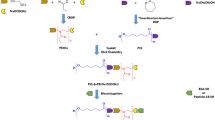
Abstract
To prepare amphiphilic block copolymers consisting of biocompatible and biodegradable segments, herein we report synthesis of diblock copolymers having ε-caprolactone (ε-CL) repeating units in one block and amino acid-based acrylate monomers in another segment. The block copolymers were prepared by a combination of metal-free ring-opening polymerization (ROP) of ε-CL and reversible addition-fragmentation chain transfer (RAFT) polymerization of tert-butyloxycarbonyl (Boc)-alanine/Boc-leucine based acrylate monomers. The ROP of ε-CL was initiated with diphenyl phosphate (DPP) as a metal-free catalyst in conjunction with a heterofunctional initiator, benzyl-2-hydroxyethyl carbonotrithioate, produced trithiocarbonate terminated poly(ε-caprolactone) (PCL). This was further employed as macro-chain transfer agent for the synthesis of side chain amino acid containing block via RAFT. Deprotection of Boc group pendants from the block copolymers under acidic conditions at room temperature provided pH responsive block copolymers with positively charged cationic primary amine functionalities. Furthermore, self-assembling nature of these block copolymers in aqueous medium was examined through dynamic light scattering (DLS) and field emission-scanning electron microscopy (FE-SEM).
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aliphatic polyesters, such as poly(ε-caprolactone) (PCL), poly(D,L-lactide), poly(trimethylene carbonate), polyglycolide, and their block copolymers possess excellent biodegradability and biocompatibility [1, 2]. Recently, these polyester polymers have drawn significant interest for their potential utilizations in various biomedical and pharmaceutical fields e.g., in drug delivery systems, as tissue engineering scaffolds, as surgical sutures and implants, and in temporary/permanent artificial organ devices, etc [3, 4]. Over the last few decades, a great deal of efforts has been made to develop functional biomaterials based on aliphatic polyesters with precisely controlled polymer architecture, composition, molecular weight, molecular weight distribution, and chain end functionality [5, 6]. Generally, polyesters are synthesized by ring-opening polymerization (ROP) of cyclic esters using the combination of organometallic catalysts or metal complex initiators such as stannous octoate (Sn(Oct)2) or stannous triflate (Sn(OTf)2) [7, 8]. Metal residues are very hard to remove completely from the synthesized polymers and remain in the polymeric materials as impurities and thus are not suitable for clinical use [9, 10]. Recently, a versatile metal-free strategy using organocatalysis has been developed for ROP of cyclic ester monomers to generate polyesters [11, 12]. ROP using organocatalysts has several advantages over metal catalysts [13, 14], such as shorter polymerization time, excellent molecular weight control, easy to remove the catalyst from the resultant polymer by simple washing or precipitation methods and ambient polymerization temperature that avoids deleterious side reactions like trans-esterification [15, 16]. Several organocatalysts e.g., N-heterocyclic carbene, phosphine, thioureas, guanidies, phosphazenes, HCl·Et2O complex, trifluoromethanesulfonic acid, methanesulfonic acid, and alcohol derivatives have been used for metal free ROP [17, 18]. In few recent reports, diphenyl phosphate was used as catalyst for the ROP of ε-CL, δ-valerolactone and other cyclic esters using aliphatic and aromatic primary hydroxyl groups as initiators [14, 19].
Amphiphilic block copolymers (PEG-b-P(DIPEMA-co-BzMA), poly(ε-caprolactone)21-b-poly(a-OEGMA)11, PEG-b-PCL(COOH), PEG-b-PCL(NH2), PEG-b-PCL(OH)) have been studied as exciting macromolecular analogues of natural amphiphiles for their potential applications in diverse fields including biomaterials, biomedicines, catalysis, photovoltaic materials, microelectronic, etc [20, 21]. Over the past few decades several controlled polymerization methods enabled facile design of block copolymers with targeted molecular weights, functionalities and morphologies [22]. Recently, a combination of two different polymerization techniques, e.g. ROP-atom transfer radical polymerization (ATRP) [23], cationic polymerization-ATRP [24] cationic polymerization-reversible addition-fragmentation chain transfer (RAFT) polymerization [25], etc. is being developed for the synthesis of block copolymers to afford polymers with interesting properties [26]. Importantly, combination of ROP and RAFT polymerization has been used for the synthesis of amphiphilic block copolymers having PCL and poly(N-vinylpyrrolidone) segments [27, 28]. de Freitas and coworkers reported synthesis of polystyrene-b-polycaprolactone block copolymers by simultaneous RAFT and ROP processes [29]. Synthesis of biodegradable PCL-b-poly(3-((tert-butoxycarbonyl)amino)propyl acrylate)-b-poly(polyethyleneglycolmethylether acrylate) triblock copolymers via combination of enzyme catalyzed ROP using benzyl-2-hydroxyethyl carbonotrithioate as initiator and RAFT method was reported [30].
Since past few decades, naturally occurring amino acids in the main/side chains of the polymer backbone have been utilized to develop amphiphilic block copolymers due to its excellent physical and chemical characteristics [31, 32]. Sun and Gao reported side chain amino acid containing cationic homopolymers which showed low cytotoxicity, and strong binding affinity with DNA [33]. Recently, De and coworkers reported controlled synthesis of side chain alanine, phenylalanine [34], leucine, isoleucine[35], tryptophan [36] containing pH-responsive cationic polymers with controlled molecular weights, narrow dispersity (Đ), and precise chain-end functionality via RAFT technique [37]. Because of the biodegradable and biocompatible nature are exhibited by PCL, and biocompatible with ionic nature of side-chain amino acid containing polymers, we have devoted our effort for the synthesis of well-defined diblock copolymers consisting of PCL and side chain amino acid containing block via combination of metal free organocatalyzed ROP and RAFT polymerization. In the present study, PCL-b-poly(Boc-L-alanine acryloyloxyethyl ester) (PCL-b-P(Boc-Ala-HEA) and PCL-b-poly(Boc-L-leucineacryloyloxyethyl ester) (PCL-b-P(Boc-Leu-HEA) block copolymers have been synthesized via combination of ROP and RAFT followed by deprotection of Boc groups to give pH responsive, cationic amphiphilic block copolymers. These amphiphilic block copolymers undergo aggregation in aqueous media to form multimicellar aggregates.
Experimental Section
Materials
Boc-L-alanine (Boc-L-Ala-OH, 99%), Boc-L-leucine (Boc-L-Leu-OH, 99%), and trifluoroacetic acid (TFA, 99.5%) were purchased from Sisco Research Laboratories Pvt. Ltd., India and used as received. ε-Caprolactone (ε-CL, 97%, Sigma) was distilled over calcium hydride (CaH2) under reduced pressure. Diphenyl phosphate (DPP, 99%), 4-dimethylaminopyridine (DMAP, 99%), dicyclohexylcarbodiimide (DCC, 99%), 2-hydroxyethylacrylate (HEA, 97%) were obtained from Sigma and used without any further purification. 2,2′-Azobisisobutyronitrile (AIBN, Sigma, 98%) was purified by recrystallization from methanol. A weak base anion exchange resin, Amberlyst A21 (Alfa Aesar) was used as received. CDCl3 (99.8% D) and D2O (99% D) were purchased from Cambridge Isotope Laboratories, Inc., USA. The benzyl-2-hydroxyethylcarbonotrithioate (BHT) was synthesized according to previously reported procedure (Fig. S1) [38]. Boc-protected amino acid based monomers Boc-L-alanine acryloyloxyethyl ester (Boc-Ala-HEA) (Fig. S2) and Boc-L-leucine acryloyloxyethyl ester (Boc-Leu-HEA) (Fig. S3) were synthesized as described elsewhere [34]. Toluene (> 99.5%) was purchased from Spectrochem. Pvt. Ltd. Inc., India and distilled over sodium and benzophenone under N2 atmosphere. The solvents such as hexanes (mixture of isomers), methanol (MeOH), acetone, ethyl acetate, dichloromethane (DCM) were purified by standard procedures.
Characterization Techniques
Number average molecular weight (Mn) and molecular weight distribution (dispersity, Ð) of polymers were obtained from Waters size exclusion chromatography (SEC) system in tetrahydrofuran (THF) with respect to poly(methylmethacrylate) (PMMA) standards at 30 °C at a flow rate of 1.0 mL/min. The system consisted of Waters Model 515 HPLC pump, Waters Model 2414 refractive index (RI) detector, one guard and two PolarGel-M columns from Agilent Technologies. The NMR spectroscopy was conducted on a Bruker AvanceIII 500 spectrometer at 25 °C. FT-IR spectrum was recorded on KBr pellets using a Perkin-Elmer Spectrum 100 FT-IR spectrometer. The UV–Visible (UV–Vis) spectroscopic measurements were carried out on a HITACHI U-4100 UV–Vis spectrophotometer with a scan rate of 240 nm/min. Thermal behaviors of synthesized polymers were studied by differential scanning calorimetry (DSC) at a heating rate of 10 °C/min under N2 atmosphere by using a Mettler Toledo DSC1 STRe instrument. During DSC measurement, polymers were first cooled from room temperature to −10 °C and hold for 10 min at that temperature, then heated to +150 °C and again cooled to −10 °C. The glass transition temperature (Tg) was taken from the third segment, i.e. from +150 to −10 °C to make sure complete removal of trace amount moistures and other volatile solvent impurities. Average hydrodynamic diameter (Dh) of polymer aggregates was determined using a dynamic light scattering (DLS) (Zetasizer Nano ZS, Malvern Instrument Ltd., UK) instrument equipped with a He–Ne laser beam at 658 nm at a scattering angle of 173°. Samples (1.0 mg/mL) were filtered through a 0.45 μm syringe filter prior to analysis and each measurement was repeated four to five times to obtain the average value. Field emission-scanning electron microscope (FE-SEM) images were recorded using Carl Ziess Sigma SEM instrument. The SEM samples were prepared by drop casting of polymer solutions in HPLC grade methanol and water on freshly cleaved clean silicon wafer, followed by drying under high vacuum at 40 °C.
Synthesis of Macro-Chain Transfer Agent (Macro-CTA) Using ROP
A typical procedure of ROP polymerization of ε-CL is as follows: ε-CL (0.485 mL, 4.38 mmol) was added to the solution of initiator BHT (35.6 mg, 0.146 mmol) in 2 mL of dry toluene inside the glove box. DPP (36.5 mg, 0.146 mmol) was then added to the reaction mixture to initiate the polymerization. Polymerization was carried at room temperature under N2 atmosphere. After certain time, the polymerization was quenched by the addition of Amberlyst A21. The resulting polymer was isolated as yellow solid by repeated precipitation (4-6 times) in excess volume of cold methanol/hexanes (9:1, v/v) mixture and finally after drying under high vacuum at 30 °C for 12 h (yield = ~85%).
Synthesis of Block Copolymers Using RAFT
Typically, Boc-Ala-HEA (50.1 mg, 0.174 mmol), PCL4.2k (see Table 1, 50.0 mg, 11.6 μmol), AIBN (0.38 mg, 2.32 μmol, from stock solution), 200 mg DMF and a magnetic spin bar were taken in a 20 mL septum sealed glass vial, purged with dry N2 for 15 min and placed in a preheated reaction block at 65 °C. The polymerization reaction was stopped after 4 h by cooling the vial in ice-water bath and exposing the reaction mixture to air. The reaction mixture was diluted with acetone followed by precipitation from large volume of hexanes. The resulting polymer was reprecipitated three times from acetone in hexanes and dried under high vacuum at 30 °C for 6 h to obtain a pale-yellow powder. Monomer conversions were determined by gravimetric method. The polymers were named as 1a, 1b and 1c for the block copolymers from the Boc-Ala-HEA monomer and 2a, 2b and 2c for the block copolymers from the Boc-Leu-HEA monomer (see Table 2).
Deprotection of Boc Groups from Block Copolymers
In a typical example, 1.0 mL TFA was added to the solution of 0.1 g block copolymer in 1.0 mL DCM in a 20 mL glass vial. The solution was stirred for 2 h, precipitated in diethyl ether and separated by centrifugation. The precipitate was washed with diethyl ether thrice and dried under high vacuum at 40 °C for 12 h. The letter “D” was added to name the deprotected polymers, for example 1a became D1a after Boc group deprotection.
Results and Discussion
ROP of ε-CL
A series of PCL homopolymers were synthesized by ROP of ε-CL using DPP as a weak acidic catalyst and BHT as the initiator in dry toluene at room temperature under N2 atmosphere (Scheme 1). The detailed conditions of polymerization reactions are summarized in Table 1. The chemical structure of the obtained polymers was characterized by 1H NMR (Fig. S4A). Characteristics resonance signals due to PCL chain appeared at 4.05 (–CH2–CH2–OH), 3.65 (–CH2–CH2–OH), 2.30 (–OCO–CH2–CH2–), 1.65 (–OCO–CH2–CH2–CH2–CH2–) and 1.38 ppm (–OCO–CH2–CH2–CH2–CH2–). Apart from these strong resonance signals coming from PCL chain, weak peaks due to terminal end groups were observed at 7.27–7.34 (–C6H5), 4.63 (C6H5–CH2–), 4.31 (–S(C=S)S–CH2–CH2–) and 3.65 ppm (–S(C=S)S–CH2–CH2–). Number average molecular weight (Mn,NMR) of PCL was estimated from 1H NMR chain end analysis by comparing the peak area of main chain protons at 2.30 ppm (–OCO–CH2–CH2–) with chain end protons at 4.63 ppm (C6H5–CH2–) from the initiator moiety. The Mn,NMR values are reported in Table 1. Presence of initiator moiety at the polymer chain end indicates that the polymerization was initiated by BHT and trithiocatbonate moiety is remained intact in the polymer chain end.
Number average molecular weights (Mn,SEC) and Ð values for the polymers were determined by SEC and results are listed in Table 1. Unimodal SEC RI traces (Fig. 1) and narrow Ð values are obtained for polymers synthesized at [ε-CL]0/[BHT]0 = 30 and 50, where no shoulder and tailing indicated polymerization reaction proceeded in a controlled manner even at high monomer conversions. However, at [ε-CL]0/[BHT]0 = 70 and higher values, RI traces showed shoulders at higher molecular weight side, indicating bimolecular terminations. Nevertheless, the Mn,SEC values of PCL polymers increased almost linearly with the increasing [ε-CL]0/[BHT]0 values and Mn,SEC values matched reasonably well with the theoretical molecular weights (Mn,Theo) obtained from monomer conversion (Table 1), indicating good control over the ROP of ε-CL. The Mn,NMR values are shown in Table 1, which are also in good agreement with the corresponding Mn,Theo values. Makiguchi and coworkers have already reported that the weak acid organocatalyzed ROP of cyclic esters in the presence of alcohol proceeds via the activated monomer mechanism to produce well-defined polyesters [39]. In our study, 1H NMR and SEC analysis confirmed that BHT acted as suitable initiator in presence of DPP catalyst for the ROP of ε-CL.
Synthesis of Block Copolymers via RAFT
Since the trithiocarbonate moiety of BHT is intact in the PCL, it was further employed as macro-CTA for the RAFT polymerization of Boc-Ala-HEA and Boc-Leu-HEA (Scheme 1). Since PCL with Mn,SEC = 4200 g/mol (Run 1, Table 1) showed unimodal curve in Fig. 1, PCL4.2k-b-P(Boc-Ala-HEA) and PCL4.2k-b-P(Boc-Leu-HEA) block copolymers were synthesized via RAFT polymerization in DMF using PCL4.2k as macro-CTA and AIBN as radical initiator at 65 °C. The details of block copolymer synthesis are summarized in Table 2, where we used a fixed ratio of [PCL4.2k macro-CTA]/[AIBN] = 5/1, but [Monomer (M)]/[PCL4.2k macro-CTA] ratios were varied from 15/1 to 50/1 with an aim to control molecular weight of block copolymers. Block copolymerizations were stopped at monomer conversion of 70-80% to get high end-group retention and avoid bimolecular termination reactions. Unimodal SEC RI traces with narrow Ð values (1.14–1.25) of the block copolymers without shoulder in the high molecular weight region was observed, indicating no bimolecular coupling reactions occurred even when higher [M]/[PCL4.2kmacro-CTA] ratios were used (Fig. 2). It is also observed that the RI traces shifted towards lower elution volume with increasing [M]/[PCL4.2k macro-CTA] ratios (Fig. 2), indicating linear increase in the molecular weights with the increase of [M]/[PCL4.2k macro-CTA] ratio. The Mn,SEC values for these block copolymers matched well with the corresponding Mn,Theo values calculated based on monomer conversion (Table 2). The observed difference can be due to the different hydrodynamic diameter of these block copolymers and polymer standards, which we used to calibrate the SEC system [40]. These observations revealed the controlled character of the RAFT polymerization of Boc-Ala/Leu-HEA in the presence of PCL4.2k macro-CTA.
Chemical structures of block copolymers were characterized by 1H NMR spectroscopy (Figs. S4B and S5B), where all the characteristic resonance signals corresponding to both the blocks were clearly observed. Peaks due to the P(Boc-Ala-HEA) block were observed at 4.42-4.16 (–O–CH2–CH2–O– and chiral proton), 1.43 (–O–C(CH3)3) and 1.50 (–CH3) ppm (Fig. S4B), and resonance signals for the P(Boc-Leu-HEA) segment appeared at 4.42–4.16 (–O–CH2–CH2–O– and chiral proton), 1.43 (–O–C(CH3)3) and 0.94 (–CH2(CH3)2) ppm (Fig. S5B). The integration ratios of protons at 4.42–4.16 ppm (–O–CH2–CH2–O– and chiral proton) from Boc-Ala/Leu-HEA units of P(Boc-Ala/Leu-HEA) segments to the chain end C6H5–CH2- protons at 4.61 ppm from the BHT chain end allowed us to determine the number average degrees of polymerization (DPn,Boc-Ala/Leu-HEA) for the amino acid block. Next, Mn,NMR values for these block copolymers were determined using the following equation: Mn,NMR = [(DPn,Boc-Ala/Leu-HEA × MWBoc-Ala/Leu-HEA) + molecular weight of PCL4.2k macro-CTA (4200 g/mol)], where MWBoc-Ala/Leu-HEA is the molecular weight of Boc-Ala/Leu-HEA monomer. The obtained Mn,NMR values are presented in Table 2, also matched nicely with the corresponding Mn,Theo values. Note that the calculated molecular weight of the block copolymers from 1H NMR assumes complete blocking. Nevertheless, PCL macro-CTA can be successfully employed for the synthesis of side-chain amino acid based block copolymers with controlled Mn, narrow Ð and intact chain ends.
Deprotection of Boc Groups
To prepare amphiphilic block copolymers, Boc groups from the pendant amino acid moieties in the PCL4.2k-b-P(Boc-Ala-HEA) and PCL4.2k-b-P(Boc-Leu-HEA) block copolymers were deprotected in the presence of TFA at room temperature to yield PCL-b-P(H3N+-Ala-HEA) and PCL-b-P(H3N+-Leu-HEA), respectively (Scheme 1). Although Boc protected block copolymers were insoluble in water, Boc deprotected polymers are nicely soluble in aqueous medium because of the high –NH3+ functionalities in the deprotected block copolymers. Quantitative expulsion of Boc groups from the block copolymers was confirmed by 1H NMR study, where the resonance signal due to Boc group at 1.43 ppm (Figs. S4B and S5B) disappeared in the Boc deprotected block copolymers (Figs. S4C and S5C). Additional evidence of the Boc deprotection was obtained from FT-IR analysis. Comparison of FT-IR spectra of diblock copolymers before and after Boc-group removal showed that the absorption band at 1518 cm-1 (Fig. S6A and S6B) due to the –N–H (2°) deformation coming from 1a and 2a are vanished and a small new band appeared at 1529 cm−1, respectively, in case of corresponding Boc deprotected polymers D1a and D2a for the primary ammonium groups (–NH3+) (Fig. S6C and S6D).
Thermal Behavior of Block Copolymers
DSC thermograms of PCL4.2k, 1a, 2a, and corresponding Boc deprotected block copolymers D1a and D2a are shown in Fig. 3. PCL4.2k showed melting temperature at 62 °C, which is close to the unmodified PCL segment [41]. The block copolymers 1a and 2a also showed melting peaks at 60 and 61 °C, respectively, with somewhat lower intensity of the peak compared to the PCL. Since both 1a and 2a contain amorphous amino acid segments, which might be affecting the ordered conformation of the PCL segment. In the case of deprotected block copolymers, Tm was not observed. Thus, expulsion of bulkier Boc groups made the block copolymers more flexible, which are unable to attain crystalline structure.
pH Responsiveness of Block Copolymers
Since primary amine groups (-NH2) of pendant amino acid units in the PCL-b-P(H3N+-Ala-HEA) and PCL-b-P(H3N+-Leu-HEA) can be protonated/deprotonated depending upon the solution pH, they are expected to show pH responsive phase transition behavior in aqueous medium. This hydrophilic-hydrophobic phase transition was monitored by using UV-Vis spectroscopy at room temperature by % transmittance (%T) measurement at 500 nm [42]. Block copolymers were dissolved in deionized water (2 mg/mL) and the initial pH of the solution was adjusted to 3.5 by adding 0.1 N HCl solution. Then, the pH of the polymer solution was gradually increased approximately by 0.5 pH units by adding 0.1 N NaOH. The %T of the polymer solutions was recorded at different pH values at 500 nm and reduction of 50 %T of the polymer solutions were considered as phase transition pH of the polymers in aqueous medium. The D1a and D2a showed phase transition pH at 9.3 and 6.2, respectively (Fig. 4). Note that the phase transition pH for the P(H3N+-Ala-HEA) and P(H3N+-Leu-HEA) homopolymers are reported respectively as 9.6 and 6.4 [32]. Slight reduction in phase transition pH values for D1a and D2a could be due to the presence of hydrophobic PCL block. Nevertheless, these hydrophilic-hydrophobic phase transitions were found to be reversible in nature depending upon the pH of the aqueous medium.
Self-Assembly Behavior of Block Copolymers
Depending on chemical compositions, molecular architectures and net content of hydrophobic/hydrophilic residues, amphiphilic block copolymers can self-assemble into various kinds of morphologies such as spherical micelles, vesicles, worm-like micelles, tadpole and other shapes in a solution as well as in solid state [43]. PCL block in D1a and D2a block copolymers is insoluble in water, whereas P(H3N+-Ala-HEA) and P(H3N+-Leu-HEA) segments are nicely soluble in water (Table S1). Therefore, we have investigated the self-assembly behavior of D1a and D2a block copolymers in aqueous medium.
First, the D1a and D2a solutions (1.0 mg/mL) in methanol and water were investigated by DLS at 25 °C. Since both the blocks of Boc deprotected block copolymers are soluble in methanol, the average hydrodynamic diameter (Dh) of D1a (Fig. 5a) and D2a (Fig. 5b) were found to be 4 and 2 nm, respectively. This observation indicated that D1a and D2a were in the form of unimer conformation in methanol. However, the D1a and D2a showed average Dh values of 92 ± 4 and 80 ± 3 nm respectively in aqueous medium, indicating formation of higher order structures. To examine the stability of self-assembled aggregated nanoparticles in water, time dependent DLS measurement was carried out (data not shown here). We did not observe a considerable change to their sizes even after 7 days, indicating the stability of aggregated particles in water.
Further supporting evidence of self-assembled aggregation of Boc deprotected block copolymers was obtained from 1H NMR studies. Since CDCl3 is a good solvent for both the PCL and P(H3N+-Ala/Leu-HEA) blocks, resonance signals from all the protons in the PCL and P(H3N+-Ala/Leu-HEA) blocks are clearly seen in the corresponding 1H NMR spectra (Figs. S4B and S5B). However, in D2O (Figs. S4C and S5C), the proton signals due to P(H3N+-Ala/Leu-HEA) segment are clearly visible but the peaks from PCL block are almost suppressed. PCL moiety has limited interaction with D2O solvent and probably situated at the core of the aggregates. Whereas, P(H3N+-Ala/Leu-HEA) segment is soluble in D2O and forms corona to stabilize the self-assembled structure formed in D2O.
The morphology of D1a and D2a block copolymers was further verified by FE-SEM imaging technique. Fig. 6 shows some short of aggregated structures with average diameters of 41 and 56 nm for D1a and D2a, respectively. The discrepancy between DLS and FE-SEM sizes of D1a and D2a samples is because DLS measurements were carried out in aqueous medium, where the particles are in a hydrated state, while the FE-SEM was performed in completely dry state. The nanostructures were formed in water with hydrophobic PCL block in the core and P(H3N+-Ala/Leu-HEA) segment in the corona. Interestingly, such aggregated structures are built by the aggregation of few unimolecular micelles and this phenomenon is well-known in self-assembly aspects, also familiar as multi-micellar aggregation [44]. The formation of such aggregated structure is schematically represented in Fig. 7.
Conclusions
In summary, we have successfully prepared well-defined amphiphilic block copolymers PCL-b-P(Boc-Ala-HEA) and PCL-b-P(Boc-Leu-HEA) via combination of metal-free ROP and RAFT technique. Size exclusion chromatography and spectroscopic results clearly depicted the formation of block copolymers in a controlled manner with targeted molecular weights and narrow molecular weight distributions. Deprotection of Boc groups from the side-chain of alanine and leucine moieties resulted pH responsive block copolymers with positively charged cationic primary amine functionalities. Although, the Boc protected block copolymers showed melting temperature at about 60 °C, it was vanished after the Boc group deprotections. The amphiphilic nature of the Boc-deprotected diblock copolymers enabled formation of self-assembled aggregates in water and showed multi-micellar aggregate. Thus, these block copolymers can be used for pH-responsive drug delivery, and high degree of primary amine functionality in the block copolymers will allow further conjugation of biomolecules for potential biomedical applications [45].
References
Basterretxea A, Jehanno C, Mecerreyes D, Sardon H ( 2019) Dual organocatalysts based on ionic mixtures of acids and bases: a step toward high temperature polymerizations. ACS Macro Lett 8:1055–1062
Cha Y, Jarrett-Wilkins C, Rahman MdA, Zhu T, Sha Y, Manners I, Tang C (2019) Crystallization-driven self-assembly of metallo-polyelectrolyte block copolymers with a polycaprolactone core-forming segment. ACS Macro Lett. 8:835–840
Clément B, Grignard B, Koole L, Jérôme C, Lecomte P (2012) Metal-free strategies for the synthesis of functional and well-defined polyphosphoesters. Macromolecules 45:4476–4486
Brannigan RP, Dove AP (2017) Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 5:9–21
Jehanno C, Mezzasalma L, Sardon H, Ruipérez F, Coulembier O, Taton D (2019) Benzoic acid as an efficient organocatalyst for the statistical ring-opening co-polymerization of ε-caprolactone and l-lactide: a computational investigation. Macromolecules 52:9238–9247
Ren JM, Fu Q, Blencowe A, Qiao GG (2012) Organic catalyst-mediated ring-opening polymerization for the highly efficient synthesis of polyester-based star polymers. ACS Macro Lett. 1:681–686
Bouyahyi M, Duchateau R (2014) Metal-based catalysts for controlled ring-opening polymerization of macrolactones: high molecular weight and well-Defined copolymer architectures. Macromolecules. 2:517–524
Zhu N, Zhang Z, Feng W, Zeng Y, Li Z, Fang Z, Zhang K, Li Z, Guo K (2015) Sn(OTf)2 catalyzed continuous flow ring-opening polymerization of ε-caprolactone. RSC Adv. 5:31554–31557
Wang Q, Li H, Wei Q, Sun JZ, Wang J, Zhang XA, Qin A, Tang BZ (2013) Metal-free click polymerizations of activated azide and alkynes. Polym. Chem 4:1396–1401
Brannigan RP, Walder A, Dove AP (2014) Block copolymer materials from the organocatalytic ring-opening polymerization of a pentaerythritol-derived cyclic carbonate. J Polym Sci Part A: Polym Chem 52:2279–2286
Higuchi M, Kanazawa A, Aoshima S (2020) Tandem unzipping and scrambling reactions for the synthesis of alternating copolymers by the cationic ring-opening copolymerization of a cyclic acetal and a cyclic ester. ACS Macro Lett. 9:77–83
Clement B, Grignard B, Koole L, Jerome C, Lecomte P (2012) Metal-free strategies for the synthesis of functional and well-defined polyphosphoesters. Macromolecules 45:4476–4486
Li X, Li H, Zhao Y, Tang X, Ma S, Gong B, Li M (2015) Facile synthesis of well-defined hydrophilic polyesters as degradable poly(ethylene glycol)-like biomaterials. Polym Chem 6:6452–6456
Danko M, Basko M, Ďurkáčová S, Duda A, Mosnáček J (2018) Functional polyesters with pendant double bonds prepared by coordination-insertion and cationic ring-opening copolymerizations of ε-caprolactone with renewable tulipalin A. Macromolecules 51:3582–3596
Ercole F, Rodda AE, Meagher L, Forsythe JS, Dove AP (2014) Surface grafted poly(ε-caprolactone) prepared using organocatalysed ring-opening polymerisation followed by SI-ATRP. Polym. Chem. 5:2809–2815
Zhao J, Pahovnik D, Gnanou Y, Hadjichristidis N (2014) Phosphazene-promoted metal-free ring-opening polymerization of ethylene oxide initiated by carboxylic acid. Macromolecules 47:1693–1698
Alamri H, Zhao J, Pahovnik D, Hadjichristidis N (2014) Phosphazene-catalyzed ring-opening polymerization of ε-caprolactone: influence of solvents and initiators. Polym. Chem. 5:5471–5478
Coady J, Horn HW, Jones GO, Sardon H, Engler AC, Waymouth RM, Rice JE, Yang YY, Hedrick JL (2013) Polymerizing base sensitive cyclic carbonates using acid catalysis. ACS Macro Lett. 2:306–312
Deng Y, Zou T, Tao X, Semetey V, Trepout S, Marco S, Ling J, Li MH (2015) Poly(ε-caprolactone)-block-polysarcosine by ring-opening polymerization of sarcosine N-thiocarboxyanhydride: synthesis and thermoresponsive self-assembly. Biomacromolecules 16:3265–3274
Jose L, Hwang AR, Lee C, Shim K, Song JK, Soo S, An A, Paika H (2020) Nitrilotriacetic acid-end-functionalized polycaprolactone as a template for polymer–protein nanocarriers. Polym Chem 11:1580–1588
Delplace V, Nicolas J (2015) Degradable vinyl polymers for biomedical applications. Nat. Chem. 7:771–784
Wang S, Kesava SV, Gomez ED, Robertson ML (2013) Sustainable thermoplastic elastomers derived from fatty acids. Macromolecules 46:7202–7212
Kryuchkov MA, Christophe Detrembleur C, Bazuin G (2014) Linear amphiphilicdiblock copolymers of lactide and 2-dimethylaminoethyl methacrylate using bifunctional-initiator and one-pot approaches. Polymer 55:2316–2324
Lenoir S, Pagnoulle C, Detrembleur C, Galleni M, Jerome R (2006) New antibacterial cationic surfactants prepared by atom transfer radical polymerization. J. Polym. Sci. Part A: Polym. Chem. 44:1214–1224
Bauri K, De P, Shah PN, Li R, Faust R (2013) Polyisobutylene-based helical block copolymers with pH-responsive cationic side-Chain amino acid moieties by tandem living polymerizations. Macromolecules 46:5861–5870
Schmid C, Weidner S, Falkenhagen J, Barner-Kowollik C (2012) In-depth LCCC-(GELC)-SEC characterization of ABA block copolymers generated by a mechanistic switch from RAFT to ROP. Macromolecules 45:87–99
Mishra AK, Patel VK, Vishwakarma NK, Biswas CS, Raula M, Misra A, Mandal TK, Ray B (2011) Synthesis of well-defined amphiphilicpoly(ε-caprolactone)-b-poly(N-vinylpyrrolidone) block Copolymers via the combination of ROP and xanthate-mediated RAFT polymerization. Macromolecules 44:2465–2473
Kang HU, Yu YC, Shin SJ, Kim J, Youk JH (2013) One-Pot synthesis of poly(N-vinylpyrrolidone)-b-poly(ε-caprolactone) block copolymers using a dual initiator for RAFT polymerization and ROP. Macromolecules 46:1291–1295
de Freitas AGO, Trindade SG, Muraro PIR, Schmidt V, Satti AJ, Villar MA, Ciolino AE, Giacomelli C (2013) Controlled One-pot synthesis of polystyrene-block-polycaprolactone copolymers by simultaneous RAFT and ROP. Macromol Chem Phys 214:2336–2344
Scarano W, de Souza P, Stenzel MH (2015) Dual-drug delivery of curcumin and platinum drugs in polymeric micelles enhances the synergistic effects: a double act for the treatment of multidrug-resistant cancer. Biomater Sci 3:163–174
Roy SG, De P (2014) pH responsive polymers with amino acids in the side chains and their potential applications. J Appl Polym Sci 131:41084
Bauri K, Roy SG, De P (2016) Sidechain aminoacidderived cationic chiral polymers by controlled radical polymerization. Macromol Chem Phys 217:365–379
Sun H, Gao C (2010) Facile Synthesis of multiamino vinyl poly(amino acid)s for promising bioapplications. Biomacromolecules 11:3609–3616
Kumar S, Acharya R, Chatterji U, De P (2013) Controlled synthesis of pH responsive cationic polymers containing side-chain peptide moieties via RAFT polymerization and their self-assembly. J Mater Chem B 1:946–957
Bauri K, Roy SG, Pant S, De P (2013) Controlled synthesis of amino acid-based pH-responsive chiral polymers and self-assembly of their block copolymers. Langmuir 29:2764–2774
Roy SG, Acharya R, Chatterji U, De P (2013) RAFT polymerization of methacrylates containing a tryptophan moiety: controlled synthesis of biocompatible fluorescent cationic chiral polymers with smart pH-responsiveness. Polym Chem 4:1141–1152
Bauri K, Nandi M, De P (2018) Amino acid-derived stimuli-responsive polymers and their applications. Polym Chem 9:1257–1287
Skey J, O’Reilly RK (2008) Facile one pot synthesis of a range of reversible addition–fragmentation chain transfer (RAFT) agents, Chem Commun 4183–4185
Makiguchi K, Satoh T, Kakuchi T (2011) Diphenylphosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules 44:1999–2005
Batiste C, Meyersohn MS, Watts A, Hillmyer MA (2020) Efficient polymerization of methyl-ε-caprolactone mixtures to access sustainable aliphatic polyesters. Macromolecules 53:1795–1808
Zhai S, Ma Y, Chen Y, Li D, Cao J, Liu Y, Cai M, Xie X, Chen Y, Luo X (2014) Synthesis of an amphiphilic block copolymer containing zwitterionicsulfobetaine as a novel pH-sensitive drug carrier. Polym Chem 5:1285–1297
Shimoboji T, Ding ZL, Stayton PS, Hoffman AS (2002) Photoswitching of ligand association with a photoresponsive polymer−protein conjugate. Bioconjug Chem 13:915–919
Haldar U, Nandi M, Ruidas B, De P (2015) Controlled synthesis of amino-acid based tadpole-shaped organic/inorganic hybrid polymers and their self-assembly in aqueous media. Euro Polym J 67:274–283
Saha A, Paira TK, Biswas M, Jana S, Banerjee S, Mandal TK (2015) Combined atom-transfer radical polymerization and ring-opening polymerization to design polymer–polypeptide copolymer conjugates toward self-aggregated hybrid micro/nanospheres for dye encapsulation. J Polym Sci Part A: Polym Chem 53:2313–2319
Li M, Song W, Tang Z, Lv S, Lin L, Sun H, Li Q, Yang Y, Hong H, Chen X (2013) Nanoscaledpoly(l-glutamic acid)/doxorubicin-amphiphilecomplex as pH-responsive drug delivery system for effective treatment of non small cell lung cancer. ACS Appl Mater Interfaces 5:1781–1792
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azmeera, V., Haldar, U., Roy, S.G. et al. Block Copolymers of Poly(ε-caprolactone) with pH-Responsive Side-Chain Amino Acid Moieties. J Polym Environ 29, 209–218 (2021). https://doi.org/10.1007/s10924-020-01872-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01872-y