Abstract
Biocompatible chitosan/PVP blends of different weight ratios were prepared by the solution casting method. The blends were characterized for morphology, structural, functional group, and thermal analysis. SEM images revealed the homogeneous mixing of both polymers at a certain weight ratio. XRD results show that the crystallinity of the blend decreases with an increase in PVP concentration. The blends were also characterized for the evaluation of physical properties like contact angle and water vapor transmission rate. A nanoindentation test was performed to characterize the mechanical strength, elastic modulus as well as hardness of these films. The water vapor transmission rate and hydrophilicity of the blend significantly decrease with an increase in the concentration of PVP. The blend with CH/PVP (75:25) showed the most satisfactory mechanical strength as compared to all tested ratios. Furthermore, these blends had good transparency and thermal stability that can be the alternative of petroleum-based synthetic products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the 1980’s an excessive use of petrochemical-based polymers, have made them a major source of pollutants after use due to their non biodegradability. So, researchers and govt. agencies show greater concern about the environmental problem caused by the use of non-biodegradable plastic material for various purposes [1,2,3]. In this regard, the use of natural resources is being explored to produce biodegradable material instead of non-biodegradable petrochemical-based counterparts [3]. After the use of biopolymers, it is desired to be degraded in a reasonable period without affecting the environment [4, 5]. Biopolymers such as starch [6], chitosan [7], cellulose [8] have been considered as an effective alternative of conventionally used polymers due to its abundant, renewable, environmentally friendly, and biodegradability.
Chitosan is a natural biopolymer, polycationic, deacetylated derivative of chitin, a major component of crustacean outer skeletons. It is a linear polysaccharide made up of repeating units of β-(1–4)-linked 2-acetamido-2-deoxy-D-glucopyranose units [9, 10]. Owing to its biodegradable, non-toxic, excellent film-forming ability, strong antimicrobial and antifungal activity, chitosan based material plays an important role in biotechnology, pharmaceutics, biomedicine, cosmetic, wastewater treatment, food science, and packaging, etc. [11,12,13]. Chitosan has been widely used to prepare active biodegradable film due to its good thermal and chemical resistant property and extend the shelf life of food and prevent contamination. Despite several advantages and unique properties chitosan films have some disadvantages associated like a poor barrier to water vapor, gas, poor mechanical, and thermal property limit its use for various applications [14, 15]. Therefore, to get the desired results polymer blending is the most efficient way to get the desired characteristics in the film. Various synthetic and a natural polymer such as poly N-vinyl pyrrolidone [16], polyethylene oxide [17], starch [18], cellulose [19], collagen [20], carboxymethyl cellulose [21], and cellulose acetate [22] are used to blend with chitosan to enhance the mechanical and barrier property of it.
Poly (vinylpyrrolidone) (PVP) is a synthetic polymer, known for its biocompatibility, non-toxicity, and hydrophilic properties, frequently used in controlled drug release, wound dressings, and tissue engineering [14, 23]. Also, PVP is a water-soluble polymer that has beneficial effects on absorbency, viscosity, solubilization, condensation, and is capable of forming blend with chitosan. PVP can form hydrogen bonding with chitosan by bond formation between the amino and hydroxyl group of the chitosan and carbonyl group of PVP [24, 25]. The blend of chitosan and PVP has been widely used in biotechnology and chemical technology. Aldana et al. reported the fabrication of chitosan/genipin/PVP film for the controlled release of drugs [26]. Smitha et al. reported the formation of PVP/chitosan blends as memberanes for methanol fuel cell applications. and study the methanol permeability [27]. However, in these research papers transparency, nanoindentation, and water vapor permeability of the film were not evaluated.
It is important to study the nano and micro scale mechanical properties of chitosan and chitosan/PVP films since they have been widely used for biomedical applications. At very small scales the standard methods do not help in determining the mechanical properties of the material. The nanoindentation technique is a very accurate technique utilized for the measurement of mechanical properties of materials at a small length scale with high precession in load and displacement. For soft materials, high loads are avoided to prevent deformation; the full range of force from micro newton to millinewton can be applied using nanoindentation. Hardness (H), elastic modulus (E), and other mechanical properties can be determined using this technique.
The current research work aims to blend chitosan and PVP, at the different ratios for succesfull production of film containing the properties of both the polymers as to achieve the desired results. These films were characterized regarding their morphology, structural, optical properties, thermal stability, mechanical performance, water vapor permeability, to anticipate their potential application for food packaging as well as biomedical film.
Experimental
Materials
Chitosan (low molecular weight), Polyvinylpyrrolidone (PVP) (K-30, Mw = 40 kDa), acetic acid of analytical grade were procured from fisher scientific. All chemicals were used without further purification. Double distilled water was used throughout the experiment.
Preparation of Chitosan/PVP Blend
The solution casting method was used to prepare the blend of different concentrations of chitosan and PVP. Figure 1a shows the scheme to prepare the chitosan/PVP blend and b shows the proposed reaction mechanism to prepare the chitosan/PVP blend. Different concentration of chitosan was dissolved in 1 N acetic acid solution for 5 h at room temperature with continuous stirring for complete mixing. Afterward, the various quantity of PVP was added with constant stirring for 24 h to get a homogeneous solution. The resulting solution was then centrifuged at 3000 rpm for 15 min to remove the air bubbles and to remove the undissolved particles. The solution was then cast onto a glass petri dish and dried at 50 °C in a hot air oven for 12 h. The dried films were peeled from the petri dishes and stored in polybags in a desiccator for future use. The chitosan/PVP blend of 100:0, 75:25, 50:50, 25:75 has been prepared.
Characterizations
Surface morphology of chitosan/PVP blended films was examined using scanning electron microscopy (Model: EVO18 Make: ZEISS). X-ray diffraction pattern of each of chitosan/PVP blend was measured using X-ray diffractometer (Model: Miniflex PDXL, Make:Rigaku) to evaluate crystallinity in the film. The functional group analysis of prepared blends was measured using an attenuated total reflectance fourier transform (ATR-FTIR) spectrometer (Model: Nicolet iS5N FT-NIR Spectrometer, Model: Thermo Scientific) in the wavelength range of 500 cm−1 to 4000 cm−1. The transmittance spectra of the chitosan/PVP blend were scanned from 200 to 800 nm wavelength using a UV–Vis spectrophotometer (Model: double beam spectrophotometer U-2900/2910, Make: HITACHI). The hydrophilicity of prepared blends was measured using a drop shape analyzer (Model: DSA25 Make: Kruss). The thermal stability of films was measured using the TGA instrument (TGA Q50/Q500, Make: TA instruments) in the temperature range of 30ºC to 600ºC under continuous nitrogen flow. The water vapor transmission rate of blends was measured by the gravimetric technique according to the ASTM E 96/E 96M-05 (water method) at the temperature of 32 ± 2 °C and relative humidity of 50 ± 2%. The nanoindentation experiment on the blends was performed using anton parr nano-indentation system at room temperature.
Result and Discussion
Scanning Electron Microscopy
To determine surface morphology, surface characteristics, SEM image of the CH/PVP blends were evaluated. Figure 2a shows the SEM image of pristine chitosan film. Figure 2b, c shows the smooth and even surface of blends (75:25, 50:50) indicating homogeneous blending between chitosan and polyvinylpyrrolidone. The smooth and even surface of the blend is might be due to the semicrystalline nature of the polymer. However, 2d shows phase separation might be due to the poor compatibility between chitosan and PVP at a certain weight ratio [14, 28].
X-Ray Diffraction
X-ray diffraction was performed to determine the crystalline structure of the chitosan/PVP blend (Fig. 3). XRD peak of pristine chitosan shows its characteristics peak at 2θ = 9.16°,11.30°,16.09°,18.13°,22.94° indicating semi-crystalline nature. Whilst with the increase in the concentration of PVP the peaks become broader. In particular, till chitosan/PVP (50/50 mass/mass) blend shows semicrystalline nature after that blend becomes more amorphous. The intensity of the peak also decreased with an increase in the concentration of PVP due to enhancing complexation between chitosan and PVP [29]. The crystallinity of blend with low PVP concentration can explain the enhanced mechanical properties exhibited by the blends, as well as the high glass transition temperature (Tg). It is a well-known fact that the degree of crystallinity has a positive effect on both of them.
Fourier Transform Infrared Spectroscopy
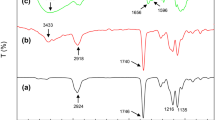
FTIR spectra of chitosan and its blend with PVP at different ration is shown in Fig. 4. Chitosan and PVP are strongly interacted with each other and form a homogeneous phase because of strong hydrogen bonding. In the chitosan spectra, the peak at 3424 cm−1 and 3267 cm−1 are attributed to the OH and NH2 group, respectively. The peak at 2921 cm−1 and 2874 cm−1 corresponds to the asymmetric and symmetric stretching of CH2 group vibration.1646 cm−1 and 1537 cm−1 corresponds to the C–O stretch of an acetyl group, N–H bending vibration of secondary amide.The presence of sharp peak at 1298 cm−1 in Chitosan/PVP (25:75, 50:50) spectra corresponds to the stretching vibration of C–N (because of pyrrolidone structure). The presence of peak at 1537 cm−1 and 1298 cm−1 in Chitosan/PVP blend confirmed the homogeneous blending of chitosan and PVP. The peak position at 3434 cm−1 in pristine chitosan is shifted to a lower frequency in the chitosan/PVP blend confirming the interaction of chitosan and PVP by means of intermolecular hydrogen bonding. Moreover, shifting of carbonyl bond from 1646 cm−1 to lower frequency confirming the interaction between chitosan and PVP [15, 24, 28].
UV–Vis Spectroscopy
UV–Vis spectra of all blends are shown in Fig. 5. Pristine chitosan film shows the optical transmittance of 60% whereas, with an increase in the concentration of PVP in chitosan/PVP blend transmittance increased up to 85% which indicated the homogeneous mixing of chitosan and PVP. The highly transparent film is suitable for transparent packaging as well as biomedical applications. Figure 6 provides camera images of CH/PVP blends. It can be seen from images that the blends have excellent transparency. The highly transparent film is suitable for various applications.
Contact Angle
Figure 7 shows the contact angle of pristine chitosan and blend of different concentrations of chitosan and PVP. It shows that the contact angle of pristine chitosan is 79.3° whilst with the increase in the concentration of PVP in the blend the contact angle decreases. The hydrophilicity of PVP is mainly due to hydrophilic amino and carboxyl groups and hence results in a decrease in the contact angle of the film when blended with chitosan [14, 30].
Water Vapor Transmission Rate
The water vapor transmission rate is the most important feature of the blend for its application in the packaging industry. It works on the principle of the diffusion process in which water vapor condenses and then liquid water diffuse through the film. Figure 8 shows the water vapor transmission rate of pristine chitosan and blend of different concentrations. Due to the hydrophilic nature of PVP water vapor transmission of blend with increasing concentration of PVP shows a significant increase in water vapor transmission. However, the blend with CH/PVP (75:25) shows lesser water vapor transmission compared to other compositions of chitosan, and PVP might be due to the strong intermolecular force of interactions between chitosan and PVP molecules results in the shorter intermolecular distance forming more compact films [24].
Thermogravimetric Analysis
The thermal stability of the pristine chitosan and CH/PVP blend of different compositions was studied by mean integral (TGA) and results are shown in Fig. 9. The results from the TGA shows the weight loss vs temperature for the prepared blend. Pristine chitosan and blend of CH/PVP shows an initial weight loss of 15% in the region of 50–180 °C due to the evaporation of absorbed water molecule.The initial onset temperature (Tonset) for degradation was observed at 223.57 °C with 18% weight loss and offset temperature(Toffset) was about 500 °C. Pristine chitosan shows degradation at 180–400 °C due to the depolymerization of the chitosan backbone through cleavage of glycosidic linkages and above 400 °C due to the decomposition of carbonaceous matter [15]. The blend of CH/PVP with different concentrations show degradation in the region of 180–390 °C due to the degradation of chitosan and 390–480 °C due to the decomposition of PVP. At 500 °C, the residue of the CH/PVP blend is less than pristine chitosan due to the fastest degradation of PVP. In CH/PVP (75:25) blend no residue is left at the temperature of 500 °C because of the fastest degradation of PVP [28].
Nanoindentation
Figure 10 shows the graphs for quasi-static nanoindentation studies on the CH/PVP blend of 100:0, 75:25, 50:50, 25:75 ratios (using a Berkovich tip).
Figure 10a shows Force -penetration depth (F-d) curves for CH/PVP Blends. The area for indentation testing was carefully chosen using an optical microscope and indentation was done in the load control mode. The effect of surface roughness was avoided by fixing the contact load as 30 μN. F-d curves show that pristine chitosan film has some amount of plastic deformation which increases with an increase in the concentration of PVP in the blend. However, the sample with 75:25 ration shows a lesser plastic area in comparison with the pristine chitosan sample also the holding capacity of the sample shows an increase with the increase in PVP concentration.
The elastic modulus (Er) and hardness (H) Fig. 10b, c for CH/PVP blend of 100:0, 75:25, 50:50, 25:75 ratios samples were calculated using parameters obtained from indentation force–displacement curves. The properties of samples were calculated using sneddon's elastic contact theory, the reduced modulus was calculated using the formula (1)
where S (S = dP/dh) is the unloading stiffness, which is calculated using the slope of the unloading curve of F-d data. β depends on the geometry of the indenter, which is called the correction factor. For our berkovich tip β = 1.034. For berkovich indenter projected contact area (Ac) depend on contact depth (hc) using the formula (2)
where h max is maximum displacement, and constant ε is taken as 0.75 for berkovich indenter.
The elastic modulus of the sample was calculated using reduced modulus Er in the formula
where E is elastic modulus, ν is poison ratio, and subscript s is for sample, and i is for indenter.
In our study, the elastic modulus of berkovich indenter Ei is 1141.00 GPa, and the poison ratio is 0.07. By using the contact area, the hardness of the sample can be obtained using an Eq. (5)
Pristine chitosan shows the hardness of 74.41 MPa. The blend with chitosan/PVP (75:25) shows an increased hardness of 112.5 MPa compared to pristine chitosan film. The increase in hardness is probably due to the strong interaction between chitosan and PVP through intermolecular hydrogen bonding. Whilst with an increase in the concentration of PVP into chitosan PVP blend hardness decreases. This could be due to the rigid and fragile nature of PVP and the added PVP destroyed the continuity of chitosan molecules, resulting in a decrease in the blended polymer strength [27, 28]
Conclusions
High-performance novel polymer blends were successfully prepared by solution casting of chitosan and polyvinylpyrrolidone (PVP) at different weight ratios. FT-IR analysis of blended polymers confirmed the compatibility and miscibility of both the polymers. The strong intermolecular hydrogen bonding interaction between amino and hydroxyl group of chitosan with the carbonyl group of PVP, results in the formation of a new biocompatible homogeneous blend. Contact angle measurement revealed that with the increase in the concentration of PVP in the blend the hydrophilicity of blends increased. TGA analysis shows that the blends are thermally stable. The water vapor transmission rate of blends increased with an increase in the concentration of PVP. Nanoindentation study also reveals that the mechanical strength shows a decrease in hardness and an increase in elastic modulus as the concentration of PVP increases, with an exception for the sample having a blend ratio of 75:25, wherein an increase in hardness but a decrease in elastic modulus is observed. An increase in holding time and plastic area is also observed with increasing concentration of PVP. Such results could be explained due to structural modification in the synthesized composites due to interpolymeric complexes with physical linkage with the participation of PVP. Moreover, the blends are highly transparent and its transparency increases with an increase in the concentration of PVP that can be utilized for transparent packaging as well as for the biomedical applications.
References
Sapper M, Talens P, Chiralt A (2019) Improving functional properties of cassava starch-based films by incorporating xanthan, gellan, or pullulan gums. Int J Polym Sci 2019:1–9. https://doi.org/10.1155/2019/5367164
Azeredo HMC, Mattoso LHC, Avena-Bustillos RJ et al (2010) Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J Food Sci 75:1–7. https://doi.org/10.1111/j.1750-3841.2009.01386.x
Merino D, Alvarez VA (2020) Green microcomposites from renewable resources: effect of seaweed (Undaria pinnatifida) as filler on corn starch-chitosan film properties. J Polym Environ 28:500–516. https://doi.org/10.1007/s10924-019-01622-9
Abdul Khalil HPS, Saurabh CK, Adnan AS et al (2016) A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr Polym 150:216–226. https://doi.org/10.1016/j.carbpol.2016.05.028
Mendes JF, Paschoalin RT, Carmona VB et al (2016) Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr Polym 137:452–458. https://doi.org/10.1016/j.carbpol.2015.10.093
Reddy N, Yang Y (2010) Citric acid cross-linking of starch films. Food Chem 118:702–711. https://doi.org/10.1016/j.foodchem.2009.05.050
Kumar R, Rai B, Kumar G (2019) A simple approach for the synthesis of cellulose nanofiber reinforced chitosan/PVP bio nanocomposite film for packaging. J Polym Environ 27:2963–2973. https://doi.org/10.1007/s10924-019-01588-8
Kumar R, Kumari S, Rai B et al (2020) A facile chemical approach to isolate cellulose nanofibers from jute fibers. J Polym Environ 28:2761–2770. https://doi.org/10.1007/s10924-020-01808-6
Priyadarshi R, Sauraj KB, Negi YS (2018) Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr Polym 195:329–338. https://doi.org/10.1016/j.carbpol.2018.04.089
Transistors OT, Du IdB, Hu S et al (2017) Eco-friendly and biodegradable biopolymer chitosan/Y2O3 composite materials in flexible organic thin-film transistors. Materials (Basel) 10:1026. https://doi.org/10.3390/ma10091026
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49:780–792. https://doi.org/10.1016/j.eurpolymj.2012.12.009
Miao J, Liu H, Li Y, Zhang X (2018) Biodegradable transparent substrate based on edible starch—chitosan embedded with nature-inspired three-dimensionally interconnected conductive nanocomposites for wearable green electronics. ACS Appl Mater Interfaces 10:23037–23047. https://doi.org/10.1021/acsami.8b04291
Karavas E, Georgarakis E, Bikiaris D (2006) Adjusting drug release by using miscible polymer blends as effective drug carries. J Therm Anal Calorim 84:125–133. https://doi.org/10.1007/s10973-005-7193-7
Hasan A, Waibhaw G, Tiwari S et al (2017) Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J Biomed Mater Res A 105:2391–2404. https://doi.org/10.1002/jbm.a.36097
Poonguzhali R, Khaleel Basha S, Sugantha Kumari V (2018) Novel asymmetric chitosan/PVP/nanocellulose wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 112:1300–1309. https://doi.org/10.1016/j.ijbiomac.2018.02.073
García MC, Aldana AA, Tártara LI et al (2017) Bioadhesive and biocompatible films as wound dressing materials based on a novel dendronized chitosan loaded with ciprofloxacin. Carbohydr Polym 175:75–86. https://doi.org/10.1016/j.carbpol.2017.07.053
Al-naamani L, Dobretsov S, Dutta J (2016) Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov Food Sci Emerg Technol 38:231–237. https://doi.org/10.1016/j.ifset.2016.10.010
El Miri N, Abdelouahdi K, Barakat A et al (2015) Bio-nanocomposite films reinforced with cellulose nanocrystals: rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films. Carbohydr Polym 129:156–167. https://doi.org/10.1016/j.carbpol.2015.04.051
Endes C, Camarero-Espinosa S, Mueller S et al (2016) A critical review of the current knowledge regarding the biological impact of nanocellulose. J Nanobiotechnol 14:78. https://doi.org/10.1186/s12951-016-0230-9
Moreno S, Baniasadi M, Mohammed S et al (2015) Biocompatible collagen films as substrates for flexible implantable electronics. Adv Electron Mater 1:1–8. https://doi.org/10.1002/aelm.201500154
Franco TS, Amezcua RMJ, Rodrìguez AV et al (2020) Carboxymethyl and nanofibrillated cellulose as additives on the preparation of chitosan biocomposites: their influence over films characteristics. J Polym Environ 28:676–688. https://doi.org/10.1007/s10924-019-01639-0
Nair SS, Zhu J, Deng Y, Ragauskas AJ (2014) High performance green barriers based on nanocellulose. Sustain Chem Process 2:23. https://doi.org/10.1186/s40508-014-0023-0
Lim JI, Kang MJ, Lee WK (2014) Lotus-leaf-like structured chitosan-polyvinyl pyrrolidone films as an anti-adhesion barrier. Appl Surf Sci 320:614–619. https://doi.org/10.1016/j.apsusc.2014.09.087
Li J, Zivanovic S, Davidson PM, Kit K (2010) Characterization and comparison of chitosan/PVP and chitosan/PEO blend films. Carbohydr Polym 79:786–791. https://doi.org/10.1016/j.carbpol.2009.09.028
Lewandowska K (2017) Surface properties of chitosan composites with poly(N-vinylpyrrolidone) and montmorillonite. Polym Sci A 59:215–222. https://doi.org/10.1134/S0965545X17020043
Aldana AA, González A, Strumia MC, Martinelli M (2012) Preparation and characterization of chitosan/genipin / poly (N-vinyl-2-pyrrolidone ) films for controlled release drugs. Mater Chem Phys 134:317–324. https://doi.org/10.1016/j.matchemphys.2012.02.071
Smitha B, Sridhar S, Khan AA (2006) Chitosan–poly(vinyl pyrrolidone) blends as membranes for direct methanol fuel cell applications. J Power Sources 159:846–854. https://doi.org/10.1016/j.jpowsour.2005.12.032
Yeh J-T, Chen C-L, Huang KS et al (2006) Synthesis, characterization, and application of PVP/chitosan blended polymers. J Appl Polym Sci 101:885–891. https://doi.org/10.1002/app.23517
Duan W, Chen C, Jiang L, Li GH (2008) Preparation and characterization of the graft copolymer of chitosan with poly[rosin-(2-acryloyloxy)ethyl ester]. Carbohydr Polym 73:582–586. https://doi.org/10.1016/j.carbpol.2007.12.025
Poonguzhali R, Basha SK, Kumari VS (2018) Nanostarch reinforced with chitosan/poly (vinyl pyrrolidone) blend for in vitro wound healing application. Polym - Plast Technol Eng 57:1400–1410. https://doi.org/10.1080/03602559.2017.1381255
Acknowledgements
The authors would like to thank Guru Gobind Singh Indraprasta University, India for providing all te neccesory facilities required for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Mishra, I. & Kumar, G. Synthesis and Evaluation of Mechanical Property of Chitosan/PVP Blend Through Nanoindentation-A Nanoscale Study. J Polym Environ 29, 3770–3778 (2021). https://doi.org/10.1007/s10924-021-02143-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02143-0