Abstract
In this study, Acrylic acid (AA), acrylamide (AM), and 16,16-dimethylheptadecan-1-amine (PJM-T) were copolymerized using gamma irradiation with 60Co γ-rays at a dose of 25 KGy to form a novel composite; Poly(acrylic acid-co-acrylamide/16,16-dimethylheptadecan-1-amine P(AA-co-AM/PJM-T). P(AA-co-AM/PJM-T) is characterized by different physicochemical techniques and used as a sorbent for rare earth elements from monazite. The optimum pH for the sorption process at 25 ℃ is 4.5 and the equilibrium attained at 60 min. Different kinetics and isothermal models is applied. The maximum adsorption capacity is 182.15 ± 3.73 mg g−1 at 25 ℃. The sorption reaction regulates a pseudo 2nd order mechanism and the process is spontaneous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last centuries, REE has gained considerable importance due to the valuable need for these elements and their compounds in several fields. The physical and chemical properties of REEs make it suitable for electric, optical, and metallurgical technological fields [1,2,3]. REEs could be used in special alloys, magnets, catalysts, electric applications [4, 5], nuclear technologies, communications systems [6,7,8], medical purposes, and agricultural application [9].
Many ores for REEs are; monazite, lanthanite, xenotime, bastnäsite, allanite, loparite and phosphate rocks [10]. Monazite could be a REE (PO4)3− that's frequently found with other valuable minerals like ilmenite, rutile, zircon and cassiterite. There are 2 steps during the recovery of the REEs from its monazite ore. The 1st step is the decomposition of the ore and purification of REE compounds. In the 2nd step, this compound is separated into individual REE products by liquid–liquid extraction and/or ion exchange.
Several methods such as; precipitation, ion exchange, coagulation, flocculation, liquid–liquid extraction [11], solid—phase extraction [12], ionic liquid extraction [13], biosorption, and classical adsorption on different sorbents have been used for separating different lanthanides from aqueous solutions. These chemical methods of separation rely up on the concentration of REEs. At higher REEs concentrations, precipitation as hydroxide or as oxalate is favourable. In addition to solvent extraction [12] is employed. At lower REEs concentrations, ion exchange resin [14], nano filtration membrane [15], synthetic composite, [16] and bio-adsorption [17] are strongly recommended.
Among them, the adsorption method is utilized in treatment of REEs solutions due to its multiple superiorities of easy operation, low cost, high availability, and favorable retention efficiency [18].
16, 16-Dimethylheptadecan-1-amine (Primene JM-T; a primary amine) is especially employed in REE retention by solvent extraction and/or ion exchange. This can be due to its unique physical and chemical properties, resistance to oxidation, Fluidity and low viscosity at different temperatures, color stability. Primene JM-T extracts negative or anionic species [19]. However; Primene JM-T is incredibly toxic to aquatic life and expensive. Thus, it is important to develop a novel sorbent to solve this defect.
Advanced sorption materials [20,21,22] and organic sorbents [23,24,25] like poly acrylic acid P (AA) which is cheap and non toxic. P(AA) was studied by authors efficiently in the adsorption of heavy metals [26,27,28,29]. Furthermore, P(AA) encompasses a great potential for adsorbing REE ions owing to its accessible carboxylic groups [30]. Enhancement of the hydrophilic character of poly carboxylic acids groups by polymerization with other hydrophilic monomers like; maleic acid) SiO2/Al2O3 for sorption of Cesium, [31] acrylamide as N-donor functional moieties is studied. Authors declare that N-donor compounds have good sorption behaviour [32].
However, there's a limitation in studying co-polymerization of Primene JM-T with hydrophilic polymers.
In the present paper; REEs are extracted from monazite ore by treatment with alkaline solution. Separation of REEs from (PO4)3− and then from the impurities by direct precipitation are performed. The rare earth cake is still impure; should undergo further purification processes. A novel composite poly (acrylic acid –co -acrylamide/16, 16-dimethylheptadecan-1-amine) composite was used for this purpose after optimization of the sorption affecting factors like; pH, contact time, concentration and resin concentration. The mechanism of the sorption reaction is proposed by applying different kinetic models; pseudo 1st order, pseudo 2nd order, Elvoich and intra-particle diffusion. Furthermore, four isothermal models; Langmuir, Freundlich, Flory–Huggins and Temkin isotherm is applied.
Experimental
Reagents and Methodology
All reagents used during this work were of analytical grade and used without further purification. Acrylic acid (AA) got from Elf Chem. Co., ATOFINA, and France. Acrylamide (AM) was supplied by Fuchen Chemical Co., Ltd., Tianjin, China. Methylene bis-acrylamide (MBA) was supplied from (Merck, Germany). 16, 16-dimethylheptadecan-1-amine (PJM-T) is purchased from Adwic.
Monazite concentrate (monazite content about 90%) is supplied by nuclear materials authority, Cairo-Egypt. Double distilled water was used for the preparation of the composite. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were supplied from ADWIC (Egypt) for pH—adjustment.
Co-polymer Preparation
A general procedure for grafting copolymerization of Acrylic acid (AA) and Acrylamide (AM) with Methylene bis-acrylamide (MBA) as acrosslinker using gamma irradiation technique was conducted as follows: 10% of prepared monomer composition (60 AA:40 AM) was dissolved in degassed, distilled water. Then added to the total solution, 0.5 mass % MBA as a cross linker and 2% PJM-T were added to the reaction mixture then shaken overnight in a glass tube. The mixture is stirred at 400 rpm at room temperature for 2 h and ultrasonically treated for 10 min, after that it was subjected to Co-60 gamma rays irradiation (25 kGy). The irradiated product (grafted copolymer) was cut into small pieces and washed by acetone for removal of excess of unreacted monomers, washed with double distilled water and dried in an oven at 60 °C to a constant weight and then ground to the desired mesh size (< 300 μm) and keep for further use.
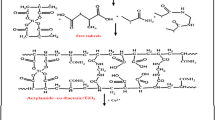
Figure 1 is a schematic diagram summarizes the preparation of P(AA-co-AM/PJM-T) composite. 10 ml of PJM-T mixed with 30 ml AA and 20 g acrylamide, 2.5 g MBA in 500 ml deoxygenated water.
Gamma Radiation
Radiation is a very convenient method for graft initiation. It has advantages compared to chemical method as it is more efficient, economical, wide applicability and easily prepared [33]. Gamma Irradiation was carried out using the Co-60 gamma cell. A cell of type yMC-20 (Russia) was used at the Cyclotron Project, Inshas site, Egypt. The monomers were exposed to a dose of gamma radiation 25 kGy. Gamma radiation leads to the formation of free radicals, so it acts as an initiator for the polymerization process. These free radicals are active sites that cross linked with other monomers to get the grafted copolymer.
Predicted Mechanism for Polymerization Process
Possible mechanism reactions for the graft polymerization of P(AA-co-AM/PJM-T) in the presence of MBA as cross linker can be predicted as shown in Fig. 2 [34].
Preparation of REEs Solutions
Monazite was digested by alkaline method (caustic soda) [35]. 15 g of the digested REEs hydroxide cake was dissolved carefully in 50 ml conc. HCl acid in water bath at 85 °C. The initial concentration of REEs solutions is 1608 mg l−1 and different concentrations from REEs were prepared via dilution of the stock standard solution for all batches adsorption trials. The stock solution keep in dark glass bottle and pH is monitored and adjusted continuously using small amounts of NH3 or HCl to avoid metal precipitation.
Instruments
The surface morphology of the prepared composites was examined using a scanning electron microscope (JEOL JSM-5400, Japan). The FT-IR measurements were performed using a Bomen Miclson FT-IR spectrophotometer, model MB157, from Canada. Differential thermal and thermo-gravimetric analyses were carried out using Shimadzu DT-60, Japan. The Samples were exposed to temperature up to 800 ℃ with a heating rate of 20 ℃ min−1 in the presence of nitrogen atmosphere to avoid thermal oxidation of the powder samples.. Shimadzu X-ray diffraction (XRD), model XD-Dl, Kyoto, Japan, was used to investigate the crystalline structure, with a diffraction angle (2θ) within the range 4–70°. Pore size distribution and corresponding porosity calculated with the aid of pore-sizer chromatech 9320, USA. The concentration of REEs solutions is determined by Inductive Coupled Plasma Optical Emission Spectrometer (Prodig Axial high dispersion ICP-OES model, USA and UV- spectrophotometer (single beam multi-cells-positions model SP-8001.
Point of Zero Charge
Point of zero charge (pHpzc) is that the pH at which the surface charge is zero [36]. The surface charge at pH lower than (pHpzc) is positive and that at pH higher than (pHpzc) is negative. The small value of pHpzc is considered as sign for an efficient sorbent because it gives a verity of pH for the sorption of cations. pHpzc can be determined practically by adjusting pH for a series of flasks each one contains 0.5 g of the adsorbent with 20 ml of 0.01 M (NaCl) and the pH was adjusted from 1.0 to 7.0 (pHinitial). The solution was shaken for 24 h and the final pH of the solutions was measured (pHfinal). pHpzc is determined by plotting pHinitial against ∆pH (pHfinal–pHinitial).
Sorption Studies
Sorption studies were carried out onto P(AA-co-AM/PJM-T) using Egyptian high-grade monazite sample. Sorption parameters. i.e. pH (2–4.5), contact time (15–120), initial concentration (200–2000 mg l−1) and resin ratio (V/m) varied to select the best conditions for sorption. 0.1 g of the sorbent contacted with 20 ml of the adsorbate solution and after sorption time; samples were filtered and thus separated from the solution.
The Sorption efficiency of the REE ions at each interval of time is given by Eq. (1).
Adsorbed amount q (mg g−1) is calculated using Eq. (2):
where q, is the amount sorbed (mg g−1). Co, Cf are the initial and final concentrations of the REE ions, respectively. m is the weight of the P(AA-co-AM/PJM-T) (g) and V is that the volume of solution (l).
Kinetic Modeling
Kinetic modelling can give a proposal for the mechanism of the sorption reaction. Four kinetics models are applied through this study; Pseudo 1st order, pseudo 2nd order, Elovich model and intra-particle diffusion model.
Pseudo 1st Order & Pseudo 2nd Order Models
The pseudo-first order equation is given by Eq. (3) [37]
where qe and qt are the sorbed amounts of REE ions; (mg g−1) at equilibrium time and at any time t, respectively; k1 (min−1) is the 1st order rate constant.
The pseudo-second order is given by the Eq. (4) [38]
where k2 (g mg−1 min−1) is the 2nd order rate constant.
Elovich Model
It’s applied for chemisorptions reaction and give by Eq. (5) [39]
where α and β are the Elovich coefficients. α (mg g−1 min−1) represents initial adsorption rate and β expressed desorption constant (g mg−1) during any experiment related to the activation energy involved in chemisorptions.
Intra- particle diffusion model
The intra-particle diffusion model is applied using Eq. (6) [40]
where kid is the intra-particle diffusion rate constant (mg g−1 min−1/2) and C is the intercept.
Isotherm Modeling
Four isotherm models are applied through this study; Langmuir [41], Freundlich [42], Flory–Huggins [43] and Temkin isotherm [44].
Langmuir Model
By Eq. (7), Langmuir isothermes model could be applied
where Q0 is the Langmuir adsorption capacity (mg g−1), b is the constant related to free energy of sorption where ∆Go (b α e−ΔG/RT) and Ce is the equilibrium concentration.
Freundlich Model
Freundlich model is illustrated by Linear Eq. (8)
where, Kf is Freundlich constants and n, related to adsorption capacity and intensity.
Flory–Huggins Model
Flory–Huggins Eq. (9) and the linear relation is given by Eq. (10):
where, θ = 1–CeCo, the degree of surface coverage, nFH the number of occupied sites, and KFH, the Flory Huggins equilibrium constant (Lmol−1). The equilibrium constant KFH is used to know the spontaneity of ∆Go with the aid of Eq. (11):
where ∆Go is the standard free energy, R is the universal gas constant, 8.314 Jmol−1 K−1, and T is temperature in Kelvin.
Temkin Model
Temkin assumes a linear variation of the adsorption heat with the degree of overlap. The model is given by the Eqs. (12, 13) [44]
where: \({B}_{T}=\frac{RT}{{b}_{T}}\text{,}\) AT, bT are constants, AT (L mol−1) is related to the maximum binding energy, bT is said to be the adsorption heat.
The loaded P(AA-co-AM/PJM-T) by REE was desorbed in several concentrations (0.01, 0.1, 0.5 and 1 M) of HCl as desorbing solution at room temperature for an hour. The mixture was filtrate to isolate the P(AA-co-AM/PJM-T) composite from the liquid phase, and then the concentration of REE ions measured. The desorption efficiency % was calculated using Eq. (14):
where Caq is related to the concentration of REE within the aqueous phase, Cs is that the concentration of REE within the P (AA-co-AM/PJM-T) composite.
Result and Discussion
Characteristics of the Adsorbent
Adsorbent Morphology
The morphology for P(AA-co-AM/PJM-T) composite Fig. 3a shows a dense packed morphology with well definite dimensions of the polymeric matrix. Sorption of rare earth ions onto P(AA-co-AM/PJM-T) (Fig. 3b) leads to fracture surface i.e. The smoothing of the surface decreases.
FT-IR Analysis
FT-IR analysis in Fig. 4a showed the characteristic absorption peaks for the functional groups of P(AA-co-AM/PJM-T) composite. The two broad absorption signals at 3439 cm−1 [45] and 1641 cm−1 were attributed to OH of AA. However, the absorption peak at 3213 cm−1 is assigned to NH for PJM-T and acrylamide. CH group is represented by the absorption peak at 2960 cm−1. A peak at 1735 is assigned to C = O [45] while that at 1456 cm−1 belongs to stretching band of CN and 1166 cm−1 is another peak related to NH group [46]. The signal at 1661 cm−1 is the characteristics (N–H) stretching peak [47]. Two existed signals at 1078–1128 cm−1 are attributed to the 2nd stretch OH group. The peaks at range 1166–811 are due to C–H stretching of CHO group that at range 579–525 assigned to CH2 aliphatic compounds. For loaded P(AA-co-AM/PJM-T) with REEs in Fig. 4b; the intensity of bands of –OH and –NH decrease due the participating of these groups in the coordination of REEs. CO as well as CN bands are shifted to 1732, 1407 cm−1, respectively. In any cases, binding of REEs strongly affects the chemical environment of N−, OH− groups. Several bands in the range 900–400 related to REEs-O stretching bands. Figure 4c for P(AA-co-AM), The broad and strong absorption band near 3435 cm−1 represents the stretching vibration of –OH group in AA and -NH group in AM the peak at 1646 cm−1 was the stretching vibration band of carbonyl group in AM. The 1459 cm−1 is related to the stretching vibration of C–N bond in graft copolymer. The absorbance at 2924.5 cm−1 is assigned to –C–H stretching of the acrylate unit.
Energy Dispersive (EDX) Spectroscopy
The energy dispersive spectra of P(AA-co-AM/PJM-T) composite are shown in Fig. 5. The spectrum of the composite revealed an intense carbon band, oxygen and nitrogen bands due to the main constituents of acrylic acid chains, acrylamide and PJM-T of the copolymer. The composite has the highest percentage of carbon followed by nitrogen and oxygen.
X-ray Diffraction Analysis
X ray diffraction may be a tool for recognition of the degree of crystalline of P(AA-co-AM/PJM-T) composite. Figure 6 clarify that P(AA-co-AM/PJM-T) is amorphous structure with non crystalline peak at 2θ = 20.4 such as atactic structures of PAA [48].
Thermogravimetric Analysis
Thermogravimetric analysis is depicted in Fig. 7a. The thermal stability of P(AA-co-AM/PJM-T) composite decrease because the temperature is raised and also the composite losses its weight in three decomposition steps. The 1st step starts from 170 ℃ to 222 ℃ with weight loss of 1.94%. This step accompanied by endothermic peak at 220 ℃ due to loss of adsorbed water. The second step starts at 340 ℃ with 42.3% weight loss. The endothermic peak at 340 ℃ attributed to loss of water from PAA and formation of poly acrylic acid anhydride [39]. Then the third step accelerated from 400 ℃ to 800 ℃ with 92.07% weight loss and can be ascribed to the degradation of composite. An exothermic peak at 528 ℃ because of burning of organic substances [49].
DSC provides qualitative and quantitative information to the thermal properties of P (AA-co-AM/PJM-T) such as the melting and degradation temperatures, glass transition temperature, melt and crystallization enthalpy, specific and latent heats, polymorphism, and purity of the materials. Figure 7b shows the DSC curve of PAA. As can be seen from this curve, there are an endothermic peak at 220 °C, at which the sample consumes energy values 304.6 J/g, respectively. In addition, the curve shows two exothermic peaks at 154.4 °C and 458 °C with emitted heat values of − 81.71 and − 16.81, respectively.. The endothermic peak at 220 °C due to water loss while the exothermic peak at 400 °C corresponds to degradation of composite. The first exothermic peak at 154.4 due to amorphous aggregate of the composite.
Surface Characteristics
Surface characteristics of any sorbent give an indication for its efficiency for the sorption process. Total pore area includes macro, meso and micro pores. However, the average pore diameter is 119.4 nm (more than 50 nm) closely related to the presence of macropores. The presence of macrospores responsible for the cracking of the surface. The difference between apparent density and bulk density is reasonable evidence for existence of open pores available for sorption process. The results for surface characteristics are listed in Table 1.
Sorption Study
Effect of [H+] Concentration
The sorption process is extremely affected by the change in pH parameter due to ionization of surface functional groups. Different sorption experiment within pH range (2–4.5) performed to determine the optimum pH. Figure 8 represents the relation between amount sorbed (qe) and pH and it is obvious that as the pH increase; the sorbed amount increases. This is owing to the increase of the total net negative charges of the sorbent surface attracting metal ions and a less competition between H+ and REE ions [50].
The pHZPC (pH of zero point charge) is an important physicochemical parameter depends on the surface chemistry of the sorbent. P(AA-co-AM/PJM-T) composite has a negative surface charge related to Acrylic acid. The pH of the point of zero charge of P(AA-co-AM/PJM-T) composite is 3 shown in Fig. 9; the charge of the surface is positive at pH < 3. At pH values above this given value, the electric charge of the adsorbent is (−ve) and can attract the REE ions (+ ve). So, it's necessary to choose the working pH > 3.
The optimum pH is chosen at pH 4.5 due to formation of rare earth hydroxides precipitate at higher pH. This is confirmed by Hydra/Medusa chemical equilibrium software [51] starting with rare earth ions (III) concentration 200 mg l−1, 25 ℃ and different [H+] concentration (1–12). Figure 10 illustrates rare earth ions (III) predominate at pH up to 5. In Fig. 10a Ce(III), Nd(III), Sm(III) and Pr(III) are predominate species at pH < 6. At pH > 6, the predominate species are Ce(OH)3, Nd(OH)3, Sm(OH)3, Pr(OH)3 and complex ions like Ce(OH)2+, Ce(OH)+2, Nd(OH)2+, Nd(OH)+2, Sm(OH)2+, Sm(OH)+2, Pr(OH)2+, Pr(OH)+2 at pH > 10 the predominate species are Ce(OH)4−, Sm(OH)4−, Pr(OH)4.
The same in Fig. 10b; the predominate species are Eu(III), Gd(III), Dy(III), Tb (III) while the Lu(III) is predominate species at pH < 5 (Fig. 10c).
Effect of Contact Time
The effect of time on adsorption process studied at intervals (15–120 min) and represented in Fig. 11. The equilibrium time of the sorption reaction was reached rapidly at nearly an hour. The sorbed amount increases at time interval (15–60 min) due to the gradual occupancy of accessible sites on abundant availability of active sites on P(AA-co-AM/PJM-T) [52]. At 60 min, the equilibrium time, complete occupancy of the P(AA-co-AM/PJM-T). The optimum sorbed amount is 36 mgg−1.
Effect of Concentration
The effect of initial REE ions concentration on the sorbed amount is shown in Fig. 12. The concentrations in the range from 200 to 2000 ppm of the REE ions have been studied. The adsorbed amount (qe) of rare earth ions was found to decrease as the REE ions concentration increase. This is owing to P(AA-co-AM/PJM-T) as a sorbents have a fixed number of active sites and at higher concentrations of rare earth ions, the driving force to P(AA-co-AM/PJM-T) surface/liquid interface increases till the active sites become saturated [53, 54].
Four models of sorption: Langmuir, Freundlich, Flory–Huggins and Temkin, were applied to identify the fit model to adsorption reaction.
Effect of V/m Ratio
Impacted volume to weight ratio (V/m) on adsorption of RE ions was studied in the range from 0.2–0.5 Lg−1 to evaluate the preferred volume/weight of sorbent for high uptake percentage. As present in Fig. 13, the uptake efficiency inversely related with V/m ratio. Thus, the best V/m ratio was chosen at 0.2.
Kinetic Modeling
Pseudo 1st –Order & Pseudo 2nd Order
The sorption reaction is more fitted with the kinetic model that has a highest correlation factor R2 and the qe(cal) identity with qe(exp.). Figure 14a, b illustrates the plots for pseudo 1st order fitting and pseudo 2nd order fitting. Table 2 lists the values of qe(exp.), k1 for pseudo 1st order, k2 for pseudo 2nd order and the correlation coefficient (R2) of the two models. From the result, pseudo 1st order plot shows a straight line with correlation factor R2 = 0.987 proposing that pseudo 1st order model is applicable. However, R2 for pseudo 2nd order plot is 0.999 which is more than that of pseudo 1st order. The calculated adsorption capacity is identical with the experimental one for both models. So, it's clear that, the sorption reaction fit pseudo 2nd order kinetic model and the adsorption process can be regulated by the chemisorptions process.
Elovich Model
Figure 14c depicts Elovich model fitting. The calculated parameters for Elovich, (α, β) in Table 2 show that the sorption reaction mechanism can be interpreted by Elovich equation. The correlation factor (R2) is greater than 0.98. The data shows that Elovich model involves chemisorptions of the prepared composite toward rare earth ions ensure pseudo 2nd order model.
Intra-particle Diffusion Model
The relation between qt and t½ for the adsorption of rare earth ions belongs to the intra particle diffusion fitting, Fig. 14d the straight lines with intercept i.e. not crossing the origin point; this means that the film diffusion mechanism is dominated during this study.
The thickness of the surface extrapolated from the intercept describes the participation of the surface adsorption within the rate-determining step. The great value of the intercept, the greater is its participation. It’s obvious that the intra-particle diffusion kinetic model for the rare earth adsorption reaction occurred through 3 stages. The primary stage (0–15 min) is that the mass transfer of rare earth ions and this occurs instantaneously and this stage is not considered by the kinetic. The second stage from (20–60 min; is the film diffusion of the rare earth ions from the solution to the surface of the composite and this the rate determining step. The third one (from 60 to 100 min) is that the equilibrium saturation. Table 3 shows the coefficients calculated from the second part of the linear plot.
Isotherm Modeling
Langmuir Isotherm Modeling
The relation of Langmuir isotherm and its calculated parameters are shown in Fig. 15a and Table 4, respectively. The linear relation with correlation factor (R2 = 0.995) assumes the applicability of the sorption process to Langmuir model. The monolayer adsorption capacity at 25 ℃ and pH 4.5 is 182.15 ± 3.73mg g−1. Furthermore, the value of RL shows in Table 4, 0.295 is more than 0 and less than 1. This implies that, the sorption reaction of rare earth ions onto P (AA-co-AM/PJM-T) at 25 ℃ is favourable.
Freundlich Isotherm Modeling
Applying Freundlich Eq. (8). The linear plot in Fig. 15b indicates a probability for the sorption reaction fitting to Freundlich. The calculated parameters for Freundlich isotherm in Table 4 clarify that the value of 1/n is not up to unity; this means that, the sorption process is concentration dependent. The values of correlation coefficient R2, (0.91) are less than that of the Langmuir model (0.995); thus, Langmuir isotherm is more regulated for the sorption process.
Flory–Huggins Isotherm Modelling
Figure 15c represents the graphical representation of Flory–Huggins model and the obtained characteristic constants values of this model are presented in Table 4. ∆Go calculated using the values of the equilibrium constant KFH; and found to be −14.393 kJ/mol indicates a spontaneous nature of the sorption process.
Temkin Isotherm Modeling
AT and BT depicted from the intercept and the slope of the plot applying Temkin Eq. (13) in Fig. 15d. The data illustrated in Table 4, indicate that bT, is 8.305 kJmol−1 which confirm chemical adsorption. Value of correction factor (R2) is (0.9) which gives a probability for regulation with the model.
Desorption studies
Desorption process is an important process to low the cost and decrease the need to continual supply of the adsorbent. Thus, the more desorption percentage is the more efficient process and a remark or the good sorbent [55]. The desorption efficiency was studied (Fig. 16a). For HNO3, H2SO4, HCL, Na2CO3 and distilled H2O. The desorption efficiencies of sorbed rare earth ions is in the order H2SO4 > HNO3 > HCl >> > Na2CO3 > H2O, this may due to lanthanide contraction [56] and the affinity of the composite for RE3+ is effected by pH. The pHpzc of the P(AA-co-AM/PJM-T) composite is 3. This means the surface charge is positive at pH < 3 and surface affinity to RE3+ decrease due to repulsion [57]. The Ka value of the acids is in the order HCl > H2SO4 > HNO3 > H2O, Na2CO3 is a strong alkaline compound. Exception for the desorption efficiency using HCl due to common ion of Cl− and use of HCl in dissolving of the digested RE3+ hydroxide in caustic soda process. Considering the economy, H2SO4 is chosen for desorption process.
Desorption processes with H2SO4 (Fig. 16b) has been worked using various concentrations 0.25, 0.5, 0.75 and 1 M. Among these concentrations 0.75 M shows desorption efficiency percent 99.8% equal to 1 M concentration. As the concentration of [H+] increases, H+ compete RE3+ and ion exchange takes place. So, it's recommended to use 0.75 M H2SO4 as an eluent for P(AA-co-AM/PJM-T)/RE ion desorption.
Regeneration of the Adsorbent
The adsorbent synthesis or the sorption process cost can be minimized by effectively reusability of adsorbent materials. As a result, in real applications, the estimation of adsorbent stability and reusability is critical. For assessing the practical application of P(AA-co-AM/PJM-T) composite, we evaluated its reusability by recovering REEs from the prepared stock solution followed by REE release and sorbent regeneration. The desorption agent in this investigation was 0.75 M H2SO4 solution, and the desorption time was 1 h. The reusability performance (adsorption–desorption–adsorption) was depicted in Fig. 17. The sorption capacity of P(AA-co-AM/PJM-T) composite decreased after the first cycle from 36 mg g−1 to 20 mg g−1. After second cycle, the sorption capacity decreased from 20 mg g−1 to 8 mg g−1 then further decrease to 2.4 mg g−1 in the third cycle. The sorption capacity did not change in the subsequent cycles fourth and fifth cycle. It can be inferred that both ion exchange and the covalent bonding between metal ions and composite play a role in the adsorption process.
Real Application
Concentration of rare earth ions (RE3+) liquor produced from monazite digestion is measured before sorption process with the aid of Ion coupled plasma. The result illustrated in Table 5. The concentration of (RE3+) after sorption process is measured by shaking 20 ml of the liquor digested solution for 24 h at 25 ℃ with 0.1 g of P(AA-co-AM/PJM-T) and pH adjusted at 4.5. The sorption efficiency of RE3+ onto P(AA-co-AM/PJM-T) is shown in Table 5 and illustrated in Fig. 18. The results are confirmed by the energy dispersive X-ray spectra of loaded P(AA-co-AM/PJM-T) composite with REEs (Fig. 19a). Beside Carbon, nitrogen and oxygen bands, the main constituents of P(AA-co-AM/PJM-T), the spectrum shows La, Ce, Nd, Pr, Sm, Gd, Yb, and Er, Dy bands besides Si, Cl, Ca and Al consistent with the elemental formula of monazite [58].
The roughness of the surface of P(AA-co-AM/PJM-T) becomes rough at the end of the sorption processes as depicted in Fig. 19b. Furthermore, the mapping images of loaded P(AA-co-AM/PJM-T) composite with REEs indicate that REEs ions are adequately adsorbed at the surface of the composite and they are distributed uniformly. La, Ce, Nd, and Pr are taken as examples of REEs to confirm sorption process.
Comparison with Other Adsorbents
There are several adsorbents that are used for RE3+ sorption from different minerals [59,60,61,62,63,64,65]. Compared the maximum sorption capacity (qmax) of other RE3+ adsorbents with (qmax) of the P(AA-co-AM/PJM-T) adsorbent is listed in Table 6. It was observed that the adsorption capacity of P(AA-co-AM/PJM-T) adsorbent has reasonable adsorption capacities of 182.15 ± 3.73 mg g−1 for RE3+. Thus, P(AA-co-AM/PJM-T) showed high potential for sorption of RE3+ could be used for recovery of REE from its ores.
Conclusion
Poly (acrylic acid-co-acrylamide/16, 16-dimethylheptadecan-1-amine) [P (AA-co-AM/PJM-T)] was successfully co-polymerized using gamma irradiation with 60Co γ-rays at a dose of 25 KGy. The prepared composite characterized by several physic chemical methods and its applicability for adsorbing REEs elements from acidic aqueous solutions was investigated. The best adsorption parameters at 25 ℃ of REEs ions are; 0.1 g [P (AA-co-AM/PJM-T)] with 20 ml REE ions solution, contact time 60 min, pH = 4.5. Kinetic and isotherm models applied. The results indicated that, the sorption reaction is regulated by pseudo 2nd order model than pseudo 1st order model. The adsorption mechanism was checked for Langmuir, Freundlich, Flory–Huggins and Temkin isotherm models. Langmuir adsorption capacity of rare earth ions onto P(AA-co-AM/PJM-T) is 182.15 ± 3.73 mg g−1. P(AA-co-AM/PJM-T) applied on liquor solution of the digested monazite with total concentration of for rare earth ions 1608 mg L−1 and the sorption efficiencies (%) of the single ions range from 95.2% for Eu3+ and 77.7% for Lu3+. Thus, [P (AA-co-AM/PJM-T)] is high selective for REEs.
Data Availability
All the data used for this work are publicly available.
References
An F, Gao B, Huang X, Zhang Y, Li Y, Xu Y, Zhang Z, Gao J, Chen Z (2013) Selectively removal of Al (III) from Pr(III) and Nd(III) rare earth solution using surface imprinted polymer. React Funct Polym 73(1):60–65. https://doi.org/10.1016/j.reactfunctpolym.2012.08.022
Celik I, Kara D, Karadas C, Fisher A, Hill SJ (2015) A novel ligandless-dispersive liquid–liquid microextraction method for matrix elimination and the preconcentration of rare earth elements from natural waters. Talanta 134:476–481. https://doi.org/10.1016/j.talanta.2014.11.063
Unal YS, Eroglu AE, Shahwan T (2013) Removal of aqueous rare earth elements (REEs) using nano-iron based materials. J Ind Eng Chem 19:898–907. https://doi.org/10.1016/j.jiec.2012.11.005
Yon H, Kim C, Chung K, Kim S, Lee J, Kumar JR (2016) Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: waste recycling strategies for permanent magnet processing. Hydrometallurgy 165(1):27–43. https://doi.org/10.1016/j.hydromet.2016.01.028
Parhi PK, Park KH, Nam CW, Park JT (2015) Liquid–liquid extraction and separation of total rare earth (RE) metals from polymetallic manganese nodule leaching solution. J Rare Earths 33:207–213. https://doi.org/10.1016/S1002-0721(14)60404-X
Ashour RM, Abdel-Magied AF, Abdel-khalek AA, Helaly OS, Ali MM (2016) Preparation and characterization of magnetic iron oxide nanoparticles functionalized by l-cysteine: adsorption and desorption behavior for rare earth metal ions. J Environ Chem Eng 4:3114–3121. https://doi.org/10.1016/j.jece.2016.06.022
Ashour RM, El-sayed R, Abdel-Magied AF, Abdel-khalek AA, Ali MM, Forsberg K, Uheida A, Muhammed M, Dutta J (2017) Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: kinetic and thermodynamic studies. Chem Eng J 327:286–296. https://doi.org/10.1016/j.cej.2017.06.101
Gok C (2014) Neodymium and samarium recovery by magnetic nano-hydroxyapatite. J Radioanal Nucl Chem 301:641–651. https://doi.org/10.1007/s10967-014-3193-z
Liang P, Liu Y, Guo L (2005) Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim Acta B 60:125–129. https://doi.org/10.1016/j.sab.2004.11.010
Goodenough KM, Wall F, Merriman D (2018) The rare earth elements: demand, global resources, and challenges for resourcing future generations. Nat Resour Res 27:201
Lima AT, Ottosen L (2021) Recovering rare earth elements from contaminated soils: critical overview of current remediation technologies. Chemosphere 265:129163. https://doi.org/10.1016/j.chemosphere.2020.129163
Tianchi L, Ji C (2021) Extraction and separation of heavy rare earth elements (2021) a review. Sep Purif Technol 276:119263. https://doi.org/10.1016/j.seppur.2021.119263
Quijada-Maldonado E, Romero J (2021) Solvent extraction of rare-earth elements with ionic liquids: toward a selective and sustainable extraction of these valuable elements. Curr Opin Green Sustain Chem 27:2452–2236. https://doi.org/10.1016/j.cogsc.2020.100428
Ang KL, Li D, Nikoloski AN (2017) The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 1. Anionic and Cationic Resins Hydrometall 174:147–155
Elkhansa E, Afnan M, MhdAmmar H, Alaa H (2021) Recovery of rare earth elements from waste streams using membrane processes: an overview. Hydrometallurgy 204:105706. https://doi.org/10.1016/j.hydromet.2021.105706
Xu X, Jiang X, Jiao F, ChenX YuJ (2018) Tunable assembly of porous three-dimensional graphene oxide-corn zein composites with strong mechanical properties for adsorption of rare earth elements. J Taiwan Inst Chem Eng 85:106–114
Gupta NK, Gupta A, Ramteke P, Sahoo H, Sengupta A (2019) Biosorption-a green method for the preconcentration of rare earth elements (REEs) from waste solutions: a review. J Mol Liq 274:48–164. https://doi.org/10.1016/j.molliq.2018.10.134
Veliscek-Carolan J, Jolliffe K, Hanley T (2013) Selective sorption of actinides by Titania nanoparticles covalently functionalized with simple organic ligands. Appl Mater Interfaces 5(22):11984–11994. https://doi.org/10.1021/am403727x
Desouky OA, Daher AM, Abdel-Monem YK, Galhoum AA (2009) Liquid–liquid extraction of yttrium using primene-JMT from acidic sulfate solutions. Hydrometallurgy 96(4):313–317. https://doi.org/10.1016/j.hydromet.2008.11.009
Janczura M, Luliński P, Sobiech M (2021) Imprinting technology for effective sorbent fabrication: current state-of-art and future prospects. Materials 14(8):1850. https://doi.org/10.3390/ma14081850
García-Otero N, Teijeiro-Valiño C, Otero-Romaní J, Peña-Vázquez E, Moreda-Piñeiro A, Bermejo-Barrera P (2009) On-line ionic imprinted polymer selective solid-phase extraction of nickel and lead from seawater and their determination by inductively coupled plasma-optical emission spectrometry. Anal Bioanal Chem 395(4):1107–1115. https://doi.org/10.1007/s00216-009-3044-x
Li-pan JIA, Jiang-kiang H, Ze-long MA, Xu-heng LIU, Xing-yu C, Jiang-tao LI, Li-hua HE, Zhong-wei Z (2020) Research and development trends of hydrometallurgy: an overview based on hydrometallurgy literature from 1975 to 2019. Trans Nonferrous Metals Soc China 30(11):1003–6326. https://doi.org/10.1016/S1003-6326(20)65450-4
Han Z, Xiao-Kun O, Li-Ye Y (2021) Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J Mol Liq 324:115122. https://doi.org/10.1016/j.molliq.2020.115122
Xu X, Ouyang X, Yang LY (2020) Adsorption of Pb (II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114523
Zhang H, Omer AM, Hu Z, Yang LY, Ji C, Ouyang X (2019) Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int J Biol Macromol 135:490–500. https://doi.org/10.1016/j.ijbiomac.2019.05.185
Wang X, Wang A (2010) Adsorption characteristics of chitosan-gPoly (acrylic acid) hydrogel composite for Hg (II) ions from aqueous solution. Sep Sci Technol 45:2086–2094
Rivas BL, Peric IM, Munoz C, Alvear R (2011) Poly (Nhydroxymethyl acrylamide-co-acrylic acid) and poly (N-hydroxymethyl acrylamide –co acrylamidoglycolic acid): synthesis, characterization, and metal ion removal properties. Polym Bull 68:391–403. https://doi.org/10.1007/s00289-011-0551-7
Guclu G, Al E, Emik S, Iyim TB, Ozgumus S, Ozyurek M (2010) Removal of Cu2+ and Pb2+ ions from aqueous solutions by starch-graft-acrylic acid/montmorillonite superabsorbent nanocomposite hydrogels. Polym Bull 65:333–346. https://doi.org/10.1007/s00289-009-0217-x
Cavus S, Gurdag G, Sozgen K, Gurkaynaka MA (2009) The preparation and characterization of poly (acrylic acid-co-methacrylamide) gel and its use in the non-competitive heavy metal removal. Polym Adv Technol 20:165–172. https://doi.org/10.1002/pat.1248
Wei J, Xiao Y, Yang C, Cai Y, Luo W, Luo T, Li H, Yang Z (2021) Dense polyacrylic acid-immobilized polypropylene non-woven fabrics prepared via UV-induced photo graft technique for the recovery of rare earth ions from aqueous solution. J Polym Environ. https://doi.org/10.1007/s10924-021-02068-8
Attallah MF, Allan KF, Mahmoud MR (2016) Synthesis of poly (acrylic acid–maleic acid) SiO2/Al2O3 as novel composite material for cesium removal from acidic solutions. J Radioanal Nucl Chem 307(2):1231–1241
Vincent B, Julien M, Alain G, Xianyu D, Jean-Jacques R, Sophie M (2020) Acidic polymeric sorbents for the removal of metallic pollution in water: a review. React Funct Polym 152:104599. https://doi.org/10.1016/j.reactfunctpolym.2020.104599
Cheremisinoff P (1997) Handbook of engineering polymeric materials, 1st edn. CRC Press, Boca Raton. https://doi.org/10.1201/9781482292183
Heidari S, Esmaeilzadeh F, Mowla D, Ghasemi S (2018) Synthesis of an efficient copolymer of acrylamide and acrylic acid and determination of its swelling behavior. J Petrol Explor Prod Technol 8:1331–1340. https://doi.org/10.1007/s13202-017-0428-x
Labib SH, Shahr El-Din AM, Allan KF, Attallah MF (2020) Synthesis of highly defcient nano SrCoOx for the purifcation of lanthanides from monazite concentrate. J Radioanal Nucl Chem 323:1179–1188. https://doi.org/10.1007/s10967-020-07031-w
Hassan HS, Attia LamisA, Dakroury GA (2020) Exploration of the parameters affecting the radioactive europium removal from aqueous solutions by activated carbon-epoxy composite. Appl Radiat Isot 164:109278. https://doi.org/10.1016/j.apradiso.2020.109278
Lagergren S (1898) About the theory of so-called adsorption of soluble substance. Kungliga Svenska Vetenskaps-Akademiens Handlingar 24:1–39
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Cheung CW, Porter JF, Mckay G (2000) Sorption kinetics for the removal of copper and zinc from effluents using bone char. J Sep Purif Technol 19(1–2):55–64 https://doi.org/10.1016/S1383-5866(99)00073-8
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Weidner E, Ciesielczyk F (2019) Removal of hazardous oxyanions from the environment using metal-oxide-based. Materials 12:927
Foo KY, Hameed BH (2010) Review: insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133(1–3):304–308
Todica M, Razvan S, Pop C, Loredana O (2015) IR and Raman investigation of some poly(acrylic) acid gels in aqueous and neutralized state. Acta Physica Polonica A 128:128–135. https://doi.org/10.1693/APhysPolA.128.128
Shahid SA, Qidwai AA, Anwar F, Ullah I, Rashid U (2012) Improvement in the water retention characteristics of sandy loam soil using a newly synthesized poly(acrylamide-co-acrylic Acid)/AlZnFe2O4 superabsorbent hydrogel nanocomposite material. Molecules 17(8):9397–9412. https://doi.org/10.3390/molecules17089397
Lu Y, Wang Z, Ouyang X, Ji C, Liu Y, Huang F, Yang L (2019) Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.10.044
Chalal S, Haddadine N, Bouslah N, Benaboura A (2012) Preparation of Poly (acrylic acid)/silver nanocomposite by simultaneous polymerization-reduction approach for antimicrobial application. J Polym Res. https://doi.org/10.1007/s10965-012-0024-1
Moharram M, Mousa A (2007) Study of the interaction of poly (acrylic acid) and poly (acrylic acid-poly acrylamide) complex with bone powders and hydroxyapatite by using TGA and DSC. J Appl Polym Sci 105:3220–3227. https://doi.org/10.1002/app.26267
Xiong C, Liu X, Yao C (2008) Effect of pH on sorption for RE (III) and sorption behaviors of Sm (III) by D152 resin. J Rare Earths 26:851–856. https://doi.org/10.1016/S1002-0721(09)60020-X
Puigdomenech I (2013) Make equilibrium diagrams using sophisticated algorithms (MEDUSA). In: Inorganic chemistry. Royal Institute of Technology, Stockholm, Sweden. http://www.kemi.kth.se/medusa. https://sites.google.com/site/chemdiagr/
Hendy A, Khozamy E, Mahmoud G, Saad E, Serror S (2019) Implementation of carboxymethyl cellulose/acrylic acid/titanium dioxide nanocomposite hydrogel in remediation of Cd(II), Zn(II) and Pb(II) for water treatment application. Egypt J Chem 62(10):1785–1798. https://doi.org/10.21608/ejchem.2019.11622.1739
Akpomie KG, Dawodu FA, Adebowale KO (2015) Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alex Eng J 54:757–767. https://doi.org/10.1016/j.aej.2015.03.025ISSN1110-0168
Ouyang D, Zhuo Y, Hu L, Zeng Q, Hu Y, He Z (2019) Research on the adsorption behavior of heavy metal ions by porous material Prepared with silicate tailings. Minerals 9(5):291. https://doi.org/10.3390/min9050291
Ponou O, Wang LP, Dodbiba G, Okaya K, Fujita T, Mitsuhashi K, Atarashi T, Satoh G, Noda M (2014) Recovery of rare earth elements from aqueous solution obtained from Vietnamese clay minerals using dried and carbonized parachlorella. J Environ Chem Eng 2:1070–1081. https://doi.org/10.1016/j.jece.2014.04.002
Coppin F, Berger G, Bauer A, Castet S, Loubet M (2002) Sorption of lanthanides on smectite and kaolinite. Chem Geol 182:57–68. https://doi.org/10.1016/S0009-541(01)00283-2
Shan X, Lian J, Wen B (2002) Effect of organic acids on adsorption and desorption of rare earth elements. Chemosphere 47(7):701–710
Zglinicki K, Szamałek K, Wołkowicz S (2021) Critical minerals from post-processing tailing. A case study from Bangka Island Indonesia. Minerals 11(4):352. https://doi.org/10.3390/min11040352
Cao X, Wang Q, Wang S, Man R (2020) Preparation of a novel polystyrene-poly(hydroxamic acid) copolymer and its adsorption properties for rare earth metal ions. Polymers 12(9):1905. https://doi.org/10.3390/polym12091905
Gallardo K, Castillo R, Mancilla N, Remonsellez F (2020) Biosorption of rare-earth elements from aqueous solutions using walnut shell. Front Chem Eng. https://doi.org/10.3389/fceng.2020.00004
Negrea A, Gabor A, Davidescu CM, Ciopec M, Negrea P, Duteanu N, Barbulescu A (2018) Rare earth elements removal from water using natural polymers. Sci Rep. https://doi.org/10.1038/s41598-017-18623
Zhao L, Duan X, Azhar MR, Sun H, Fang X, Wang S (2020) Selective adsorption of rare earth ions from aqueous solution on metal-organic framework HKUST-1. Chem Eng J Adv. https://doi.org/10.1016/j.ceja.2020.100009
Kosheleva A, Atamaniuk I, Politaeva N, Kuchta K (2018) Adsorption of rare earth elements using bio-based sorbents. MATEC Web Conf 245:18001. https://doi.org/10.1051/matecconf/201824518001
Babua C, Binnemans K, Roosen J (2018) EDTA-functionalized activated carbon for the adsorption of rare earths from aqueous solutions. Ind Eng Chem Res 57(5):1487–1497. https://doi.org/10.1021/acs.iecr.7b04274
Dupont D, Brullot W, Bloemen M, Verbiest TM, Binnemans K (2014) Selective uptake of rare earths from aqueous solutions by EDTA functionalized magnetic and nonmagnetic nanoparticles. ACS Appl Mater Interfaces 6(7):4980–4988. https://doi.org/10.1021/am406027y
Li J, Gong A, Li F, Qiu L, Zhang W, Gao G, Liu Y, Li J (2018) Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2–DODGA nanoparticles for adsorption of 16 rare earth elements. RSC Adv 8(68):39149–39161. https://doi.org/10.1039/c8ra07762b
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation, data collection and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and not under consideration for publication elsewhere.
Consent to Participate
All of the authors consented to participate in the drafting of this manuscript.
Consent for Publication
All of the authors consent to publish this manuscript.
Research Involving Human Participants and/or Animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A.H., Dakroury, G.A., Hagag, M.S. et al. Sorption of Some Rare Earth Elements from Acidic Solution onto Poly(acrylic acid–co-acrylamide/16, 16-dimethylheptadecan-1-amine) Composite. J Polym Environ 30, 1170–1188 (2022). https://doi.org/10.1007/s10924-021-02271-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02271-7