Abstract
Bacterial cellulose (BC) aerogel has great potential in treating oil spill and organic pollutant. However, its inherent hydrophilicity and poor rigidity limit its practical application and recyclability. In this study, elastically compressible and high oil-absorbing aerogels were developed by freeze-drying aqueous suspensions with appropriate BC concentrations, followed by a chemical vapor deposition of methyltrimethoxysilane with ammonia as catalyst. The modified aerogel shows high water contact angle of 142° and enhanced compression resistance. The effect of BC concentration on the absorption capacity and recyclability of aerogel has been investigated. The results show that the aerogel prepared with 0.3 wt% BC exhibits simultaneously high absorption capacity (121.8–284.1 g/g) and excellent recyclability. Futhermore, the aerogel could also separate chloroform-water mixture by gravity-driven filtration, giving the separation efficiency of 96.7%. Therefore, this economical green aerogel provides a feasible strategy for solving oil leakage in industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil pollution has become a big environmental problem as frequent oil spills and widespread discharge of large quantities of industrial waste oil have caused serious pollution to oceans and lakes [1,2,3,4]. Therefore, it is of great significance to explore an effective route to recover waste oil and organic solvents in water without causing secondary environmental pollution.
Aerogel is a kind of lightweight material with high porosity, and considered as one of the most ideal absorbents [5,6,7].Various aerogels made of gelatin [8], graphene [9], silica [10], cellulose nanofibers [11,12,13], chitosan [14, 15], have already been studied for oil absorption. Among them, the bacterial cellulose (BC) aerogels have attracted intensively attention. BC is a kind of natural nanocellulose produced by microorganisms, identical in chemical formula to the plant-derived cellulose. However, BC exhibits many superior properties over the plant cellulose, such as higher purity and crystallinity, better mechanical properties and degradable [16,17,18]. Therefore, the aerogels made of BC possess higher holding capacities and tensile strength than that made of plant cellulose, representing a promising material for oil/water separation [19]. As BC aerogel is inherently hydrophilic due to the presence of abundant hydroxyl groups in BC [5, 20], a hydrophobic surface modification is necessary to realize the selective absorption for oil from oil/water mixture. The modification process is usually performed using modifiers with low surface energy. Besides, the hydrophobic and oleophilic nature can also be obtained by directly carbonizing BC aerogel. Wu et al. [21] reported that the BC aerogels carbonized at 1300 °C became hydrophobic and exhibited high adsorption capacities for organic liquids (up to 312 g/g). However, the high cost and energy consumption of carbonization process is not conducive to the practical application of aerogels. Comparatively, the latter modification method is inexpensive. Using modifiers, the surface hydrophobization of cellulose aerogels were realized mainly via two different routes, gas phase reaction, and aerogel or hydrosol immersed in liquids containing modifiers. It is noted that most of the plant-derived cellulose aerogels were hydrophobic via gas phase reactions [22,23,24], while the reported BC aerogels were often hydrophobicly modified in liquids containing modifiers. Wang et al.[25] prepared a superhydrophobic BC aerogel by immersing BC hydrogel in a modifier solution containing stearic acid, giving the maximum absorption capacity of 48.2 g/g for soybean oil. Similarly, Sai et al. [26] immersed blocky BC aerogel in a solution containing trimethylchlorosilane and triethylamine, and the prepared hydrophobic BC aerogel showed a maximum absorption capacity of 185 g/g for chloroform. He et al. [27] prepared a superelastic and superhydrophobic BC/silica aerogel by immersing BC aerogel in flexible silica sol with methyltriethoxysilane (MTES) as the precursor, and the absorption capacities for the organic solvents and oils were 8–14 g/g. Although the surface modification of aerogels by chemical vapor deposition (CVD) is prone to heterogeneous growth [26, 28], the CVD method can reduce the amounts of modifiers and solvents compared with the modification in liquid. Thus, the CVD modification is adopted in this work.
Absorption capacity and recyclability are two important properties that affect the practical application of BC aerogels. From the researches mentioned above [21, 25,26,27], we note that the modified BC aerogels with modifiers need to further improve their adsorption capacities compared with the carbonized BC aerogels. Normally, hydrophobic aerogel with higher porosity owns higher absorption capacity for oil [26]. And the porosity is critically determined by the preparation conditions during freeze-drying process, such as solvent [25], feature of suspension [29], along with cooling rate and temperature gradient [30]. Here, only BC concentration and surface energy of the mold are considered. The mold with lower surface energy tend to increase the freezing delay time and degree of supercooling due to a smaller contact area with the aqueous suspension. This will result in smaller ice nuclei uniformly formed in the whole suspension [31]. In addition, it is understandable that the suspension with lower BC concentration tends to form more ice crystals during freeze process, leading to a higher porosity of BC aerogel. On the other hand, the increase in porosity will reduce the mechanical strength of BC aerogel, which is harmful to its recyclability in practical application. Therefore, it is necessary to explore an appropriate BC concentration for preparing aerogels to aspire excellent performance. To the best of our knowledge, the effects of BC concentration on absorption capacity and recyclability of aerogels have rarely been studied.
Here, elastically compressible and highly oil-absorbing aerogels were developed by freeze-drying the suspension with low BC concentration (0.2, 0.3 and 0.4 wt%), followed by a CVD modification with methyltrimethoxysilane as the modifier and ammonia as the catalyst. The influence of BC concentration on the performances of aerogel were investigated. A polytetrafluoroethylene (Teflon) mold possessing low surface energy was used for freeze-drying so as to form BC aerogels with homogenized porous structure. This recyclable and hydrophobic BC aerogel displays great advantages in cost and performances.
Experimental
Materials
The bacterial cellulose hydrogel with 2.1 wt% BC concentration was purchased from Guilinqihong Technology Co., LTD. NH4OH (≥ 25%), anhydrous ethanol (≥ 99.7%), and chloroform (≥ 99%) were all purchased from Guoyao Chemical Reagent Co., LTD. Methyltrimethoxysilane (MTMS, ≥ 98%), dimethyl silicone oil (AR) and NaOH (AR) were purchased from Aladdin. Pump oil was purchased from Lichen Technology Co., LTD and engine oil was purchased from Mobil Universal 4 T. Dichloromethane, petroleum ether, castor oil, and soybean oil were all purchased from the local market. All experiments were conducted using deionized water (DI).
Preparation of BC Aerogel
The hydrogel of bacterial cellulose was cut into small pieces, and then purified through soaking in DI at room temperature for 5 h and subsequently in 1% NaOH solution at 80 °C for 8 h. The purified cellulose block was washed repeatedly with alcohol and DI water until the solution was neutral. Three types of uniform cellulose suspensions with BC concentration of 0.2 wt%, 0.3 wt% and 0.4 wt%, were obtained by stirring the mixture of BC block and DI at a high speed of 20,000 r/min. 10 g suspension was poured into a Teflon cylindrical mold (φ = 3 cm), pre-frozen at − 60 °C for 12 h, and then freezing-dried for 48 h. The BC aerogels prepared at different BC concentrations were denoted as DB-0.2, DB-0.3 and DB-0.4.
Modified BC Aerogels
The surface of the BC aerogel was modified via the CVD method. The BC aerogel (30 mg) was put into a sealed dryer together with two small glass bottles containing 5 mL MTMS and 7 mL NH4OH, respectively, and then heated at 75 °C for 3.5 h to complete the vapor deposition. Later, the bacterial cellulose aerogel was taken out of the dryer and maintained at 60 °C for 12 h. The modified BC aerogels were denoted as DBS-0.2, DBS-0.3 and DBS-0.4.
Characterization
The morphology of aerogel was characterized by using field emission scanning electron microscope (Sigma-500). Fourier transform infrared spectrometer (Nicolet 5700) with a resolution of 4 cm−1, was used to determine the change of the groups in the modified samples. X-ray diffractometer (Miniflex-600) was used to analyze the crystal structure of the samples at a scanning speed of 2°/min. The surface chemical properties of the samples were identified by using X-ray photoelectron spectrometer (XPS, Thermo Scientific™ K-Alpha™). The static contact Angle was measured by the contact angle analyzer (SL200B). The stress–strain curves of the BC aerogels were tested by the universal testing machine (CMT4202). N2 absorption–desorption isotherms were measured by using Micromeritics 3Flex instrument.
Results and Discussion
The Structure and Morphology of BC Aerogels
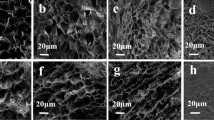
The apparent densities of DB-0.3, DBS-0.2, DBS-0.3 and DBS-0.4 are 3.15 mg/cm3, 4.25 mg/cm3, 6.10 mg/cm3 and 8.54 mg/cm3, respectively. The SEM images of these samples are shown in Fig. 1a–d. From the images at low magnification, it can be seen that all the samples exhibit plenty of interlinked BC sheets, among which irregularly shaped macro-pores are distributed. These pores present nonuniform sizes, mainly in the micron scale. As the BC concentration increases from 0.2 wt% to 0.3 wt% and 0.4 wt%, more small macro-pores below 100 μm and fewer big macro-pores above 200 μm are observed (Fig. 1b–d). The image of DB-0.3 at high magnification (inset of Fig. 1a) presents that the BC sheets are composed of compactly entangled BC fibers, among which a large amount of nanopores with diameter of 1–50 nm are dispersed. In addition, the insets of Fig. 1b–d show that there are plenty of micro-nanometer silica particles grown on the BC sheets of the aerogels modified by MTMS. These particles are mainly nanorods and doughnut-shaped. In Fig. 1e, f, the energy dispersive X-ray energy spectrum (EDS) of DBS-0.3 shows the existence of Si element as well as C and O elements, proving that the BC aerogel was successfully modified by MTMS.
Figure 2a shows the nitrogen absorption–desorption isotherms of DBS-0.3. It can be seen that DBS-0.3 presents a type-IV isotherm curve with a clear hysteresis loop in the 0.5–0.95 relative pressure range, indicating the presence of a mesoporous structure. Moreover, the isotherm curve shows no adsorption saturation at high relative pressure of 0.9–1.0, suggesting the existence of macropores. Figure 2b shows the Barrett-Joyner-Halenda (BJH) and Horvath-Kawazoe (HK) pore size distributions obtained from the absorption branch. It can be seen that the micropore sizes and mesopore sizes of the DBS-0.3 are mainly in the range of 0.8–1.2 nm and 2–10 nm, respectively, which is consistent with the SEM observation for the nanopores formed by entangled BC fibers.
The XPS test was carried out to explore the surface chemical constituent of DB-0.3 and DBS-0.3, as shown in Fig. 3a–d. The DB-0.3 is composed of C and O elements, while DBS-0.3 contains C, O and Si elements. For the DBS-0.3 aerogel, the spectrum of O 1 s shows two peaks at 533 eV and 534 eV, respectively, corresponding to C–O and Si–O components. In addition, the Si–O bond at 103 eV and Si–C bond at 104 eV were observed in the Si 2p spectrum of DBS-0.3 (Fig. 3d) [11]. These results demonstrate the presence of the silanization reaction between MTMS and hydroxyl group of cellulose.
The changes of the chemical groups of aerogels after modification were analyzed by the infrared spectroscopy. As shown in Fig. 4a, both DB-0.3 and DBS-0.3 aerogels show four vibration absorption bands in the range of 3348–3350 cm−1 (–OH), 2915–2917 cm−1 (C–H), 1631–1634 cm−1 (C = O) and 1059–1060 cm−1 (C–O), revealing that BC has abundant oxygen containing groups such as hydroxyl groups and carbon chains[25]. It can also be observed that the intensity of the absorption band around 3349 cm−1 significantly decreases after modification. This should be due to the decrease of hydroxyl groups in BC induced by the reaction between MTMS and the hydroxyl of cellulose. In addition, the modified aerogel DBS-0.3 exhibits new vibration bands at 781 cm−1 (Si–O–Si) and 1274 cm−1 (Si–CH3), which also means the Si-CH3 groups were grafted on BC[32]. Owing to low surface energy, these Si-CH3 groups on the surface of BC are benefit to enhancing the surface hydrophobicity of aerogel. Figure 4b shows the X-ray diffraction spectra of DB-0.3 and DBS-0.3. The original DB-0.3 shows three typical diffraction peaks at 15°, 17° and 22.8°, respectively corresponding to the (1 \(\overline{1}\) 0), (110) and (020) reflection planes of the cellulose crystal with cellulose Ι type structure[26]. In the X-ray diffraction spectrum of DBS-0.3, two diffraction peaks at 15° and 17° disappear and the peaks at 22.8° become weakened, resulting from the decreased mass fraction of cellulose in DBS-0.3. We noted that the mass of DB-0.3 increased by about one times after modification with MTMS, due to the growth of silica on BC fibers.
Compressibility of BC Aerogel
Figure 5a, b give the compressive stress–strain curves and the loading–unloading resilience test curve of the aerogels. As shown in Fig. 5a, the compressive stress first presents a gentle rising with increasing strain, and then rises abruptly when the compressive strain exceeds 60%. This is because the interlaced structure of BC became densified due to the squeezed and contracted pores. Thanks to the micro-meso-macro porous and interleaving network structure of the aerogels, the force required to compress the aerogels to perform 50% strain is less than 2 kPa, indicating the good flexibility of these aerogels. Compared with the modified samples, DB-0.3 shows much lower stress in the strain range of 60–80%, and its poor rigidity is detrimental to the mechanical recycling. Among the three modified aerogels, DBS-0.4 exhibits the highest stress within the abruptly increasing stress region, attributed to it having the most compact structure due to the high BC concentration. It can be concluded that silane modification and increasing BC concentration can both improve the compressive strength of aerogel. Figure 5b shows that the compressed DBS-0.3 aerogel can be immediately restored to the original height after unloading the weight. Moreover, about 98% of the original height can be restored after 300 cyclic loading–unloading resilience tests. Therefore, DBS-0.3 has been proved to have excellent flexibility and elastic recovery ability, which makes it possible to recycle the adsorbed aerogels by squeezing.
Absorption for Various Oils and Organic Solvents
The wettability changes of aerogel after modification are shown in Fig. 6a–d. Due to the large amount of hydroxyl groups on the surface of pristine BC, the water and engine oil droplets are able to quickly spread over the aerogel surface, suggesting that the original aerogel has high affinity to engine oil and water (Fig. 6a). For the modified aerogel, the surface hydrophobicity was observed (Fig. 6b). In order to further investigate whether the interior shows oleophilic-hydrophobicity, the modified aerogel was cut along the diameter direction, and the wettability of the transverse section is shown in Fig. 6c, d. It can be seen that oil droplets spreads over the aerogel surface, but water droplets remain the spherical shape and the water contact angle is 142°. These results indicate the oleophilic-hydrophobicity of the whole aerogel instead of just the surface, has been achieved by the facile CVD of MTMS.
By virtue of its good hydrophobicity, the modified BC aerogel can realize absorption for oil from water. As shown in Fig. 7a, DBS-0.3 can absorb engine oil (dyed red) floating on the water surface within 10 s. Figure 7b shows that DBS-0.3 can rapidly absorb chloroform and repelled water when being immersed in a mixed aqueous solution containing chloroform (dyed red) at the bottom. Therefore, DBS-0.3 exhibits highly efficient oil-absorption. Besides, the aerogel can also rapidly absorb chloroform from HCl solution (pH5), NaOH solution (pH12) and 5% NaCl solution and maintain its original shape, exhibiting good oil absorption ability independent of the solution property. The absorption capacity of DBS-0.3 for various oils and organic solvents is showed in Fig. 7c. The DBS-0.3 aerogel exhibits good absorption ability and the obtained absorption capacity is 121.8–284.1 g/g. Moreover, Fig. 7d shows that the absorption capacity of DBS-0.3 is linearly positively correlated with the density of the absorbed liquid, indicating that the absorption ability of aerogel is mainly determined by its pore volume. As the pore volume of a given aerogel is constant, the liquid with higher density will present higher mass per unit volume, resulting in increasing the absorption capacity of the aerogel. As given in Table 1, it can be seen that the absorption capacity of the aerogel DBS-0.3 prepared in this work is superior to most of the previously reported aerogels due to low cost and high absorption capacity, and so it is easier to achieve large-scale waste oil treatment in industry.
Separation for Oil/Water Mixture by Filtration
A test to separate oil/water mixture by filtration was carried out via plugging DBS-0.3 into the bottom of a funnel, in which dyed chloroform (10 g) and DI (10 g) were poured. As shown in Fig. 8, driven by the gravity, the dyed red chloroform can quickly flow through the pores of DBS-0.3 aerogel to the beaker below, while the dyed blue DI is hold in the funnel due to the good hydrophobicity of DBS-0.3 aerogel. The separation efficiency can be calculated by the ratio of the mass of separated chloroform (Mt) in the beaker to the original mass (M0) of chloroform. In this work, the obtained separation efficiency is up to 96.7%.
Effect of BC Concentration on the Absorption and Recyclability of Aerogels
The excellent recyclability of aerogel is very important for practical applications. In this work, the recyclabilities of DBS aerogels with different BC concentrations were investigated. As shown in Fig. 9a, the absorbed oil was released by simply squeezing the saturated adsorbed DBS aeroge, and then the flattened DBS aerogel was re-immersed in the engine oil. After a few seconds of absorption, the re-saturated DBS aerogel was restored to its original shape. The absorption capacity of the recycled DBS aerogel can be calculated based on the following equation:
where M0 is the mass of the original DBS aerogel, Ma and Mb are the mass of the flattened aerogel and saturated aerogel in the nth squeeze-absorption cycle, respectively. The absorption capacity of DBS-0.2, DBS-0.3 and DBS-0.4 for engine oil are shown in Fig. 9b–d. It can be seen that the maximum absorption capacity and the recyclability are quite different for the three DBS aerogels with different BC concentrations. Although the DBS-0.2 has the highest maximum absorption capacity, some breakage of aerogel can be observed in the cycling process, resulting in rapidly-decreasing absorption capacity with increasing cycling times. Compared with DBS-0.2, the DBS-0.3 aerogel could maintain its integrity of appearance, showing lower maximum absorption capacity and better recyclability with an absorption capacity maintained above 150.0 g/g throughout the 10 squeezing-absorbing cycles. This is consistent with the result of stress–strain test that DBS-0.3 has a higher ability to bear compressive deformation. Among the three DBS aerogels, DBS-0.4 shows the most stable absorption capacity throughout the 10 cycles. However, its absorption capacity is far lower than that of DBS-0.3, attributed to the lower porosity caused by higher BC concentration. As a consequence, the DBS aerogel with 0.3 wt% BC concentration exhibits the best comprehensive performance, simultaneously its high absorption and excellent recyclability provide a feasible strategy for solving oil leakage in industry.
Conclusions
The lipophilic-hydrophobic aerogels have been successfully developed by freeze-drying the aqueous suspensions with different BC concentrations (0.2, 0.3 and 0.4 wt%), followed by a simple modification via chemical vapor deposition. The absorption capacity of aerogel for oil increases with decreasing BC concentration, but the recyclability shows an opposite trend. The DBS-0.3 aerogel achieves high absorption capacity (121.8–284.1 g/g) for various oils and organic solvents (soybean oil, castor oil, pump oil, engine oil, dichloromethane, chloroform, dimethyl silicone oil, petroleum ether and anhydrous ethanol). Owing to its excellent elastically compressibility, DBS-0.3 can be recycled through a simple squeezing, and the absorption capacity for engine oil is above 150 g/g during 10 cycles. It can also be used to rapidly separate the chloroform-water mixture with a separation efficiency of up to 96.7%. This economical green aerogel is promising for treating oil leakage and purifying water.
References
Zhang J, Meng Z, Liu J, Schlaich C, Yu Z, Deng X (2017) Breath figure lithography for the construction of a hierarchical structure in sponges and their applications to oil/water separation. J Mater Chem A 5(31):16369–16375. https://doi.org/10.1039/C7TA02751F
Wang Y, He Y, Fan Y, Li H, Yu H, Yu J et al (2021) A robust anti-fouling multifunctional aerogel inspired by seaweed for efficient water purification. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.118153
Viswanathan K, Wang S (2021) Experimental investigation on the application of preheated fish oil ethyl ester as a fuel in diesel engine. Fuel. https://doi.org/10.1016/j.fuel.2020.119244
Li J, Xu C, Guo C, Tian H, Zha F, Guo L (2018) Underoil superhydrophilic desert sand layer for efficient gravity-directed water-in-oil emulsions separation with high flux. J Mater Chem A 6(1):223–230. https://doi.org/10.1039/c7ta08076j
Wang FP, Zhao XJ, Wahid F, Zhao XQ, Qin XT, Bai H et al (2021) Sustainable, superhydrophobic membranes based on bacterial cellulose for gravity-driven oil/water separation. Carbohydr Polym 253:117220. https://doi.org/10.1016/j.carbpol.2020.117220
Oshima T, Sakamoto T, Ohe K, Baba Y (2014) Cellulose aerogel regenerated from ionic liquid solution for immobilized metal affinity adsorption. Carbohydr Polym 103:62–69. https://doi.org/10.1016/j.carbpol.2013.12.021
Long S, Feng Y, Liu Y, Zheng L, Gan L, Liu J et al (2021) Renewable and robust biomass carbon aerogel derived from deep eutectic solvents modified cellulose nanofiber under a low carbonization temperature for oil-water separation. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.117577
Jiang J, Zhang Q, Zhan X, Chen F (2019) A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem Eng J 358:1539–1551. https://doi.org/10.1016/j.cej.2018.10.144
Zhan C, Jana SC (2020) Shrinkage reduced polyimide-graphene oxide composite aerogel for oil absorption. Micropor Mesopor Mat. https://doi.org/10.1016/j.micromeso.2020.110501
Zhang X, Zhang T, Yi Z, Yan L, Liu S, Yao X et al (2020) Multiscale mullite fiber/whisker reinforced silica aerogel nanocomposites with enhanced compressive strength and thermal insulation performance. Ceram Int 46(18):28561–28568. https://doi.org/10.1016/j.ceramint.2020.08.013
Lu Y, Niu Z, Yuan W (2019) Multifunctional magnetic superhydrophobic carbonaceous aerogel with micro/nano-scale hierarchical structures for environmental remediation and energy storage. Appl Surf Sci 480:851–860. https://doi.org/10.1016/j.apsusc.2019.03.060
Karzar Jeddi M, Laitinen O, Liimatainen H (2019) Magnetic superabsorbents based on nanocellulose aerobeads for selective removal of oils and organic solvents. Mater Design. https://doi.org/10.1016/j.matdes.2019.108115
Yang J, Xia Y, Xu P, Chen B (2018) Super-elastic and highly hydrophobic/superoleophilic sodium alginate/cellulose aerogel for oil/water separation. Cellulose 25(6):3533–3544. https://doi.org/10.1007/s10570-018-1801-8
Hu J, Zhu J, Ge S, Jiang C, Guo T, Peng T et al (2020) Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil−water separation. Surf Coat Tech. https://doi.org/10.1016/j.surfcoat.2020.125361
Yang H, Zhang S, Yan J (2020) Chitosan-reinforced MFC/NFC aerogel and antibacterial property. Adv Polym Tech 2020:1–9. https://doi.org/10.1155/2020/7890215
Cheng Z, Li J, Wang B, Zeng J, Xu J, Gao W et al (2020) Scalable and robust bacterial cellulose carbon aerogels as reusable absorbents for high-efficiency oil/water separation. ACS Appl Bio Mater 3(11):7483–7491. https://doi.org/10.1021/acsabm.0c00708
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40(7):3941–3994. https://doi.org/10.1039/c0cs00108b
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D et al (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed Engl 50(24):5438–5466. https://doi.org/10.1002/anie.201001273
Hou G-Y, Lyu Z-Y, Tang Y-P, Cao H-Z, Zheng G-Q (2020) Preparation of flexible composite electrode with bacterial cellulose (BC)-derived carbon aerogel supported low loaded NiS for methanol electrocatalytic oxidation. Int J Hydrogen Energ 45(32):16049–16059. https://doi.org/10.1016/j.ijhydene.2020.04.005
Zhang X, Zhao X, Xue T, Yang F, Fan W, Liu T (2020) Bidirectional anisotropic polyimide/bacterial cellulose aerogels by freeze-drying for super-thermal insulation. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123963
Wu ZY, Li C, Liang HW, Chen JF, Yu SH (2013) Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew Chem Int Ed Engl 52(10):2925–2929. https://doi.org/10.1002/anie.201209676
Zhu W, Yao Y, Zhang Y, Jiang H, Wang Z, Chen W et al (2020) Preparation of an amine-modified cellulose nanocrystal aerogel by chemical vapor deposition and its application in CO2 capture. Ind Eng Chem Res 59(38):16660–16668. https://doi.org/10.1021/acs.iecr.0c02687
Wang J, Liu S (2019) Remodeling of raw cotton fiber into flexible, squeezing-resistant macroporous cellulose aerogel with high oil retention capability for oil/water separation. Sep Purif Technol 221:303–310. https://doi.org/10.1016/j.seppur.2019.03.097
Yi L, Xia Y, Tan Z, Fang X, Zhao L, Wu H et al (2020) Design of tubelike aerogels with macropores from bamboo fungus for fast oil/water separation. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.121558
Wang Q, Tian D, Hu J, Huang M, Shen F, Zeng Y et al (2020) Harvesting bacterial cellulose from kitchen waste to prepare superhydrophobic aerogel for recovering waste cooking oil toward a closed-loop biorefinery. ACS Sustain Chem Eng 8(35):13400–13407. https://doi.org/10.1021/acssuschemeng.0c04212
Sai H, Fu R, Xing L, Xiang J, Li Z, Li F et al (2015) Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl Mater Interfaces 7(13):7373–7381. https://doi.org/10.1021/acsami.5b00846
He J, Zhao H, Li X, Su D, Zhang F, Ji H et al (2018) Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. J Hazard Mater 346:199–207. https://doi.org/10.1016/j.jhazmat.2017.12.045
Liu H, Geng B, Chen Y, Wang H (2016) Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain Chem Eng 5(1):49–66. https://doi.org/10.1021/acssuschemeng.6b02301
Wang J, Zhang W, Zhang C (2019) Versatile fabrication of anisotropic and superhydrophobic aerogels for highly selective oil absorption. Carbon 155:16–24. https://doi.org/10.1016/j.carbon.2019.08.049
Jiménez-Saelices C, Seantier B, Cathala B, Grohens Y (2017) Effect of freeze-drying parameters on the microstructure and thermal insulating properties of nanofibrillated cellulose aerogels. J Sol-Gel Sci Techn 84(3):475–485. https://doi.org/10.1007/s10971-017-4451-7
Azimi Yancheshme A, Momen G, Jafari Aminabadi R (2020) Mechanisms of ice formation and propagation on superhydrophobic surfaces: A review. Adv Colloid Interfac 279:102155. https://doi.org/10.1016/j.cis.2020.102155
Dilamian M, Noroozi B (2021) Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr Polym 251:117016. https://doi.org/10.1016/j.carbpol.2020.117016
Li L, Hu T, Sun H, Zhang J, Wang A (2017) Pressure-sensitive and conductive carbon aerogels from poplars catkins for selective oil absorption and oil/water separation. ACS Appl Mater Interfaces 9(21):18001–18007. https://doi.org/10.1021/acsami.7b04687
Lu J, Xu D, Wei J, Yan S, Xiao R (2017) Superoleophilic and flexible thermoplastic polymer nanofiber aerogels for removal of oils and organic solvents. ACS Appl Mater Interfaces 9(30):25533–25541. https://doi.org/10.1021/acsami.7b07004
Wang NN, Wang H, Wang YY, Wei YH, Si JY, Yuen ACY et al (2019) Robust, lightweight, hydrophobic, and fire-retarded Polyimide/MXene Aerogels for effective oil/water separation. ACS Appl Mater Interfaces 11(43):40512–40523. https://doi.org/10.1021/acsami.9b14265
Xiao J, Lv W, Song Y, Zheng Q (2018) Graphene/nanofiber aerogels: Performance regulation towards multiple applications in dye adsorption and oil/water separation. Chem Eng J 338:202–210. https://doi.org/10.1016/j.cej.2017.12.156
Li Z, Zhong L, Zhang T, Qiu F, Yue X, Yang D (2019) Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustain Chem Eng 7(11):9984–9994. https://doi.org/10.1021/acssuschemeng.9b01122
Acknowledgements
This work is financially supported by Fujian Provincial Nature Science Foundation of China (Grant 2018J01755).
Author information
Authors and Affiliations
Contributions
Zhuofeng Yan: Conceptualization, Verification, Writing manuscript. Qian Liu: Resources, Investigation, Software. Kaixiao Zhu: Resources. Xiangqi Li: Supervision, Writing—review. Xiao Wu: Reviewing & editing manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, Z., Zhu, K., Li, X. et al. Recyclable Bacterial Cellulose Aerogel for Oil and Water Separation. J Polym Environ 30, 2774–2784 (2022). https://doi.org/10.1007/s10924-021-02369-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02369-y