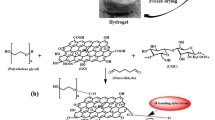
Abstract
A promising sorbent was prepared by a combination of different eco-friendly functional groups such as ethylenediaminetetraacetic acid with 3-aminopropyltriethoxysilane (EDTA/Si) and polyacrylamide grafted carboxymethyl cellulose (AmCMC) with magnetic graphene oxide (mGO) and N,N-Methylene bis-acrylamide as a crosslinker for efficient removal of lead ions (Pb2+) from aqueous solutions. The prepared sorbent was characterized by X-ray diffraction (XRD), infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), the scanning electron microscope (SEM), Vibrating sample magnetometer (VSM), and Zeta-potential measurements. The oxidation of graphite was confirmed by XRD and transmission electron microscope (TEM). The adsorption conditions such as contact time, pH, sorbent dosage, and initial Pb2+ concentrations were investigated to optimize the adsorption efficiency of the prepared sorbent. EDTA/Si/mGO/AmCMC was found to be an ideal sorbent for Pb2+ removal with a higher adsorption capacity due to the successive link of chelating groups onto AmCMC. From the data, at pH = 5.3 after 120 min, EDTA/Si/mGO/AmCMC has high removal efficiency of Pb2+ (92%) compared with AmCMC (46%). For deep information, Kinetic and isotherm models were applied for the adsorption process. From the data, the harmony of data fitting with the pseudo-second-order kinetic model and the Langmuir isotherm model. According to the Langmuir adsorption isotherms, the maximum adsorption capacity of 0.15 g EDTA/Si/mGO/AmCMC at pH = 5.3 after 120 min is 86 mg/g.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
1.
EDTA functionalized mGO were synthesized.
-
2.
Grafted CMC hydrogel embedded with EDTA functionalized mGO was prepared.
-
3.
The prepared adsorbent has high adsorption capacity for Pb+2.
-
4.
The adsorption process fitted with pseudo-second-order and Langmuir models.
Introduction
Water shortage is a great challenge that faces the entire world, specifically in the Middle East. At the same time, the drinking water is a major burden in many countries due to scarcity of water as a result of an absence of water sources and/or due to water pollution. So, discovering new resources of water became an essential issue [1, 2]. Consequently, the researchers try to find new approaches to preserve water from pollution, treat or reuse water, as well as extract groundwater [3,4,5,6]. The entire removal of pollutants in the resources of water protects the environment as well as stops the transfer of toxic contaminant in food chains. One of the most common pollutants in water are heavy metals which adversely affect the environmental and human health [7]. Many conventional approaches have been employed to remove heavy metals from water such as chemical precipitation, ion-exchange, reverse osmosis, filtration, membrane technologies, and electrochemical methods [8, 9]. However, the sorption process is one of the most productive, simply flexible, and most cost-effective methods [10]. Several sorbents have been studied for metal removals like inorganic materials, activated carbon, zeolite, and resins but these sorbents have been suffering from either low efficiencies or low adsorption capacities [11,12,13,14]. Therefore, in recent years a great effort to prepare new sorbents and develop new techniques has been made. New as well as eco-friendly materials and composites were used in the sorption processes such as cellulose, carrageenan, and hydrogels [15, 16]. Hydrogels are 3D cross linked polymers that can absorb a lot of water into their structure [17]. So, it looks like a promising heavy metal removal. Various natural biopolymers can be used to prepare hydrogel such as chitosan, cellulose and carboxymethyl cellulose [18, 19]. Cellulose is a green natural, non-toxic, biodegradable, and biocompatible polymer that has been reported to be a promising material used in a vast number of applications [4, 5, 20, 21]. Its characteristics can be altered by physical and/or chemical modifications depending on the end product needed [22, 23]. The most widespread derivative of cellulose is carboxymethylcellulose (CMC). It is a water-soluble anionic polysaccharide that has great importance in the industry and our everyday life [24, 25]. It is commonly utilized in many fields of industrial applications such as cosmetics, pharmaceuticals, and packaging [22, 26].
Among other eco-sorbents, carbon-based nanomaterials have been studied as superior sorbents for the removal of metal pollutants from aqueous solutions with high capacity and selectivity [27]. Nanomaterials have great impact in wastewater treatment applications in the recent years due to their remarkable properties. Graphene oxide (GO) is one of these materials that holds promising properties due to its unique structure [28]. It is composed of a single layer of graphene bound to oxygen in the form of carboxyl, hydroxyl, or epoxy groups. Due to its mechanical, electronic, optical, and thermal properties, GO has a major role in many applications such as energy storage devices, flexible electronics, sensors, and solar cells [18, 29].
In recent years, magnetic and surface-modified magnetic composites have been developed and used for metal removal due to their rapid separation from an aqueous solution and led to increase adsorption capacity and chemical stability of adsorbentsn [30, 31].The introduction of the huge amount of OH groups onto the magnetic surface during the preparation process makes magnetite become hydrophilic in an aqueous solution. On the other hand, the chelating characteristic of ethylenediaminetetraacetic acid (EDTA) may be used to synthesize EDTA-functionalized magnetic particles [32]. When magnetic particles reacted with EDTA and aminopropyl triethoxysilane, the hydrolysis of the trialkoxy groups generates Si–OH groups that react with Fe-OH of magnetic particles surface. Accordingly, EDTA can link to the iron oxide surface through Si–O–Fe bonds and chelating groups are introduced onto the magnetic surface [32, 33].
Accordingly, this contribution presents the synthesis of a new sorbent by embedded EDTA modified magnetic graphene oxide onto polyacrylamide grafted CMC with N,N-Methylene bis-acrylamide as a crosslinker. The prepared sorbent was characterized by several tools such as XRD, FTIR, SEM, TEM, TGA, VSM, and Zeta-potential. The efficiency of the prepared sorbent has been investigated for the removal of Pb2+. The changing of the adsorption conditions was studied to investigate the isotherms and kinetics of the adsorption process.
Materials and Methods
Materials
Sodium carboxymethyl cellulose (CMC) medium viscosity, 98.5% with DS = 0.70–0.85 was purchased from Fluka. Sulfuric Acid (99.9%) was acquired from Sigma Aldrich, USA. Graphite powder (99.9%) was received from Fisher Scientific, UK. Potassium permanganate (KMnO4), (> 99%), and hydrogen peroxide were provided from Bio Basic Canada Inc. and Carl Roth GmbH, respectively. Potassium persulfate (PPS), and ferric chloride (FeCl3), 3-aminopropyltriethoxysilane (APTES) were purchased from Fluka. Polyacrylamide, N,N-Methylene bis-acrylamide (MBAm) were purchased from Merck. Potassium Permanganate, Sodium Nitrate (NaNO3), (99.99%) was bought from Sd Fine-CHE Mlimited, India. Lead nitrate salt [Pb(NO3)2], (99%) was brought from Loba Chemie Co., India. All chemicals were used as received without further treatment.
Methods
Preparation of Graphene Oxide (GO)
To a solution of 7.5 g sodium nitrate in 230 mL of concentrated sulfuric acid, 15 g of graphite powder was added with decreasing the temperature to 0 °C. This following by slow addition of 45 g of potassium permanganate with raise in the temperature to the ambient value. After 3 h under vigorous stirring, 700 mL of distilled water was added and the temperature was kept at 98 °C. After 20 min of stirring, hot distilled water (2100 mL) was added followed by 150 mL of hydrogen peroxide to avoid effervescence. The suspended graphite oxide was sonicated for 30 min, filtered out, and washed with hot distilled water until pH ~ 7, collected (the brown paste), and dried under vacuum at 60 °C [29, 34].
Preparation of Magnetic Graphene Oxide (GO/Fe3O4) (mGO)
The solvothermal method was used to load the magnetite onto GO. A mixture of GO (1 g) and ethylene glycol (150 mL) was ultrasonicated for 2.5 h and the temperature was settled up to 30 °C. FeCl3⋅6H2O (3.2 g) and sodium acetate (6.0 g) were added to the mixture and stirred for 40 min. The mixture was heated to 200 °C and for 8 h in a Teflon-lined stainless-steel autoclave. The precipitate (mGO) was magnetically separated, washed with distilled water and ethanol, dried in a vacuum oven at 50 °C for 2 days.
Modification of mGO by EDTA and APTES
In a round bottom flask, 1 g mGO was dispersed in 40 mL of toluene followed by the addition of 0.9 mL of 3-aminopropyltriethoxysilane (APTES). The mixture was refluxed in a water bath at 90 °C for 2 h with rotated at 30 rpm (revolutions/minute). 500 mg EDTA and 50 mL pyridine were added after cooling the mixture to room temperature. Again, the mixture was refluxed in a water bath for 2 h with rotation at 30 rpm. To adjust the pH, 100 mL of sodium bicarbonate (0.5 M) was added after cooling the mixture to room temperature. The supernatant was decanted and the particles were recovered by using a magnet (EDTA/Si/mGO). The EDTA/Si/mGO particles were rinsed twice by deionized water and retaining the particles with the magnet. The same was performed twice with ethanol and then diethyl ether and dried the particles at room temperature for 24 h, and stored in desiccators [33].
Preparation of Sorbent
The loading of EDTA/Si/mGO onto polyacrylamide grafted carboxymethyl cellulose (AmCMC) was performed using a surface deposition-crosslinking method. In a typical preparation, a calculated amount of potassium persulfate (KPS) (0.22 mM ≈ 0.06 g/1 g CMC) was added to an aqueous solution of CMC and heated at 60 °C. After 15 min 2 g PAm and MBAm (0.8 mM, 0.12 g/1 g CMC) were added and the mixture was kept at 80 °C for 2 h with stirring. The calculated amount of EDTA/Si/mGO (0.2 g/1 g CMC) was added before the addition of the crosslinker. The mixture was frozen in a deep-freezer followed by the freeze-drying process (ALPHA 1–2/LD PLUS, Martin Christ, Germany).
Characterization
FT-IR spectra were recorded in the range of 400–4000 cm−1on (Shimadzu 8400S) FT-IR Spectrophotometer. The XRD patterns were investigated on a Diano X-ray diffractometer using CoKα radiation source energized at 45 kV and a Philips X-ray diffractometer (PW 1930 generator, PW 1820 goniometer) with CuK radiation source (λ = 0.15418 nm), at a diffraction angle range of 2θ from 10 to 70° in reflection mode. The surface morphology was analyzed using scanning electron microscope (SEM) FEI IN SPECTS Company, Philips, Holland, environmental scanning without coating. Furthermore, transmission electron microscope (TEM) images of GO were taken with a JEOL JEM-2100 electron microscopy at an acceleration voltage of 120 kV. The thermal stability was carried out using a TGA Perkin-Elmer (STA6000), with a heating rate (10 °C /min). The temperature ranged from room temperature up to 800 °C under air atmosphere (50 mL/min). The magnetic property of material can be characterized by studying its magnetization versus the magnetic field (M–H loop). This loop can be used as an indicator for the presence of an ordered magnetic structure using a vibrating sample magnetometer (VSM, Lakeshore 7410, USA) with a maximum applied a magnetic field of 20 kOe at room temperature. To reveal the point of zero charge (PZC) of the sorbent, zeta potentials (Zetasizer Nano S, Malvern Instruments, UK) at different pH (3–9) were measured.
Sorption Studies
Sorbents Comparative Study
A comparative adsorption study was estimated to identify the best efficient sorbent by adding the prepared sorbents to 100 ml of 100 mg/L Pb+2 solutions. The mixtures were shaken for (30, 60, and 120 min) then, the solutions were filtered and the remaining Pb+2 concentrations were determined by the atomic absorption spectrometer (Varian SpectrAA220). The removal efficiency (R%) was calculated using the following equation:
where; Co and Ct are the initial concentration and the remaining concentration of Pb+2 (mg/L) after contact time (t), respectively.
Effect of Contact Time on the Removal Efficiency
The batch experiments have been employed to study the influence of contact time on the removal efficiency by adding 0.15 g of EDTA/Si/mGO/AmCMC to 100 mL (1.5 g/L) of Pb+2 solutions (100 mg/L) and shacked at room temperature for a certain time (5–120 min). The regular procedure of filtration and analysis was next applied to calculate the removal efficiency (R%).
Effect of pH on the Removal Efficiency
The influence of different pH (3–9) on the removal efficiency (R%) was studied using 100 mg/L of Pb+2. The pH of the solutions was adjusted by NaOH and HCl. The solutions were filtered after a contact time of 120 min and the residual Pb+2 concentrations were measured.
Effect of Sorbent Dosage on the Removal Efficiency
The effect of sorbent dosage (0.05, 0.1, 0.15, 0.2, and 0.25 g) in the removal efficiency of Pb+2 at pH 5.3 for 120 min was estimated. The R% was calculated from Eq. (1) and the amount of Pb+2 uptakes (q) by the sorbent was calculated as follows:
where, Co and Ct are the initial and the residual ions concentration after the time (t), respectively. V is the volume of solution (ml), and M is the sorbent mass (g).
Effect of Initial Pb2+ Concentrations on the Removal Efficiency
The removal study at different concentrations of Pb2+ (10, 25, 50, 100, and 200 mg/L) was also investigated at optimum conditions for contact time (120 min), R% and q were calculated.
Kinetic and Isotherm Studies
Four different kinetic models namely; pseudo-first-order [35], pseudo-second-order [36], intra-particle diffusion [37], and Elovich [38] were used to identify the sorption kinetics of Pb2+ from aqueous solution. Furthermore, Langmuir [39], Freundlich [40], Temkin [40], and the Dubinin–Radushkevich (D-R) [41] adsorption isotherms were applied to study how the adsorbate molecules interact with the sorbent particles. The equations and constants of the kinetic models and sorption isotherms were listed clearly in the Table 1.
Results and Discussion
Preparation and Characterization of Sorbents
In this study, a crosslinked carboxymethyl cellulose grafted polyacrylamide (AmCMC) embedded with magnetic graphene oxide (mGO) and ethylene diamine tetra acetic acid as well as 3-aminopropyltriethoxysilane (EDTA/Si) was prepared as a sorbent (EDTA/Si/mGO/AmCMC) (Scheme 1). In the first, graphite was oxidized to GO by the Hummer method to introduce oxygenated functionalities such as carbonyl, carboxylic, and hydroxyl onto graphite layers, makes it hydrophilic and expand the layer separation.
The structure of the synthesized GO, as well as that of the parent graphite, was studied by XRD analysis. As shown in Fig. 1A, the XRD pattern of the graphite shows a sharp peak at 2θ ~ 26°andrelatively weak intensity at 2θ ~ 54°, corresponding to the (002) and (004) reflection planes of natural graphite, respectively. These peaks disappeared after oxidation and a new peak at 2θ ~ 10° due to (002) plane of GO appeared. The characteristic peak of GO at 2θ ~ 11° confirmed the successful chemical oxidation of graphite into GO and the formation of oxide groups. Consequently, increased the d-spacing and caused peak shift of graphite to a lower degree [42].More confirmation of graphite oxidation is the TEM image of GO (Fig. 1B) it was observed that the GO was formed by a layered structure due to the oxidation of the graphite. From the electron diffraction pattern (Fig. 1C), GO particles have a typical diffuse ring with uniform sizes that have limited aggregation.
To increase the adsorption efficiency of GO and facilitate the separation of sorbent from the solution, the surface of GO was modified by environmentally functional groups that is magnetic nanoparticles followed by EDTA. The magnetite was loaded onto GO by solvothermal and new significant diffraction peaks appeared at 2θ ~ 29, 35, 43, 53, 57, and 62° are matched well with the data from the JCPDS card (19–0629) for Fe3O4 which attributed to the crystal planes of Fe3O4. However, the intensity of the diffraction peak of GO (at 2θ ~ 10°) was decreased, which means that the loading of magnetite destroyed the layer stacking of GO sheets [43].
Since EDTA is a tetradentate ligand has two nitrogen atoms and two oxygen atoms in separate four carboxyl groups. It can act as scavenge-chelating agents which can capture metals through several powerful bonds. Accordingly, EDTA/Si/mGO was synthesized through a chemical reaction between the OH– groups of hydroxyl and carboxyl on the surface of mGO and alkoxy groups of silanes. From X-ray diffraction (Fig. 2A) the peak of GO (at 2θ ~ 10°) was not observed in EDTA/Si/mGO and no phase change of magnetite occurred through EDTA modification. The peak at 2θ ~ 15° and the broad peak at 2θ ~ 23° in EDTA/Si/mGO could be related to EDTA, suggesting that EDTA was well distributed on the mGO [44].
After loading of magnetite onto GO and modified with EDTA/Si giving EDTA/Si/mGO, the sorbent composite was prepared by loading the EDTA/Si/mGO onto polyacrylamide grafted CMC during the grafting process. The disappearance of most peaks of EDTA/Si/mGO on the X-ray diffraction of sorbent composite and appear new peaks at 2θ ~ 17 and 27° which could be related to acrylamide grafted CMC suggesting that EDTA/Si/mGO was well distributed on the polyacrylamide grafted CMC (Fig. 2A).
The anchoring of functional groups on the GO surface and their loading onto polyacrylamide grafted CMC was approved by FTIR spectra (Fig. 2B).From the IR spectra of CMC, it showed a broad absorption band at 3350 cm−1, due to the stretching frequency of the –OH group. The band at 2920 cm−1 and a strong absorption band at 1620 cm−1 were due to C–H stretching vibration and –COO groups. The bands around 1420 and 1320 cm−1 were assigned to CH2 scissoring and –OH bending vibration, respectively while the band at 1100 cm−1 indicates the stretches of C-O group. By loading EDTA/Si/mGO onto AmCMC. The FTIR spectra of sorbent composites confirmed the well-distributed of EDTA/Si/mGO on the acrylamide grafted CMC is the decrease in band intensity of –OH groups at 3350 cm−1 due to the weakening of hydrogen bonds among the –OH groups. Furthermore, the peak at 1620 cm−1 was shifted to a higher wavenumber with the weakening of its intensity. Another remarked aspect is the disappearance of peak at 2920 cm−1 and sorbents showed a characteristic absorption band around 580 cm−1 that assigned to the stretching vibration frequency of the metal–oxygen (M–O) [45,46,47]. More confirmation is the appearance of chrematistic peaks of mGO with EDTA which are 1100, 1250, 1560, 1650, 1720, and2923 cm−1 characteristic to stretches of C–O in epoxy, hydroxyl, and carboxyl groups, C–OH stretching vibration, aromatic C=C, hydroxyl groups of alcohol and carboxyl of mGO, C–O stretching vibration of –COOH, and –CH bond stretching respectively [44]. The spectral analysis of sorbent after adsorption of Pb2+ showed that the peaks either decrease in intensity, shift, or disappear which justifies the feasibility of the adsorption process [48].
The magnetization at room temperature of the prepared composite was done by Vibrating Sample Magnetometer (VSM), ± 20 kG, to examine the possibility for the development of their magnetic properties. Figure 2C shows that magnetization increased with the magnetic field until saturation with a saturation magnetization Ms = 2.039 emu/g. Also, it shows a symmetric hysteresis loop confirming the presence of ferromagnetic behavior with a soft magnetic order where the coercivity (Hc) is equal to 18.85 G. The saturated hysteresis loops for composite confirmed the presence of magnetic order released from the magnetic particles that loaded onto GO.
Figure 2D shows the zeta potential measurements of the EDTA/Si/mGO/AmCMC composite at pH 3–9. The data presented that the point of zero charge of the sorbent at pH 3.8. This means that at pH less than 3.8 the sorbent will be positively charged inhibiting the positively charged Pb2+ adsorption while at pH above the zero-charge point, it will carry a negative charge which promoting Pb2+ removal [49].
SEM micrographs for the AmCMC (Fig. 3A) and EDTA/Si/mGO loaded AmCMC (Fig. 3B) proved the incorporation of EDTA/Si/mGO in AmCMC and resulted in remarkable changes in the surface morphology of AmCMC. The AmCMC has an interconnected laminar structure with randomly distributed pores with an average diameter range of 125 μm. By loading EDTA/Si/mGO, the surface morphology revealed the formation of a layer and high distribution of EDTA/Si/mGO throughout the surface of AmCMC.
The immobilization of EDTA/Si/mGO onto the AmCMC will be affected its thermal stability. In order to evaluate this effect, TGA analysis of the AmCMC and EDTA/Si/mGO loaded AmCMC was performed (Fig. 3C).
The thermal degradation of AmCMC occurs in three steps, the initial step started at ~ 110 °C with ~ 12% weight loss due to dehydration. The second step was from 200 to 450 °C resulting in ~ 78% weight loss which could be ascribed to the decomposition of the crosslinked polymer chains. The third step started at 750–800 °C with ~ 95% weight loss due to the pyrolysis of the cellulose backbone [50]. As we expected the thermal stability has improved by loading the EDTA/Si/mGO onto AmCMC. The TGA curve of EDTA/Si/mGO/AmCMC demonstrated three degradation steps (Fig. 3C). The sample started breaking down around ~ 400 °C with ~ 30% weight loss then further degradation at ~ 660 °C with ~ 40% weight. The decomposition was resumed at ~ 775 °C losing ~ 55% of the original sample mass due to the pyrolysis of the cellulose backbone with residual ash ~ 45%.
Sorption Study
Comparative Study on the Removal Efficiency
Figure 4A shows a comparative study on the removal efficiency of the prepared sorbents, AmCMC and EDTA/Si/mGO/AmCMC, for Pb2+ from aqueous solution after different times namely 30, 60, and 120 min. The data showed that EDTA/Si/mGO/AmCMC has the highest removal efficiency for Pb2+ over other AmCMC sorbent, which reached 86, 88, and 92% after 30, 60, and 120 min respectively. The higher removal efficiency of this sorbent can be attributed to the presence of EDTA/mGO system as Pb2+ can react with chelating groups such as –COOH, –NH2, and –OH. Due to the high removal efficiency of EDTA/Si/mGO/AmCMC sorbent, the other adsorption conditions will be studied by this sorbent only.
A The removal efficiency of Pb2+ by the prepared sorbents for 30, 60, and 120 min as contact times and sorbents dosage was 0.15 g/100 mL, (B, C) the removal efficiency of 100 mg/LPb2+, and Pseudo-second-order adsorption fitting model for Pb+2 by 0.15 g of EDTA/Si/mGO/AmCMC respectively as a function of contact time
Effect of Contact Time on the Removal Efficiency
Figure 4B displaying the effect of contact time on the removal efficiency of Pb2+ by the prepared sorbentsuch as EDTA/Si/mGO/AmCMC. It can be seen that a quick removal of Pb2+ within the first 5 min of the adsorption process was obtained. Next, a slower sorption step continued until reaching a state of equilibrium.This behavior is due to the availability of sufficient vacancies and cleating groups such as –COOH, –NH2, and –OH in the initial of the reaction but after this contact time, active sites became occupied [51]. On the other side, the kinetic studies of adsorption of Pb2+ by sorbentwere elucidated after applying the pseudo-first-order, pseudo-second-order, intra-particle diffusion model, and Elovich model. The kinetic model's constants and correlation coefficients of Pb2+ elaborate in Table 2. Interestingly from the data, the kinetics of the sorption reaction was perfectly fitted to the pseudo-second-order model (Fig. 4C) which assumes that therate of solute sorption is directly proportional to the square of the number of vacant binding sites [52]. This due to the higher correlation coefficient value (R2 = 0.9992), and the close matching between the experimental (qe = 58.67 mg/g) and calculated (qe = 58.47 mg/g) uptake from this model [4, 5].
Effect of pH on the Removal Efficiency
One of the most essential parameters which affect the removal efficiency of metal ions is pH value. Figure 5A revealing the removal efficiency of 100 mg/L Pb2+ by 0.15 g EDTA/Si/mGO/AmCM Cover the pH range of 3–9. The data clarified that as pH increase, the removal efficiency increase from 40 to 98%. At low pH values, less Pb2+ removal is observed which is attributed to the presence of a large number of protons (H+) that may have adsorbed on the prepared sorbent sites rather than Pb2+ [53]. At high pH values, the H+ competition disappears and sorbent carry a negative charge as previously reported in zeta potential measurements that increases attraction with Pb2+ thus increases the removal [49]. In alkaline media (pH higher than 8.0), it is known that precipitation plays a major role in removing metal ions which inhibits the adsorption process [9].
Effect of Sorbent Dosage on Removal Efficiency of Pb2+
The sorbent dosage used in the sorption process is also important in the removal of metal ions from a solution. Figure 5B illustrates the effect of the sorbent dose from 0.05 to 0.25 g on the removal efficiency and the uptake capacity while maintaining other parameters fixed i.e., pH = 5.3, time = 120 min, and conc. = 100 mg/L. As expected, the removal efficiency of Pb2+ increased from 74 to 96% with increasing the amount of the sorbent added which attributed to the increase of the active sites available for the Pb2+ removal [54]. Conversely, the removal efficiency of Pb2+increased, the uptake capacity decreased from 140 to 36 mg/g with increasing the sorbent dosage due to the repulsion between the few Pb2+ occurring in the solution and the high-density volume of the already adsorbed metal ions [55, 56].
Effect of the Initial Pb2+ Concentrations on the Removal Efficiency
The Pb2+ concentration affects the adsorption process, so the removal efficiency by 0.15 g of sorbentwas carried out with different Pb2+ concentrations (Fig. 6A). The data show that as the initial concentration of Pb2+ increased from 10 to 200 mg/L, the removal efficiency decreased from 98 to 60% and this recognized the lacking sufficient active sites for adsorption [57]. On the contrary, the uptake capacity increase from 3 to 80 mg/g with increasing the concentration and this attributed to the ratio between Pb2+ and sorbentt is sufficient to provide a driving force to overcome the mass transfer resistance between them [4, 5].
On the other hand, to illustrate how the Pb2+ interact with the sorbents; Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R) isotherm models were studied. The constants and correlation coefficients calculated from the isotherm models were listed in Table 2. Remarkably from the data (Fig. 6B), the sorption of Pb2+ was fitted to the Langmuir model due to the higher correlation coefficient (R2 = 0.995) value which assumes monolayer adsorption of the Pb2+ onto active sites of the sorbent's surface [58]. The value of n > 1 in Freundlich and E < 8 in (D-R) model demonstrating that the adsorption is a physical process [40]. Moreover, the separation factor (RL) values were found to be in the range from 0 to 1, which proposing favorable adsorption between Pb2+ and EDTA/Si/mGO/AmCMC.
The maximum Pb2+ sorption capacities (qmax) of the sorbent (EDTA/Si/mGO/AmCMC) which calculated from the Langmiur model were compared with different sorbents in previous studies (Table 3). The data indicated that the prepared sorbent has a good ability for the removal of Pb+2 froman aqueous solution.
Conclusion
An excellent eco-friendly sorbent was successfully prepared by the introduction of EDTA groups to the mGO surface through a salinization process followed by embedded onto AmCMC for Pb2+ removal from aqueous solution. The adsorption was studied by studying the effect of contact time, pH, sorbent dosage of sorbent, and concentrations of Pb2+ on removing efficiency of the prepared sorbent. The results illustrated that the removal efficiency of Pb2+ by EDTA/Si/mGO/AmCMC (92%) is higher than that of AmCMC (46%) after a contact time of 120 min. A great increase in the removal efficiency was detected with increasing contact time, pH, and sorbent dosage, while a significant decrease was observed with increasing the initial concentration of Pb2+. The higher correlation coefficient (R2 = 0.9992) indicated that the kinetics of the sorption reaction could be fitted to a pseudo-second-order model. Furthermore, the isothermal results indicate that the Langmuir isotherm was a much better model due to the higher correlation coefficient (R2 = 0.995) and a maximum adsorption capacity for EDTA/Si/mGO/AmCMC of 86 mg/g was obtained.
Data Availability
All relevant data are within the manuscript and available from the corresponding author upon request.
References
Li R et al (2018) Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator. Environ Sci Technol 52(19):11367–11377
Zhao F et al (2019) Super moisture-absorbent gels for all-weather atmospheric water harvesting. Adv Mater 31(10):1806446
Haggag S et al (2020) Innovative synthesis of modified cellulose derivative as a uranium adsorbent from carbonate solutions of radioactive deposits. Cellulose 27:7093–7108
Kamal K et al (2019) Methylene blue removal from wastewater using silica/corncob nanocomposite. Int J Dev 8(1):81–93
Kamal KH et al (2019) Adsorption of Fe ions by modified carrageenan beads with tricarboxy cellulose: kinetics study and four isotherm models. Desalin Water Treat 165:281–289
Yang L et al (2020) Energy harvesting from atmospheric humidity by a hydrogel-integrated ferroelectric-semiconductor system. Joule 4(1):176–188
Sardar K et al (2013) Heavy metals contamination and what are the impacts on living organisms. Greener J Environ Manage Public Safety 2(4):172–179
Abou-Zeid RE et al (2020) Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int J Biol Macromol 167:1091
Mohammad AM et al (2017) Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures. Arab J Chem 10(5):683–690
Rengaraj S et al (2002) Studies on adsorptive removal of Co (II), Cr (III) and Ni (II) by IRN77 cation-exchange resin. J Hazard Mater 92(2):185–198
Cai G-B et al (2010) Synthesis of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water. J Phys Chem 114(30):12948–12954
Fu F et al (2007) Effective removal of coordinated copper from wastewater using a new dithiocarbamate-type supramolecular heavy metal precipitant. Chemosphere 69(11):1783–1789
Rao MM et al (2009) Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J Hazard Mater 166(2–3):1006–1013
Solanki P et al (2010) Synthesis of zeolite from fly ash and removal of heavy metal ions from newly synthesized zeolite. E-J Chem 7(4):1200–1205
Frimpong RA et al (2007) Synthesis and temperature response analysis of magnetic-hydrogel nanocomposites. J Biomed Mater Res Part A 80(1):1–6
Kabir A et al (2018) Water recycling efficacies of extremely hygroscopic, antifouling hydrogels. RSC Adv 8(66):38100–38107
Abou-Yousef H et al (2021) Biocompatible hydrogel based on aldehyde-functionalized cellulose and chitosan for potential control drug release. Sustain Chem Pharm 21:100419
Pashaei-Fakhri S et al (2021) Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 270:129419
Peighambardoust SJ et al (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131
Abou-Zeid RE et al (2018) Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int J Biol Macromol 119:207–214
Sirviö J et al (2011) Dialdehyde cellulose microfibers generated from wood pulp by milling-induced periodate oxidation. Carbohyd Polym 86(1):260–265
Akl EM et al (2020) Preparation and characterization of novel antibacterial blended films based on modified carboxymethyl cellulose/phenolic compounds. Polym Bull 78:1–25
Khattab TA et al (2019) Smart microfibrillated cellulose as swab sponge-like aerogel for real-time colorimetric naked-eye sweat monitoring. Talanta 205:120166
Dacrory S et al (2018) Preparation and characterization of eco-friendly carboxymethyl cellulose antimicrobial nanocomposite hydrogels. J Renew Mater 6(5):536–547
Mohammadi R et al (2021) Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur Polym J 153:110500
Mekheimer RA et al (2011) Simple, three-component, highly efficient green synthesis of thiazolo [3, 2-a] pyridine derivatives under neat conditions. Synth Commun 41(17):2511–2516
Madadrang CJ et al (2012) Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl Mater Interfaces 4(3):1186–1193
Dacrory S et al (2021) Cyanoethyl cellulose/BaTiO3/GO flexible films with electroconductive properties. ECS J Solid State Sci Technol. https://doi.org/10.1149/2162-8777/ac1c56
Ghanem AF et al (2020) Synergistic effect of zinc oxide nanorods on the photocatalytic performance and the biological activity of graphene nano sheets. Heliyon 6(2):e03283
Foroutan R et al (2021) Adsorption mercury, cobalt, and nickel with a reclaimable and magnetic composite of hydroxyapatite/Fe3O4/polydopamine. J Environ Chem Eng 9(4):105709
Peighambardoust SJ et al (2021) Decoration of Citrus limon wood carbon with Fe3O4 to enhanced Cd2+ removal: a reclaimable and magnetic nanocomposite. Chemosphere 2021:131088
Magdalena A et al (2018) EDTA-functionalized Fe3O4 nanoparticles. J Phys Chem Solids 113:5–10
Sahoo, J. K., et al. (2017). EDTA functionalized magnetic nanoparticle as a multifunctional adsorbent for Congo red dye from contaminated water. AIP Conference Proceedings, AIP Publishing LLC
Dacrory S (2021) Antimicrobial activity, DFT Calculations, and molecular docking of dialdehyde cellulose/graphene oxide film against Covid-19. J Polym Environ 29:2248
Sharma N, Nandi BK (2013) Utilization of sugarcane baggase, an agricultural waste to remove malachite green dye from aqueous solutions. J Mater Environ Sci 4(6):1052–1065
Azizian SJJOC, Science I (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276(1):47–52
Doğan M et al (2004) Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J Hazard Mater 109(1–3):141–148
Bharathi K, Ramesh SJAWS (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3(4):773–790
Gupta S, Babu BV (2009) Removal of toxic metal Cr (VI) from aqueous solutions using sawdust as adsorbent: equilibrium, kinetics and regeneration studies. Chem Eng J 150(2–3):352–365
Senthil Kumar P et al (2014) Adsorption of basic dye onto raw and surface-modified agricultural waste. Environ Prog Sustain Energy 33(1):87–98
Dubinin M (1947) The equation of the characteristic curve of activated charcoal. Dokl Akad Nauk SSSR 55:327
Jiao X et al (2017) Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv 7(82):52337–52344
Kamel S et al (2020) Carboxymethyl cellulose-hydrogel embedded with modified magnetite nanoparticles and porous carbon: effective environmental adsorbent. Carbohydr Polym 242:116402
Marnani NN, Shahbazi A (2019) A novel environmental-friendly nanobiocomposite synthesis by EDTA and chitosan functionalized magnetic graphene oxide for high removal of Rhodamine B: adsorption mechanism and separation property. Chemosphere 218:715–725
Abou Hammad A et al (2019) A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr Polym 216:54–62
Dacrory S et al (2019) Functionalization and cross-linking of carboxymethyl cellulose in aqueous media. Cellul Chem Technol 53(1–2):23–33
Dacrory S et al (2020) In situ synthesis of Fe3O4@ cyanoethyl cellulose composite as antimicrobial and semiconducting film. Carbohydr Polym 2020:116032
Foroutan R et al (2021) Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J Hazard Mater 413:125428
Salah TA et al (2014) Development of nano-hydroxyapatite/chitosan composite for cadmium ions removal in wastewater treatment. J Taiwan Inst Chem Eng 45(4):1571–1577
El-Sayed NS et al (2019) Development of electrical conducting nanocomposite based on carboxymethyl cellulose hydrogel/silver nanoparticles@ polypyrrole. Synth Metals 250:104–114
Abd El-Aziz M et al (2018) Biodegradable grafting cellulose/clay composites for metal ions removal. Int J Biol Macromol 118:2256–2264
Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6(2):2335–2343
Boushehrian MM et al (2020) Ultrasonic assisted synthesis of Kaolin/CuFe2O4 nanocomposite for removing cationic dyes from aqueous media. J Environ Chem Eng 8(4):103869
Nasuha N et al (2010) Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater 175(1–3):126–132
Foroutan R et al (2019) Eggshell nano-particle potential for methyl violet and mercury ion removal: Surface study and field application. Adv Powder Technol 30(10):2188–2199
Foroutan R et al (2019) Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: real wastewater treatment. Environ Sci Pollut Res 26(7):6336–6347
Aksu Z, Tezer SJPB (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40(3–4):1347–1361
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Mwandira W et al (2020) Cellulose-metallothionein biosorbent for removal of Pb (II) and Zn (II) from polluted water. Chemosphere 246:125733
Aquino R et al (2018) Adsorptive removal of lead (Pb 2) ion from water using cellulose acetate/polycaprolactone reinforced nanostructured membrane. IOP Conf Ser 191(1):012139
Shafiee M et al (2019) Application of oak powder/Fe3O4 magnetic composite in toxic metals removal from aqueous solutions. Adv Powder Technol 30(3):544–554
Gupta VK et al (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35(5):1125–1134
Günay A et al (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146(1–2):362–371
Acknowledgements
The authors acknowledge the Academy of Scientifc Research and Technology (ASRT), Egypt (Grant ASRT-19-06), for financial support of the bilateral research activities.
Funding
This work was supported by the Academy of Scientific Research and Technology (ASRT), Egypt (Grant ASRT-19-06).
Author information
Authors and Affiliations
Contributions
Study conception and design: SD and KHK, Acquisition of data: SD and KHK. Analysis and interpretation of data: SK. Drafting of the manuscript: SD, KHK, and SK. Critical revision: SK. All authors approved the final version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
All the authors hereby declare that they do not have any conflict of interest about this manuscript.
Consent to Participate
All authors have participated in this work.
Consent to Publish
All authors agree to publish.
Research Involving Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dacrory, S., Kamal, K.H. & Kamel, S. EDTA-Functionalized Magnetic Graphene Oxide/Polyacrylamide Grafted Carboxymethyl Cellulose Hydrogel for Removal of Pb+2 from Aqueous Solution. J Polym Environ 30, 1833–1846 (2022). https://doi.org/10.1007/s10924-021-02310-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02310-3