Abstract
In this study, the changes in the properties of polypropylene (PP) after loading of modified pro-oxidant were studied. Different proportions of modified-cobalt stearate (CoSt) pro-oxidant were filled in PP, and composites were prepared in a twin-screw extruder through compounding technique. Five films of these composites were prepared using hot press moulding. The modified pro-oxidant loaded PP films were characterized for chemical, physical, thermal, and morphological properties (before and after the biodegradation test). The biodegradability of the modified pro-oxidant loaded PP films was measured according to ASTM D 5338, and the biodegradation intermediate products were evaluated for their eco-toxicological effect. The changes (with respect to PP) in FTIR spectra of modified pro-oxidant loaded PP were noticed before and after accelerated ageing. The tensile properties and thermal stability of modified pro-oxidant loaded PP films decreased as confirmed by universal testing machine (UTM) and thermogravimetric analysis (TGA). Using differential scanning calorimetry (DSC) and XRD analysis, it was found that the percentage crystallinity of modified pro-oxidant loaded PP films has decreased and this led to increased degradability of PP. After the biodegradability test, SEM results of modified pro-oxidant loaded PP films revealed rougher morphology than before the biodegradability test. The highest biodegradability (28.87%) was obtained in PP100T30 film containing 30 phr of modified pro-oxidant. All the eco-toxicity tests of the degraded product materials demonstrated that the degraded products were nontoxic. Hence, the prepared composites can be effectively used as biodegradable flexible packaging materials.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics have become an essential aspect of our daily lives. Globally, the production of plastic crosses 150 MT per year [1]. Polyolefin like polypropylene (PP) is one of the most inexpensive synthetic polymeric material and broadly utilized for packaging applications [2]. It is widely used in packaging films due to its properties, like flexibility, lightweight, chemical resistance, low cost, hydrophobic nature, mechanical integrity, transparency, ease of processing, and durability [2,3,4]. The overall utilization of packaging applications is anticipated to rise from 27.4 million tons in 2017 to 33.5 million tons in 2022 [5]. But, PP is not eco-friendly because of its high molecular weight, increased shelf-life, hydrophobic nature, absence of functional groups, resistance to microbial attack, and lack of degradability. Therefore, their discarding and management at the end of their life span is a reason for significant worldwide anxiety. The deposition of PP in various land areas, sea, rivers leads to economical and environmental-related, as well as plastic solid waste disposal issues [6,7,8]. Current developments and research have been mostly focused on the development of degradable as well as compostable polymeric materials to oppose these problems impacting our atmosphere. Therefore, the new material formulations that can increase the PP degradability through various methods have been very demanding for environmental issues.
Biodegradable polymeric materials can be made using pro-oxidants. They can be transition metal ions and remarkably stearate. Cobalt (CoSt), copper (CuSt), manganese (MnSt), silver (AgSt), magnesium (MgSt), and calcium (CaSt) are used in the polymeric materials to develop degradable polymer. They accelerate the degradability of polymeric materials. After the addition of pro-oxidants, surface nature changes from hydrophobic to hydrophilic, reduces molecular weight, and then the breakdown of a long chain of carbon polymeric materials by the microbial attack [9]. Also, they assist to increase abiotic and biotic degradation [9]. Pro-oxidants like cobalt stearate (Co2+), magnesium stearate (Mn2+), as well as iron stearate (Fe3+) are mostly added in the polymers to start the thermoxidation plus photo-oxidation [4].
In the degradation, hydroperoxides are formed, that can photolyse/thermolyse and resulting chain scission as well as making of low molecular weight hydrocarbons and oxidized products (for example carboxylic acid, alcohols, plus ketones) [10], after that, they are degraded biotically using the microorganisms aerobically [11,12,13] and anaerobically [14]. In aerobic conditions, microbes form CO2, biomass, and water as products [8, 15]. In anaerobic conditions, in methanogenic conditions, microorganisms from CO2, water, biomass, and methane; otherwise, in sulfidogenic conditions products like H2S, carbon dioxide, plus H2O are produced [16, 17].
Currently, various research findings show the improvement of PP degradation through different methods [18,19,20,21]. In the earlier two decades, investigators have focused more on biopolymers and blending of PP with natural polymeric materials namely starch [22,23,24,25], cellulose [26], and poly(lactic acid) [17, 27], and utilizing isolated microbes [28]. In a patent filed in 2010, transition metal ions as pro-oxidant are utilized in polymeric material [29]. The innovation gives a technique of making a degradable polymer through preparing an intercalated double hydroxide. The intercalated double hydroxide is mixed with polymeric substrate and, alternatively, minimum one additional component selected from anti-oxidants as well as decomposition vulnerable polymeric materials to generate a combination which is compounded to make the degradable polymers. It is also stated that the metal complexes were activated via light/heat to increase decomposition. Contat–Rodrigo [14] showed the oxo-degradable PP films with various concentrations (0–10% w/w) of pro-oxidant. All the films were exposed to an accelerating photo-oxidation at 55 °C with radiation of 478 W/m2 for 300 h. The obtained results suggested an increased degree of oxidation of film samples with the addition of pro-oxidants in comparison to the neat PP and confirmed the effect of pro-oxidant. Finally, they concluded that the oxidation level is increased with pro-oxidant concentration. Bensaad, Belhaneche-Bensemra [30] revealed that the tensile properties of PP have reduced because of the pro-oxidant (calcium stearate) in PP.
Subramanian et al. [9] reported the influence of metal stearates (cobalt and iron) on PP with concentration range of 0.1–0.9% w/w. They found that the tensile strength decreased with increase in metal stearate concentration. Cobalt stearate increased the degradability of PP to a greater extent as compared to ferrous stearate. The degradation rate depended on the amount of pro-oxidant and its type. Fechine et al. [31] reported the influence of UV exposure on the oxo-degradation of PP having 0–3% w/w of pro-oxidant. The samples were subjected to UV radiation by the circulating of air at 50 °C and then evaluated various properties after 120, 288, 384, and 480 h. The pro-oxidant containing samples showed higher degradability than those without pro-oxidant, due to formation of carbonyl groups, and decreased molecular weight. In previous research, the authors concluded that cobalt stearate is better than calcium stearate for biodegradability of PP/PLA/nanoclay blends and composites [27]. In this study, we have used modified-CoSt pro-oxidant, as on addition of this pro-oxidant double peroxide groups are formed and also increase in the dispersion of pro-oxidant particles in the PP matrix. The details of preparation of modified pro-oxidant are given in Sect. 2.2.
Present work aimed to investigate the impact of filling modified pro-oxidant at different concentrations on mechanical and chemical properties of the films made from modified pro-oxidant loaded PP. Biodegradability of the prepared films were also determined. Furthermore, the influence of the biodegraded intermediate products on the environment has also been tested through various eco-toxicity tests.
Experimental Methodology
Materials
Commercial PP pellets having MFI = 11 g/10 min (Grade: 1030FG), melting temperature 165 °C, and a density of 0.9 g/cm3, were purchased from Indian Oil Corporation Ltd, India. Hydrochloric acid (35% conc.) and nutrient agar were procured from Hi-Media Laboratories Pvt. Ltd., India. Microcrystalline cellulose (MCE) of ≤ 20 µm particle size and barium hydroxide with molecular weight = 315.47 were obtained from S. D. Fine Chemicals Ltd. (Mumbai, India). The seeds of Mung bean and wheat for the eco-toxicity test were procured from a local market, Patiala, India, and earthworms were collected from a local farmer, Patiala, India.
The mature compost prepared by Green-Tech Fuel Processing Plant at Chandigarh; India was utilized as a microbial source. The other impurities present in the compost were removed through < 4 mm mesh sieve. The physical, as well as chemical properties of the compost were determined as per ASTM D5338. The bulk density and C/N ratio was 0.79 g/cm3, 18.96, respectively. The pH was 6.70. The compost included total organic carbon 31.11%, potash 0.51%, phosphate 0.70%, and total nitrogen 1.64%.
Preparation of Modified Pro-oxidant
Masterbatch containing 10 phr of cobalt stearate in PP (PP100CoSt10) was made in a co-rotating twin-screw extruder (M/s Labtech Engineering Co., Ltd. Thailand) at a speed of 150 rpm in a nitrogen blanket and pelletized. This masterbatch was aged at 110 °C for 48 h. After that, it was crushed in to powder, sieved, and used as a modified pro-oxidant (T).
Preparation of Modified Pro-oxidant Loaded Polypropylene Composites
The proportions of modified pro-oxidant in PP composites are presented in Table 1. Firstly, the constituents were combined manually with varying amounts, and then mixture was fed into an extruder. The compounds of modified pro-oxidant in PP were made in co-rotating twin-screw extruder (M/s Labtech Engineering Co., Ltd. Thailand) at 150 rpm speed in a nitrogen blanket. The temperature in the feed zone to die zone varied from170 to 230 °C and it was used for extrusion. After extrusion, the continuous compounds were cut into small pellets. After that, the prepared composites were dried in a vacuum oven at 70 °C for 2 h for the removal of moisture.
Preparation of Films
After processing of composites, the films of PP and modified pro-oxidant loaded PP were casted through hot press moulding for 2 min. The temperature of 185 °C and pressure of 400 kN/m2 were used. The pellets of material were sandwiched between two aluminum sheets to avoid the sticking of melt with plates. Tap water was utilized to cool the plates. The thickness of the films was 80–85 μm.
Analytical Methods
Mechanical Properties
The mechanical properties (tensile strength and elongation at break) of the prepared film samples were monitored by UTM (Z010, Zwick-Roell, Germany). The distance between the clamps and the cross-head speed were 100 mm and 12.5 mm/min, respectively and it was selected as per ASTM D 882-91. The specimens were made in the rectangular-shape through the strip-sample cutter for mechanical properties. Five specimens of every sample were tested for (tensile strength and elongation at break) and the average results of all the samples were recorded.
Fourier Transform Infrared (FTIR) Spectral Analysis
The structural and chemical changes that take place in the PP were investigated by FTIR spectroscopy (Agilent Pro Cary 660). The FTIR analysis was done at a resolution of 4 cm−1 in the spectra scan range of 500–4000 cm−1. The obtained spectra were analyzed through Spectrum 100 software.
Thermogravimetric Analysis
TGA/DTG measurements of the modified pro-oxidant containing PP samples were performed with a thermogravimetric analyzer (TA Instruments TGA Q-500 series, USA) to determine the thermal stability. The films (5–10 mg) were heated from 30 to 700 °C at a rate of 20 °C/min under a N2 condition at a constant flow rate of 50 mL/min and the thermal profiles of films were reported. The initial decomposition temperature (Ti) of the films was considered at a 5% weight loss in the polymeric material films. The final degradation temperature (Tf) of the films was corresponding to 5% residual left in the polymer films.
Differential Scanning Calorimetry
The thermal properties of PP and modified composite films were analyzed using a DSC instrument (Setaram DSC 131evo DSC, France). In every analysis, approximately 5–10 mg of films were used through 120 μL sealed aluminum pan. The films were heated in the temperature range of 30–200 °C in an N2 condition with a heating rate of 10 °C/min. Subsequently, samples were cooled from 200 to 30 °C to know the thermal behavior. The melting temperature (Tm), crystallization temperature (Tc), as well as melt enthalpy (\(\Delta H_{m}\)) of modified pro-oxidant containing PP film samples were determined from the area under the peaks. The enthalpy of melting for 100% crystalline PP is assumed to be 163 J/ g [17]. Through the DSC results, the percentage crystallinity of the modified film samples was calculated using Eq. (1) [32]:
where \(\Delta H_{100}\) denotes the melting enthalpy of PP (100% crystalline) and \(\Delta H_{m}\) denotes the melting enthalpy of modified PP composite film samples.
X-Ray Diffraction
Philips Xpert X-ray diffractometer (Almelo, Netherlands) was utilized to characterize the modified PP films. During analysis, automatic recording and processing of the experimental data were done in this equipment. The film samples were scanned from the diffraction angle range of 5° to 60° at the rate of 5°/min. The influence of modified pro-oxidant on the crystallographic structure of PP film was evaluated. The XRD was operated at 20 mA and 40 kV by monochromatic Cu-Kα radiation of 1.54 A° of wavelength (λ). The percentage crystallinity was measured from the ratio of the area of the crystalline zone (2θ = 12°–24°) to a total area of the XRD pattern (the sum of the area of the amorphous region plus the crystalline zone at 2θ = 12°–30°) according to Eq. (2) [33].
where \(A_{amorphous}\) and \(A_{crystalline}\) designate the area of the amorphous region and crystalline zone, respectively.
Scanning Electron Microscopy
Surface topography of modified pro-oxidant containing PP film samples before and after biodegradability studies were obtained by scanning with a JEOL scanning electron microscope instrument (JSM 6510-LV, Tokyo, Japan). Before SEM analysis, to avoid the charging, the films were put on aluminium stumps with coating of 15 nm gold through an electron beam in a high vacuum automatic sputter coater (model JFC-1600, Japan). The SEM was utilized to understand all the modifications on the surfaces of the films before and after the biodegradability test.
Biodegradability Test
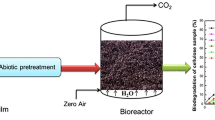
In this test, the biodegradability of pristine PP, CEL, and modified pro-oxidant loaded PP samples was determined through controlled aerobic composting as per ASTM D 53338, based on the measurement of CO2 emission by microbial respiration [34]. The films were subjected to inoculum derived from the matured compost of municipal solid waste (MSW) and are utilized for microbes as a nutrient source. All the modified films were incubated for 45 days in 1 L reactors at 58 ± 2 °C and the experiments were done in three replicates. Their average results were reported. During the experiment, humidity, temperature, and proper aerobic condition were monitored continually and closely maintained according to ASTM D 5338. Before beginning the process, the moisture was made to 50–55% in the bioreactor adding distilled water. At an interval of each 7 days, moisture content was measured and maintained constant by adding distilled water throughout the biodegradability test. The bioreactors were shaken sometimes to verify that zero air and moisture were distributed uniformly inside the bioreactors. The zero-air flow rate was 70–80 mL per minute to maintain the O2 level inside the bioreactor. During the test, sufficient porosity of inoculum was also maintained. The inoculum containing bioreactor was used as a blank control. The inoculum containing pristine PP was utilized as a negative control and inoculum plus MCE was utilized as a positive control. The theoretical mass of CO2 (CO2th) was estimated by Eq. (3)
where Wsample represents total dry solids in grams, in the films added to the bioreactors at the start of the test (g); Csample is the proportion of total organic carbon (TOC) in the films (g/g); 44 and 12 are the molecular weight of CO2 and atomic weight of carbon, respectively.
During biodegradability testing, the amount of CO2 produced was captured in 0.024 N barium hydroxide containing conical flasks and precipitated as BaCO3. The remaining Ba(OH)2 was titrated with 0.05 N HCl solution to the phenolphthalein endpoint. The cumulative carbon dioxide emission measurement was made through titration of acid–base at each two–three-day interval. The theoretical weight of CO2 (CO2th(g)) was measured from TOC analyzer [35]. TOC-VCPH (solid module) from Shimadzu, Japan was utilized with 900 °C heat. A non-dispersive infrared (NDIR) gas analyzer was used to identify carbon dioxide. The NDIR output, an analog detection signal, forms a peak; the peak area was determined through TOC-Control V software.
The percentage biodegradability of the sample was computed with the help of Eq. (4)
where CO2(t) is the cumulative amount of carbon dioxide emitted from each test film containing bioreactor (g) and CO2(b) is the mean cumulative amount of carbon dioxide emitted from the blank bioreactor (g).
Evaluation of the Eco-toxicological Effect of Biodegraded Intermediates
Three replicate bioreactors after 45 days were removed and the contents of each bioreactor were carefully combined. Before the biodegradability test, a polymeric material can be safe but may turn toxic after biodegradation. Therefore, the impact of biodegraded intermediate products on inoculum quality was measured through the following eco-toxicity tests.
Microbial Toxicity Test
After biodegradability studies, 1 g of degraded compost was taken and 10 mL disinfected deionized water was added as well as vortexed for 1 min. After that kept it untouched for 30 min. The supernatant of compost was serially diluted to a factor (10−3) with 100 µL of each diluted factors 10−1, 10−2, and 10−3 was utilized for inoculating the nutrient agar plates. All the inoculated plates were put in an incubator. The NSW-152 incubator model of Narang Scientific Works Pvt. Ltd., India was used and maintained at 37 °C for 24 h. The bacterial colony forming units (CFU) on each nutrient agar plate were counted to estimate the number of colonies per mL [36]. The CFU per mL of suspension was estimated by using Eq. (5)
Plant Growth Test
This test was completed as per the guidelines of organization of the economic co-operation and development (OECD 208) [37]. In this test, a mixture of biodegraded compost, perlite and soil was utilized in the ratio of 1:1:2. Two plants of Mung bean (Phaseolus aureus) and wheat (Tritleum aestivum) were utilized for plant growth tests. All the experiments were completed in triplicates for each test material and control. During the experiments, the conditions maintained are temperature 22 ± 10 °C, and humidity 70 ± 25%. The test was continued for 3 weeks in 8 h dark/ 16 h light cycle for the growth of plants. After 3 weeks, the grown emerged seedlings were counted. After drying at 75 °C, the dry weight of both the plants was calculated until the constant weight was achieved.
Earthworm Acute-Toxicity Test
In the earthworm test, the biodegraded compost product was used according to the guidelines of OECD 207 [38]. Three adult earthworms were taken and kept in every 250 mL pot. To enhance the moisture content up to 35%, the tap water was sprayed on the compost. For each test, the compost sample of 75 g on wet wt. basis was used. For every test compost material and control, tests were performed in triplicates. The pots were incubated in the control condition of 25 ± 2 °C, and continued illumination for 14 days. After two weeks, the worms were collected from each pot, and the mortality of worms was reported. The earthworms were pronounced dead when they failed react on gentle mechanical stimulus.
Accelerated Ageing
The pristine PP as well as modified pro-oxidant loaded PP samples were subjected to accelerated ageing as per the standard ASTM D 4329 to simulate the outdoor atmospheric conditions including UV (part of the sun light) exposure [39]. The accelerated ageing test was conducted in the controlled weather equipment (QV model of Q-lab Corporation, USA).
Results and Discussion
Characterization
Mechanical Properties
The mechanical properties (tensile strength as well as elongation at break) of the modified pro-oxidant containing PP and pristine PP film samples are shown in Fig. 1. Compared with film 1, which is only pristine PP, adding the modified pro-oxidant could decrease the tensile strength significantly from 35 to 14 MPa. However, elongation at break (%) was decreased significantly from 3.5 to 1.1%. When the content of modified pro-oxidant was enhanced from 5 to 30 phr, a considerable decrease in tensile strength and elongation at break was found from 28.4 to 14 MPa and 2.7 to 1%, respectively. The film of PP100T30 was observed to have the lowest value of tensile strength with elongation at break as compared to all other film samples as presented in Fig. 1. At 5 phr concentration, maximum tensile strength and elongation at break were 28.4 MPa and 2.7%, respectively. This drop in the values might have occurred due to modifications in the PP matrix on the loading of modified pro-oxidant to the PP matrix. The decrease in tensile characteristics could be due to the oxidation of PP by modified-CoSt pro-oxidant [40]. Mandal et al. [17] have also reported that the addition of cobalt stearate 0.2 phr in PP reduced elongation at break and tensile strength 3.6 to 3.4% and 39 to 35 MPa, respectively.
Fourier Transform Infrared (FTIR) Spectral Analysis
Figure 2 presents the FTIR spectra of the PP, PP100T5, PP100T10, PP100T20, and PP100T30 films before and after accelerated ageing. Excluding PP100T20 and PP100T30 films, almost similar spectra were found for all other samples in case of fresh samples (without giving any treatment). Peaks between 2949–2866 cm−1 are assigned to C-H stretching, peak at 1375 cm−1 shows C-H bending, and peak at 1454 cm−1 shows CH3 bending in the FTIR spectra of pristine PP. A peak observed at 1715 cm−1 is recognized to be due to modified pro-oxidant, that can be attributed as asymmetric vibration stretching of the carbonyl groups attached to the metal ion [40]. This confirmed that the modified pro-oxidant is present in the PP films. The carbonyl groups are present as a peak at 1800–1700 cm−1 [10, 41]. However, after ageing, the peak intensity at 1735 cm−1 of PP100T20 and PP100T30 films increased and which confirmed that due to UV exposure new carbonyl groups have been formed on the PP films. Increase in peak intensity and a band broadening has also been observed, which indicates the formation of some oxidized products on the increasing content of modified pro-oxidant. The carbonyl band can be assigned to C=O stretching vibrations inside aldehydes and/or esters (1734 cm−1), γ lactones (1781 cm−1), and carboxylic acid groups (1700 cm−1). Small modifications have been observed inside the carbonyl peak (C=O). The extent of modification depended on the amount of modified pro-oxidant added [30, 33, 42].
Thermogravimetric Analysis
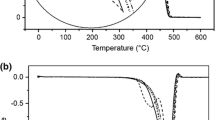
Figure 3a and b presents the thermogravimetric (TG) and differential thermogravimetric (DTG) trends for pristine PP and modified PP composite films. Table 2 summarizes the values of Ti and Tf. The values of Ti of the modified pro-oxidant containing PP film samples are lower in contrast to that of the pristine PP. It illustrates the progressive enhancement of thermal degradation in modified pro-oxidant filled PP samples because of the increased metal ion concentration [9, 43]. The thermal stability of different films PP, PP100T5, PP100T10, PP100T20, and PP100T30 is up to 375.50, 275.63, 268.47, 257.19, and 237.47 °C, respectively.
Differential Scanning Calorimetry
To monitor the influence of the modified pro-oxidant on the degradation of the pristine PP films, their percentage crystallinity was calculated from DSC analysis dada. During DSC analysis, cooling and heating cycles of modified pro-oxidant loaded PP film samples were found in the temperature range of 30–200 °C. The results are presented in Fig. 4. The values of melting enthalpy (ΔHm), melting temperature (Tm), and crystallization temperature (Tc), were estimated from the curves and noted in Table 3. The percentage crystallinity of all the films was determined from Eq. (1) and given in Table 3. The Tm values of the modified pro-oxidant loaded PP samples are lower than those of neat PP because of the presence of functional groups [17, 44]. The obtained results indicate that modified pro-oxidant enhanced the sensitivity to thermal degradation [45]. Also, the percentage crystallinity of the modified pro-oxidant loaded PP samples was found to be lower than that of the pure PP. The results clearly show that the film PP100T30 had the lowest percentage of crystallinity. This confirmed that the presence of modified pro-oxidant could decrease the crystallinity [9, 46]. Consequently, it made the modified pro-oxidant loaded PP samples more prone to microbial attacks [17, 28, 45] as the rate of degradability is influenced by the crystallinity of the films.
X-Ray Diffraction
In Fig. S1 of supplementary material, the XRD patterns of the pristine PP, with modified pro-oxidant containing PP samples are presented. The peaks of the pristine PP correspond to the α-monoclinic form as presented at 2θ = 14.1°, 16.8°, 18.6°, 21.1°, and 21.8° [17, 28, 47]. The addition of modified pro-oxidant in the PP reduced the peaks intensity indicating decreased crystallinity. Similar observations were also reported by Mandal et al. [17]. The presence of the modified pro-oxidant did not change the diffraction angles [35]. The crystallinity (%) of all the modified PP films was calculated using Eq. (2) and presented in Table S1 of Supplementary material. The crystallinity (%) of pristine PP is 76.6%. The value of the crystallinity of the other samples reduced from 76.6 to 40.50% due to the modified pro-oxidant.
Scanning Electron Microscopy
Before and after biodegradability studies, changes in the surface morphology of the PP, PP100T5, PP100T10, PP100T20, and PP100T30 composite films were investigated using a scanning electron microscopy and reported in Figs. S2 and S3 of Supplementary material. Fig. S2(a) of supplementary material displays the surface morphology of pristine PP before biodegradation. The surface of pristine PP was smooth and uniform. The surface morphology of modified pro-oxidant containing PP films before biodegradation is shown in Fig. S2(b-g) of Supplementary material. It was seen that loading of modified pro-oxidant did not significantly affect the surface morphology of pristine PP [17, 32, 45]. However, the surface roughness of modified pro-oxidant containing films was increased and it depended on pro-oxidant concentration, which was helpful for the growth of microbes [9].
The morphological changes were detected after 45 days of the composting process and depicted in Fig. S3(a) of Supplementary material. After 45 days, the surface morphology of the PP film did not change its morphology. It revealed that microbes did not consume the PP during composting processes. Fig. S3(b-g) of Supplementary material reveals the surface morphology of modified pro-oxidant containing PP films. After biodegradation (45 days), distinct changes were observed on modified pro-oxidant containing PP films, with roughness, disintegration, and cavitation on the surface, which is confirmed by the biodegradability results [25]. The surface morphology of the modified pro-oxidant containing PP films was altered, showing that it was consumed via the microorganisms during biodegradation. More pronounced peeling, exfoliation, as well as several small holes in the plate structure were detected in contrast to pristine PP. It revealed a greater degree of surface decay of modified pro-oxidant loaded PP films. This is due to modified pro-oxidant containing films becoming most fragile on degradation.
Biodegradability Test
To evaluate the biodegradability, the composite films were subjected to a respirometry test using bioreactors. Determining the amount of CO2 emitted through microbial respiration after utilization of the nutrient source of carbon and energy in the polymer films, makes it a possible to estimate % biodegradability. The percentage biodegradability of PP, PP100T5, PP100T10, PP100T20, and PP100T30 was estimated based on ASTM D 5388-15 standard protocol. Using Eq. (4), the biodegradability was estimated in terms of C–CO2 release. The microbes present in the inoculum should mineralize the degradable substrate and convert 90% carbon content of the individual films into CO2, H2O, and biomass [3, 19, 21]. Figure 5 shows the biodegradation of all the modified pro-oxidant containing PP film samples. The percentage biodegradation of all the films in enhancing order are: PP 0%, PP100T5 15.51%, PP100T10 18.88%, PP100T20 23.92%, PP100T30 28.87% and cellulose (reference) 77.22%. The biodegradability has been enhanced by modified pro-oxidant loaded PP composites because of the weak linkages, amorphous nature, chain cleavage, and different functional groups [28, 48]. The modified pro-oxidant made the PP prone to microbial attack due to its amorphous nature [9, 28, 45]. Microbes have consumed it as a nutrient source of energy and emitted CO2, biomass, and water [2, 17].
During controlled aerobic composting, the degradation of the polymer has two stages. The first stage is the abiotic hydrolysis of solid and the next stage is carbon mineralization [21, 25]. The biodegradation mechanism can be summarized. The PP reacted with O2 and produced free radicals. These produced radicals are then broken down into hydroperoxides which are further decomposed to more radicals by the metal salts through chain reaction mechanism [32, 49]. This way, the original polymeric material chain gets broken-down by microbial activity.
Eco-toxicity Tests
Microbial Toxicity Test
The numbers of colony-forming bacteria in the composted film material are presented in Table 4. It can be observed that the sample of blank gave CFU count of 0.3 × 104, and CEL gave the maximum CFU count (bacterial lawn). In all the modified pro-oxidant loaded PP films, the value of CFU count was more than the blank sample, which shows an enhanced bacterial growth in the composted samples. Therefore, the degraded intermediate products from the film samples are considered as non-toxic.
Plant Growth Toxicity Test
For the plant growth test, a middle range of pH was utilized to confirm the aptness of composted PP films. The suggested optimal range of pH is 5.5 [50] for the growth of plants. In the growth medium, a pH value very low or high will influence the solubility plus the availability of nutrients. As per suggestions, in this case, the pH was kept at 5.8–6.0, which is close to the optimal range of pH.
Mung bean and wheat plants are utilized to identify the toxic impacts of biodegraded intermediate products of modified pro-oxidant loaded PP samples [37]. After 3 weeks, the visual assessment of both the seedlings was done in the growth medium and it revealed that the average number of plant growth was 100%. In Figs. 6 and 7, no variations were seen in test sample films plus control in case of both the plants. Both plants were cut, dried, and weighed after 3 weeks of growth. The dry weight of the plants of the Mung was nearly similar within 2% in each composted sample. The same was the case for the plants of the wheat.
Earthworm Acute-Toxicity
In the post biodegradation compost, the value of mean percentage mortality of earthworms for the PP, CEL, blank (control), PP100T5, PP100T10, PP100T20, and PP100T30 was nil after the 14 days test. This revealed that the biodegradation products of different film samples were nontoxic.
Conclusions
In this study, the effect of modified pro-oxidant concentration on the degradability of PP was studied. For this aim, modified pro-oxidant was used as organic additives in PP films. The effects of modified pro-oxidant on the characteristics (physical, chemical, thermal, and morphological) of the PP films were studied. The biodegradability of these films was also evaluated. The mechanical properties (tensile strength and elongation at break) changed significantly, which can be still suitable for the packaging applications. The FTIR study confirmed the incorporation of modified pro-oxidant by a peak at 1715 cm−1 in PP. TGA results showed that the thermal stability of PP reduced with the loading of modified pro-oxidant. The XRD and DSC analysis revealed that modified pro-oxidant reduces the crystallinity of the PP and made it more susceptive to degradation.
SEM results revealed that modified pro-oxidant changed the morphology of the PP before and after the biodegradability test. After biodegradation, the surface of modified pro-oxidant containing PP films changed significantly and showing that it was consumed by the microbes, and more pronounced peeling, exfoliation, and several small holes in the films as compared to neat PP. Finally, we concluded that the degradation rate strongly depends on pro-oxidant concentration. The highest biodegradability (28.87%) could be obtained in case of PP100T30 film. All the eco-toxicity tests (plant growth, earthworm acute-toxicity, and microbial growth tests) confirmed that biodegradation intermediate materials were nontoxic. Hence, the prepared composites can be effectively applied for flexible packaging materials.
References
Singh N, Hui D, Singh R, Ahuja I, Feo L, Fraternali F (2017) Recycling of plastic solid waste: a state of art review and future applications. Compos B 115:409–422
Constantinou A, Antelava A, Bumajdad A, Manos G, Dewil R, Al-Salem S (2020) Identification of commercial oxo-biodegradable plastics: study of UV induced degradation in an effort to combat plastic waste accumulation. J Polym Environ 28:2364–2376
Kalita NK, Bhasney SM, Kalamdhad A, Katiyar V (2020) Biodegradable kinetics and behavior of bio-based polyblends under simulated aerobic composting conditions. J Environ Manag 261:110211
Hayoune F, Chelouche S, Trache D, Zitouni S, Grohens Y (2020) Thermal decomposition kinetics and lifetime prediction of a PP/PLA blend supplemented with iron stearate during artificial aging. Thermochim Acta 690:178700
Jain K, Bhunia H, Sudhakara Reddy M (2018) Degradation of polypropylene–poly-L-lactide blend by bacteria isolated from compost. Bioremed J 22(3–4):73–90
Kalita NK, Nagar MK, Mudenur C, Kalamdhad A, Katiyar V (2019) Biodegradation of modified Poly (lactic acid) based biocomposite films under thermophilic composting conditions. Polym Test 76:522–536
Pelegrini K, Maraschin TG, Brandalise RN, Piazza D (2019) Study of the degradation and recyclability of polyethylene and polypropylene present in the marine environment. J Appl Polym Sci 136:48215
Kalita NK, Bhasney SM, Mudenur C, Kalamdhad A, Katiyar V (2020) End-of-life evaluation and biodegradation of Poly (lactic acid)(PLA)/Polycaprolactone (PCL)/Microcrystalline cellulose (MCC) polyblends under composting conditions. Chemosphere 247:125875
Subramaniam M, Sharma S, Gupta A, Abdullah N (2018) Enhanced degradation properties of polypropylene integrated with iron and cobalt stearates and its synthetic application. J Appl Polym Sci 135(12):46028
Ammala A, Bateman S, Dean K, Petinakis E, Sangwan P, Wong S, Yuan Q, Yu L, Patrick C, Leong K (2011) An overview of degradable and biodegradable polyolefins. Prog Polym Sci 36(8):1015–1049
Fontanella S, Bonhomme S, Brusson JM, Pitteri S, Samuel G, Pichon G, Lacoste J, Fromageot D, Lemaire J, Delort AM (2013) Comparison of biodegradability of various polypropylene films containing pro-oxidant additives based on Mn, Mn/Fe or Co. Polym Degrad Stabil 98(4):875–884
Koutny M, Lemaire J, Delort A-M (2006) Biodegradation of polyethylene films with prooxidant additives. Chemosphere 64(8):1243–1252
Nguyen DM, Do TVV, Grillet A-C, Thuc HH, Thuc CNH (2016) Biodegradability of polymer film based on low density polyethylene and cassava starch. Int Biodeterior Biodegrad 115:257–265
Contat-Rodrigo L (2013) Thermal characterization of the oxo-degradation of polypropylene containing a pro-oxidant/pro-degradant additive. Polym Degrad Stabil 98(11):2117–2124
Al-Salem S, Sultan H, Karam H, Al-Dhafeeri A (2019) Determination of biodegradation rate of commercial oxo-biodegradable polyethylene film products using ASTM D 5988. J Polym Res 26(7):157
Arutchelvi J, Sudhakar M, Arkatkar A, Doble M, Bhaduri S, Uppara PV (2008) Biodegradation of polyethylene and polypropylene. Indian J Biotechnol 7:9–22
Mandal DK, Bhunia H, Bajpai PK, Chaudhari CV, Dubey KA, Varshney L (2018) Morphology, rheology and biodegradation of oxo-degradable polypropylene/polylactide blends. J Polym Eng 38(3):239–249
Acik G, Altinkok C, Tasdelen MA (2018) Synthesis and characterization of polypropylene-graft-poly (l-lactide) copolymers by CuAAC click chemistry. J Polym Sci A 56(22):2595–2601
Sugumaran V, Bhunia H, Narula AK (2018) Evaluation of biodegradability of potato peel powder based polyolefin biocomposites. J Polym Environ 26(5):2049–2060
Mandal DK, Bhunia H, Bajpai PK, Chaudhari C, Dubey K, Varshney L (2016) Radiation-induced grafting of acrylic acid onto polypropylene film and its biodegradability. Radiat Phys Chem 123:37–45
Sable S, Ahuja S, Bhunia H (2020) Biodegradation kinetic modeling of acrylic acid-grafted polypropylene during thermophilic phase of composting. Iran Polym J 29:735–747
Steller R, Meissner W (1998) Structure and properties of degradable polyolefin-starch blends. Polym Degrad Stabil 60(2–3):471–480
Ramis X, Cadenato A, Salla J, Morancho J, Valles A, Contat L, Ribes A (2004) Thermal degradation of polypropylene/starch-based materials with enhanced biodegradability. Polym Degrad Stabil 86(3):483–491
Morancho J, Ramis X, Fernández X, Cadenato A, Salla J, Vallés A, Contat L, Ribes A (2006) Calorimetric and thermogravimetric studies of UV-irradiated polypropylene/starch-based materials aged in soil. Polym Degrad Stabil 91(1):44–51
Leejarkpai T, Suwanmanee U, Rudeekit Y, Mungcharoen T (2011) Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag 31(6):1153–1161
Kaczmarek H, Ołdak D, Malanowski P, Chaberska H (2005) Effect of short wavelength UV-irradiation on ageing of polypropylene/cellulose compositions. Polym Degrad Stabil 88(2):189–198
Sable S, Mandal DK, Ahuja S, Bhunia H (2019) Biodegradation kinetic modeling of oxo-biodegradable polypropylene/polylactide/nanoclay blends and composites under controlled composting conditions. J Environ Manag 249:109186
Mandal DK, Bhunia H, Bajpai PK, Kumar A, Madhu G, Nando GB (2018) Biodegradation of pro-oxidant filled polypropylene films and evaluation of the ecotoxicological impact. J Polym Environ 26(3):1061–1071
Magagula B, Douglas (2010) A degradable polymeric material. Pat WO089691
Bensaad F, Belhaneche-Bensemra N (2018) Effects of calcium stearate as pro-oxidant agent on the natural aging of polypropylene. J Polym Eng 38(8):715–721
Fechine G, Rosa D, Rezende M, Demarquette N (2009) Effect of UV radiation and pro-oxidant on PP biodegradability. Polym Eng Sci 49(1):123–128
Montagna LS, Catto AL, de Camargo Forte MM, Chiellini E, Corti A, Morelli A, Santana RMC (2015) Comparative assessment of degradation in aqueous medium of polypropylene films doped with transition metal free (experimental) and transition metal containing (commercial) pro-oxidant/pro-degradant additives after exposure to controlled UV radiation. Polym Degrad Stabil 120:186–192
Moo-Tun NM, Valadez-González A, Uribe-Calderon JA (2018) Thermo-oxidative aging of low density polyethylene blown films in presence of cellulose nanocrystals and a pro-oxidant additive. Polym Bull 75(7):3149–3169
ASTM D (2015) 5338: Standard test method for determining aerobic biodegradation of plastic materials under controlled composting conditions. Incorporating Thermophilic Temperatures
Madhu G, Bhunia H, Bajpai PK, Nando GB (2016) Physico-mechanical properties and biodegradation of oxo-degradable HDPE/PLA blends. Polym Sci Ser A 58(1):57–75
Mandal DK, Bhunia H, Bajpai PK, Chaudhari CV, Dubey KA, Varshney L, Kumar A (2019) Preparation and characterization of polypropylene/polylactide blends and nanocomposites and their biodegradation study. J Thermoplast Compos Mater. https://doi.org/10.1177/0892705719850601)
OCED 208 (1984) Terrestial plant growth test: OECD guidelines for testing of chemicals
OECD 207 (1984) Earthworm, acute toxicity test: OECD guidelines for testing chemicals
ASTM D 4329 (2005): Standard practice for fluorescent UV exposure of plastics.
Islam NM, Othman N, Ahmad Z, Ismail H (2010) Effect of pro-degradant additives concentration on aging properties of polypropylene films. Polym Plast Technol Eng 49(3):272–278
Arkatkar A, Arutchelvi J, Bhaduri S, Uppara PV, Doble M (2009) Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int Biodeterior Biodegrad 63(1):106–111
Sable S, Ahuja S, Bhunia H (2020) Sudies on biodegradability of cobalt stearate filled polypropylene after abiotic treatment. J Polym Environ 28:2236–2252
Santhoskumar A, Palanivelu K (2012) A new additive formulation to enhance photo and biodegradation characteristics of polypropylene. Int J Polym Mater 61(10):793–808
Montagna LS, da Camargo Forte MM, Santana RMC (2013) Induced degradation of polypropylene with an organic pro-degradant additive. J Mater Sci Eng A 3(2A):123
Rosa D, Grillo D, Bardi M, Calil M, Guedes C, Ramires E, Frollini E (2009) Mechanical, thermal and morphological characterization of polypropylene/biodegradable polyester blends with additives. Polym Test 28(8):836–842
Pablos J, Abrusci C, Marín I, López-Marín J, Catalina F, Espí E, Corrales T (2010) Photodegradation of polyethylenes: comparative effect of Fe and Ca-stearates as pro-oxidant additives. Polym Degrad Stabil 95(10):2057–2064
Jain K, Madhu G, Bhunia H, Bajpai PK, Nando GB, Reddy MS (2015) Physico-mechanical characterization and biodegradability behavior of polypropylene/poly (L-lactide) polymer blends. J Polym Eng 35(5):407–415
Muthukumar T, Aravinthan A, Mukesh D (2010) Effect of environment on the degradation of starch and pro-oxidant blended polyolefins. Polym Degrad Stabil 95(10):1988–1993
Islam NZM, Othman N, Ahmad Z, Ismail Z (2011) Effect of pro-degradant additive on photo-oxidative aging of polypropylene film. Sains Malaysiana 40(7):803–808
Marschner H (1995) Functions of mineral nutrients: macronutrients mineral nutrition of higher plants, 2nd edn. Academic Press, Cambridge, pp 299–312
Acknowledgements
The authors are thankful to CSIR (Government of India) for financial support under project file No. 22(00745)/17/EMR-II. Special thanks to Dr. P. K. Bajpai, Retired Professor, TIET, Patiala, Punjab, India for valuable guidance. We are also expressing our gratitude to Dr. Vishal Goal, IOCL Faridabad, India for compounding facilities utilized in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sable, S., Ahuja, S. & Bhunia, H. Preparation and Characterization of Oxo-degradable Polypropylene Composites Containing a Modified Pro-oxidant. J Polym Environ 29, 721–733 (2021). https://doi.org/10.1007/s10924-020-01910-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01910-9