laoutid F, Brohez S, Dubois P (2017) Mater Sci Engineering: R: Rep 117:1–25. https://doi.org/10.1016/j.mser.2017.04.001
ISO 4589-2:2017(E) (2017) Determination of Burning Behaviour by Oxygen Index – Part 2: Ambient-Temperature Test Geneva. International Organization for Standardization
Laoutid F, Bonnaud L, Alexandre M, Lopez-Cuesta JM, Dubois P (2009) Mater Sci Engineering: R: Rep 63(3):100–125. https://doi.org/10.1016/j.mser.2008.09.002
ASTM D7309-21 (2021) Standard Test Method for Determining Flammability Characteristics of Plastics and Other. Solid Materials Using Microscale Combustion Calorimetry ASTM International
Sonnier R, Vahabi H, Ferry L, Lopez-Cuesta JM (2012) Pyrolysis-Combustion Flow Calorimetry: A Powerful Tool To Evaluate the Flame Retardancy of Polymers. Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science. ACS Symposium Series, vol 1118: American Chemical Society. p. 361 – 90. https://doi.org/10.1021/bk-2012-1118.ch024
Hao F, Geng W, Zhang J, Liu Q, Ding B (2019) Wool Text J 47(2):80–85
Huang C, He J, Liang C, Tang S, Yong Q (2019) J Forestry Eng 4(1):17–26
Lizundia E, Sipponen MH, Greca LG, Balakshin M, Tardy BL, Rojas OJ, Puglia D (2021) Green Chem 23(18):6698–6760. https://doi.org/10.1039/D1GC01684A
Song P, Cao Z, Fu S, Fang Z, Wu Q, Ye J (2011) Thermochimica acta 518(1):59–65. https://doi.org/10.1016/j.tca.2011.02.007
Canetti M, Bertini F, De Chirico A, Audisio G (2006) Polym Degrad Stab 91(3):494–498. https://doi.org/10.1016/j.polymdegradstab.2005.01.052
Fernandes DM, Winkler Hechenleitner AA, Job AE, Radovanocic E, Gómez Pineda EA (2006) Polym Degrad Stab 91(5):1192–1201. https://doi.org/10.1016/j.polymdegradstab.2005.05.024
Bai J, Xue B, Yang Y (2017) Eng Plast Application 45(7):119–123
Verdolotti L, Oliviero M, Lavorgna M, Iannace S, Camino G, Vollaro P, Frache A (2016) Polym Degrad Stab 134. https://doi.org/10.1016/j.polymdegradstab.2016.10.001. :115 – 25
Cayla A, Rault F, Giraud S, Salaün F, Sonnier R, Dumazert L (2019) Materials (Basel) 12(7). https://doi.org/10.3390/ma12071146
Costes L, Laoutid F, Aguedo M, Richel A, Brohez S, Delvosalle C, Dubois P (2016) Eur Polymer J. https://doi.org/10.1016/j.eurpolymj.2016.10.003. 84:652 – 67
Dorez G, Otazaghine B, Taguet A, Ferry L, Lopez-Cuesta JM (2013) Journal of Analytical and Applied Pyrolysis 105. https://doi.org/10.1016/j.jaap.2013.10.011
Ferry L, Dorez G, Taguet A, Otazaghine B, Lopez-Cuesta JM (2015) Polymer Degradation and Stability 113:135 – 43. https://doi.org/10.1016/j.polymdegradstab.2014.12.015
Prieur B, Meub M, Wittemann M, Klein R, Bellayer S, Fontaine G, Bourbigot S (2016) Polym Degrad Stab 127:32–43
Mendis G, Weiss S, Korey M, Boardman C, Dietenberger M, Youngblood J, Howarter J (2016) Green Mater 4(4):150–159
Fu RL, Cheng XS (2011) Adv Mater Res 236:482–485
Zhang R, Xiao X, Tai Q, Huang H, Hu Y (2012) Polym Eng Sci 52(12):2620–2626. https://doi.org/10.1002/pen.23214
Costes L, Laoutid F, Brohez S, Delvosalle C, Dubois P (2017) Eur Polymer J 94:270–285. https://doi.org/10.1016/j.eurpolymj.2017.07.018
Yu Y, Jin C, Fu S, Zhao L, Wu Q, Jiewang Y (2012) Ind Eng Chem Res 51:12367–12374. https://doi.org/10.1021/ie301953x
Zhu H, Peng Z, Chen Y, Li G, Wang L, Tang Y, Pang R, Khan ZUH, Wan P (2014) RSC Adv 4(98):55271–55279. https://doi.org/10.1039/C4RA08429B
Zhou S, Tao R, Dai P, Luo Z, He M (2020) Polym Compos 41(5):2025–2035. https://doi.org/10.1002/pc.25517
Wu W, He H, Liu T, Wei R, Cao X, Sun Q, Venkatesh S, Yuen RKK, Roy VAL, Li RKY (2018) Composites Science and Technology 168:246 – 54. https://doi.org/10.1016/j.compscitech.2018.09.024
Zhong R, Lin X, Huang S, Xiong L, Jin Y (2019) Polym Mater Sci Eng 35(12):143–148
Liu L, Huang G, Song P, Yu Y, Fu S (2016) ACS Sustain Chem Eng 4(9):4732–4742
Liu L, Qian M, Song PA, Huang G, Yu Y, Fu S (2016) ACS Sustain Chem Eng 4(4):2422–2431
Cao Z, Zhang Y, Song P, Cai Y, Guo Q, Fang Z, Peng M (2011) J Anal Appl Pyrol 92(2):339–346. https://doi.org/10.1016/j.jaap.2011.07.007
Visakh PM, Arao Y (2015)Springer, Switzerland
Jineesh A, Mohapatra S (2018) Thermal Properties of Polymer–Carbon Nanocomposites. p. 235 – 70. https://doi.org/10.1007/978-981-13-2688-2_7
Vahidi G, Bajwa DS, Shojaeiarani J, Stark N, Darabi A (2021) J Appl Polym Sci 138(12):50050. https://doi.org/10.1002/app.50050
Vahabi H, Brosse N, Latif NHA, Fatriasari W, Solihat NN, Hashimd R, Hussin MH, Laoutid F, Saeb MR (2021) Chap. 24-Nanolignin in materials science and technology-does flame retardancy matter? In: al SSKe, Biopolymeric Nanomaterials: Fundamental and Applications. Elsevier, pp 515–550
Wang X, Ji S-L, Wang X-Q, Bian H-Y, Lin L-R, Dai H-Q, Xiao H (2019) J Mater Chem C 7(45):14159–14169. https://doi.org/10.1039/C9TC04961D
Yu H, Zhan W, Liu Y (2020) Waste and Biomass Valorization 11(8):4561–9. https://doi.org/10.1007/s12649-019-00763-1
Chollet B, Lopez-Cuesta JM, Laoutid F, Ferry L (2019) Mater (Basel) 12(13). https://doi.org/10.3390/ma12132132
Lu W, Li Q, Zhang Y, Yu H, Hirose S, Hatakeyama H, Matsumoto Y, Jin Z (2018) J Wood Sci 64(3):287–293. https://doi.org/10.1007/s10086-018-1701-4
Hu W, Zhang Y, Qi Y, Wang H, Liu B, Zhao Q, Zhang J, Duan J, Zhang L, Sun Z, Liu B (2020) Macromol Mater Eng 305(5). https://doi.org/10.1002/mame.201900840
Wu Q, Ran F, Dai L, Li C, Li R, Si C (2021) Int J Biol Macromol 190:390–395. https://doi.org/10.1016/j.ijbiomac.2021.08.233
He T, Chen F, Zhu W, Yan N (2022) Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2022.04.089
Widsten P, Tamminen T, Paajanen A, Hakkarainen T, Liitiä T (2021) Holzforschung 75(6):584–590. https://doi.org/10.1515/hf-2020-0147
Granata A, Argyropoulos DS (1995) J Agric Food Chem 43(6):1538–1544. https://doi.org/10.1021/jf00054a023
Mandlekar N, Malucelli G, Cayla A, Rault F, Giraud S, Salaün F, Guan J (2018) Polym Degrad Stab 153:63–74. https://doi.org/10.1016/j.polymdegradstab.2018.04.019
Xiong X, Niu Y, Zhou Z, Ren J (2020) Polymers 12(9):1–11. https://doi.org/10.3390/polym12092007
Funding
This work was supported by the Deputy for Strengthening Research and Development, Ministry of Research and Technology in the National Competitive Research grant from the Deputy of Strengthening Research and Development, Ministry of Research and Technology/National Research and Innovation Agency 2021 Fiscal Year (95/UN5.2.3.1/PPM/KP-DRPM/2021) and Fundamental Research Grant Scheme (FRGS) provide by Ministry of Higher Education, Malaysia (FRGS/1/2018/WAB07/UPM//1).This study was supported by a cooperation agreement No: 001/Greenei-KS/VII/2021 and B-6022/III/LS.01.01/07/2021 betweem Resaerch Center for Biomaterial and PT Greenei Alam Indonesia FY 2021-2023.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by [Widya Fatriasari], [Alif Faturahman Hidayat], [Nissa Nurfajrin Solihat], [Azman Mohammad Taib], [M. Hazwan Hussin], [Lee Seng Hua] and [Syeed SaifulAzry Osman Al Edrus]. The first draft of the manuscript was written by [Nissa Nurfajrin Solihat], [Widya Fatriasari], [Azman Mohammad Taib], [M. Hazwan Hussin], [Henri Vahabi], [Lee Seng Hua], and [Muhammad Aizat Abdul Ghani] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solihat, N.N., Hidayat, A.F., Taib, M.N.A.M. et al. Recent Developments in Flame-Retardant Lignin-Based Biocomposite: Manufacturing, and characterization. J Polym Environ 30, 4517–4537 (2022). https://doi.org/10.1007/s10924-022-02494-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02494-2