Abstract
In this study, a novel biosorbent was prepared by the modification of sodium alginate with thiosalicylic acid in the presence of calcium ions (TS-A). TS-A was then characterized and its removal performance for Cu(II) and Cd(II) ions from the aqueous solutions was investigated. The effects of batch and column parameters on the biosorption were systematically investigated. The obtained data from the biosorption results were well fitted by the pseudo-second-order kinetic and Langmuir isotherm models. The maximum biosorption capacities of TS-A were found as 426.9 mg g−1 for Cu(II) ions and 592.9 mg g−1 for Cd(II) ions. In the column system, the saturation points of TS-A were obtained as 180 min and 150 min for Cu(II) and Cd(II) ions, respectively. In conclusion, TS-A has provided the removal of heavy metal ions with superior performance by a smart biosorption method, offering advantages such as rapid kinetic and high biosorption capacities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Discharging large amounts of lethal heavy metal ions including copper, cadmium, zinc, lead, mercury and chromium into the environment after industrial activities (automotive, metal plating, mining and nuclear activities etc.) are one of the main environmental problems that need to solve urgently [1]. Copper is an essential element for the human body but its acute doses cause metabolic disorders, such as hair loss, anemia, nausea, vomiting, diarrhea and jaundice [2]. The allowed limit value of Cu in drinking water by USEPA is 1.3 mg L−1 [3]. Cadmium is another highly toxic metal and it has been described as a carcinogen for the human body and a priority pollutant [4, 5]. Cadmium causes Itai-Itai, a disease in which patients exhibit a wide range of symptoms, including low-grade bone mineralization, high fracture rates, increased osteoporosis, and intense bone pain [5,6,7]. The value of the concentration limit for Cd(II) ions which is reported by the World Health Organization in drinking water is 3 μg L−1 [8]. The effective removal of these heavy metal ions from water is important for the survival of living beings. Biosorption, which is a powerful and economical method, is applied to remove pollutants by using biologically based solid supports with high biosorption capacity [9,10,11]. Therefore, it is an important objective for researchers to investigate new biosorbents with the above-mentioned properties [12,13,14]. Natural biopolymers e.g. alginate, cellulose, chitin, chitosan and agar obtained from plants, animals, and microorganisms offer a wide range of resource have not yet completely discovered for biomaterials [15,16,17]. Biopolymers are easy to use, practical and non-toxic, and have high ion-exchange capacities [17, 18]. Thus, the use of biopolymers in biosorption makes the method advantageous and superior [19].
Alginate which is an anionic polysaccharide (biopolymer) containing β-D-mannuronic and α-L-guluronic acids finds in many algae species, particularly the brown algae [20]. Alginate includes rich carboxyl functional groups that play active roles in the biosorption of heavy metal ions [21, 22]. In the presence of divalent cations, like e.g. calcium ion, alginate gels turn into a structure of high stable cross-linked chains described as the “egg-box” model [23, 24]. In this model, a pair of alginate chains can cross-link to calcium ions from guluronic blocks and also these pairs can dimerize with other pairs [25]. In the Ca-alginate gel matrix, there are free mannuronic groups that play an important role in the biosorption process. These rich functional active groups of binding sites in the alginate matrix can interact with metal ions and the easy replacement of calcium ions by other metal ions also occurs [26,27,28]. Alginate is a highly promising material for the biosorption of heavy metal ions because of its low cost and high affinity toward heavy metal ions [22].
In this study, thiosalicylic acid was used as a modification agent because of its donor atoms (O and S) which have a high complexing ability for metal ions. Sodium alginate (Na-A) solution was mixed with various concentrations of thiosalicylic acid (TS) solutions, and the modified alginate (TS-A) was subsequently obtained by gelling reaction with CaCl2. The obtained TS-A was characterized by using FT-IR, SEM, elemental and thermal analysis, and dynamic light scattering measurements. In the biosorption studies, several experimental parameters such as pH, biosorbent dosage, temperature, equilibrium time, initial metal ion concentrations, etc. in the batch system and, the flow rate and biosorbent dosage in the column system were optimized to determine the highest biosorption yields. The obtained kinetic and equilibrium data were evaluated and their suitability for various kinetic and isotherm models was investigated thus revealing the nature of the biosorption process and the amounts of the maximum biosorption for Cu(II) and Cd(II) ions by TS-A. The performance of TS-A for the biosorption of the metal ions in the synthetic wastewater was also examined in the single and binary systems.
Experimental
Preparation of Modified Biosorbents and Solutions
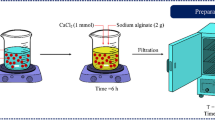
In this study, calcium alginate (Ca-A) was chosen as a biosorbent for the removal of metal ions from aqueous solutions. The preparation route of Ca-A is the mixing of 2% (w/v) CaCl2 (Merck) and 1% (w/v) sodium alginate (Na-A) (Sigma-Aldrich) solutions. To improve the biosorption capacity, the modified alginate was formed that CaCl2 solution was dripped into Na-A solution which contains thiosalicylic acid (TS) at the concentration range of 0.025–0.200 M. During the modification process, this mixture was kept in an ultrasonic bath. After that, the modified biosorbents were rinsed with deionized water for the removal of excess CaCl2 and TS, and they were dried at 80 °C for 24 h. The modified biosorbents were ground and sieved through a mesh size of 150 µm to obtain homogenous particle sizes and dried again at 80 °C for 24 h. Then, the biosorbents were kept in the glass bottles for further use. The schematic illustration of the preparation of TS-A was given in Fig. 1. The modified biosorbents were prepared with various TS solutions. Due to the high biosorption capacities resulting from the modification process, 0.10 and 0.15 M TS solutions were selected to prepare TS-A which was used for the biosorption of Cu(II) and Cd(II) ions, respectively.
1000 mg L−1 stock solutions of Cu(II) and Cd(II) ions were prepared from Cu(SO4)·5H2O (Sigma-Aldrich) and Cd(NO3)2·4H2O (Sigma-Aldrich), respectively. The diluted Cu(II) or Cd(II) ion solutions used in all experiments were prepared by these stock solutions.
Biosorbent Characterization
The prepared biosorbent was characterized by Scanning Electron Microscopy (SEM) to observe the surface morphologies (ZEISS Ultra Plus). SEM–EDX (Energy Dispersive X-Ray) analysis were also performed to identify biosorbed metal ions onto the modified alginate. An elemental analyzer (Vario EL III) was used to determine the chemical compositions of the biosorbents. FT-IR spectrophotometer (Perkin Elmer Spectrum 100) was performed to identify the surface functional groups of the biosorbents in the range of 400–4000 cm−1. Thermal behaviors of the biosorbent were examined by TG analysis by a thermal analyzer (Setaram Labsys TGDTA Model). Zeta potential measurements of the biosorbents were performed by using a Zetameter (ZEN 3600 Model Zetasizer Nano-ZS) in order to determine the surface charges.
Biosorption
Batch Studies
The biosorption experiments were applied in a batch system. The effects of the parameters such as pH, biosorbent dosage, temperature, contact time and initial metal concentration on the biosorption capability were investigated. The effect of pH was examined for Cu(II) and Cd(II) ions at the range of 1.0–6.0 and 1.0–8.0, respectively. After adding 0.5 g dm−3 TS-A into 100 mg dm−3 metal ion solutions, the medium pH was adjusted with HCl/NaOH or acetate buffer solution and measured by using a pH meter (Radiometer Analytical MeterLab pHM 220). The mixtures were then stirred for 60 min on the multiple magnetic stirrers, this operation followed by a separation process of the liquid phase from a solid phase via a simple filtration. The heavy metal ions remaining in the solutions were analyzed by Flame Atomic Absorption Spectrophotometer (AAS; PerkinElmer Model AAnalyst 800) with an air-acetylene flame at λmax = 324.8 nm for Cu and λmax = 228.8 nm for Cd, respectively. The Cu(II) and Cd(II) standard solutions were prepared from the stock solution containing 5% HNO3. The different amounts of TS-A for the biosorption of Cu(II) and Cd(II) ions at the concentration of 100 mg dm−3 were also studied at the range of 0.2–0.8 and 0.1–0.8 g dm−3, respectively. The effect of temperature on the biosorption was investigated in the range from 10 to 45 °C at the same metal ion concentration. To evaluate the biosorption kinetics, the effect of the contact time was examined at the concentrations of 150, 175 and 200 mg dm−3. In order to obtain the equilibrium data for the biosorption processes, the metal ion concentrations were chosen from 80 to 240 mg dm−3 for Cu(II) ions and 170 to 350 mg dm−3 for Cd(II) ions, respectively. In addition, the synthetic wastewater was prepared and the biosorption yield of TS-A was investigated in this media. The prepared synthetic wastewater consists of 100 mg dm−3 Cu(II) or Cd(II) ions and a mixture of these metal ions with the various components (glucose, 0.125 g; KH2PO4, 0.008 g; MgSO4·7H2O, 0.005 g; CaCl2·2H2O, 0.025 g; Na2SO4, 0.025 g; Na2CO3 0.05 g; NiCl2·6H2O, 0.005 g; CuSO4·5H2O, 0.002 g; CoCl2·6H2O, 0.001 g and ZnCl2, 0.008 g in 250 mL of deionized water) [29]. 0.5 g dm−3 of TS-A was added into the synthetic wastewater and then the pHs of the wastewaters including Cu(II) or Cd(II) and both Cu(II) and Cd(II) ions were settled at 5.5, 7.5 and 5.5, respectively. After the biosorption experiments, the solid–liquid phases were separated and, Cu(II) and Cd(II) ions remaining in the filtrates were measured by using an AAS.
Column Studies
The biosorption experiments in a column system were performed by using a 9 mm internal diameter glass column which was connected to a peristaltic pump (Ismatec ecoline) through the tubing. The column was packed by embedding TS-A between two layers of glass wool. For each experimental design, the solution containing 100 mg dm−3 metal ions of interest (Cu(II) or Cd(II)) was pumped through the column, which was conditioned at a predetermined optimum pHs and room temperature. Firstly, the flow rate of the solution was altered from 0.5 to 4.0 cm3 min−1 in order to explain the effect of flow rate on the biosorption of Cu(II) and Cd(II) ions. Biosorbent dosage is also a key factor to improve the removal efficiency of heavy metal ions from an aqueous solution. For this purpose, the range of biosorbent dosage for TS-A was selected from 0.4 to 2.0 g dm−3. The breakthrough studies were also carried out to identify the performance of TS-A in the column. The residual metal ions solution was periodically collected from the column and analyzed by using an AAS. When the final concentration of the residual solution was equal to the initial concentration of the metal ions solution, the process was terminated. For this study, the amount and percentage of metal ions biosorption from the solution are calculated for the obtained experimental data (Eqs. 1 and 2).
where qe is the amount of biosorbed metal ions on the biosorbent at equilibrium (mg g−1), C0 is the initial concentration of metal ions (mg dm−3), Ce is the equilibrium concentration of metal ions (mg dm−3), m is the amount of biosorbent (g), V is the volume of metal ions solution (dm3).
Results and Discussion
Characterization of Biosorbents
The species of functional groups on the biosorbent surface can be a significant factor in the interaction between metal ions and the biosorbent, and they are responsible for the metal ions biosorption. To identify the functional groups, FT-IR analysis was performed for thiosalicylic acid (TS), thiosalicylic acid modified alginate (TS-A) and calcium alginate (Ca-A), and their FT-IR spectra are shown in Fig. 2a.
As it can be seen from FT-IR spectrum of TS, the absorption peak at 3441 cm−1 belongs to ‒OH stretching vibration band, the stretching vibration observed at 3063 cm−1 is related to the aromatic ν(C–H) and the peaks at 2654, 2567 and 2518 cm−1 are conducted with ν(S–H) stretching vibrations. The sharp peak appearing at 1677 cm−1 is attributed to the asymmetric stretching vibration of carboxylate group of TS. Moreover, the characteristic ν(C-S) stretching vibration of TS is observed at 650 cm−1. After modification, the presence of the characteristic peaks in TS-A (ν(OH stretching) = 3441 cm−1, ν(Ar–H stretching) = 3051 cm−1, ν(S–H stretching) = 2655, 2567 and 2517 cm−1, ν(C=O stretching) = 1677 cm−1, ν(C=C ring stretching) = 1587, 1563, 1469 and 1435 cm−1, ν(C=C ring bending) = 811 cm−1, ν(C–O stretching) = 1268 cm−1, ν(disubstitute benzene stretching) = 1062 and 1042 cm−1, ν(disubstitute benzene bending) = 742 cm−1, ν(C–S) = 650 cm−1)) with respect to TS indicates the achievement of the modification process. The functional groups in TS-A are also different from those of Ca-A structure, this is another evidence for the successful modification of the biosorbent.
The FT-IR spectra of Cu(II) ions loaded TS-A (Cu-TS-A) and Cd(II) ions loaded TS-A (Cd-TS-A) are illustrated in Fig. 2b. When FTIR spectra of TS-A before and after biosorption were compared, a decrease in the intensity of some functional groups and the observation of band shift suggest that these relevant groups play a role in the biosorption process. The observed broad ‒OH stretching vibration band in TS and TS-A shifted 3370 cm−1 for Cd(II) ions loaded biosorbent but this band intensity was very low for Cu(II) ions loaded biosorbent. This result explains that the interactions between Cu(II) ions and the biosorbent occur via ‒OH groups. The peaks presented at 2655, 2567 and 2517 cm−1 for TS-A shifted to 2658 and 2565 cm−1 for Cu(II) ions loaded biosorbent and 2640 and 2546 cm−1 for Cd(II) ions loaded biosorbent. The shift of these bands further indicated the involvement of –SH of thiol groups that took place in the biosorption process. The peak intensity for Cd(II) ions loaded biosorbent is also lower than Cu(II) ions loaded biosorbent. This indicates that Cd(II) ions have more interaction with –SH of thiol group and this result also agrees with the obtained biosorbed value for Cd(II) ions from Langmuir isotherm model. Carbonyl stretching peak intensity of carboxylates of biosorbent at around 1680 cm−1 decreased after the biosorption of Cu(II) and Cd(II) ions. This peak intensity for Cd(II) ions loaded biosorbent is dramatically lower than Cu(II) ions loaded biosorbent. This situation verifies occurring the more interactions between the carboxylate groups and Cd(II) ions. The band only appeared at around 1383 cm−1 after the biosorption of Cd(II) ions indicated that the corresponding groups participated in the biosorption. The peak at 553 cm−1 for Cu(II) ions loaded biosorbent or 855 and 724 cm−1 for Cd(II) ions loaded biosorbent was only observed after the biosorption but these peaks did not appear for TS-A. Hence, it is another evidence for the presence of Cu(II) or Cd(II) ions biosorption.
Morphological changes of Ca-A and TS-A surfaces were determined by SEM analysis and shown in Fig. 3.
The surface of Ca-A can be assessed as a smooth, clean and homogenous structure from the SEM image. After the modification with TS, the SEM image revealed that the surface gained more irregular bulges and perforated appearances. The observed changes on the surface of TS-A may offer the favorable conditions for both Cu(II) and Cd(II) ions biosorption. After Cu(II) and Cd(II) ions uptake, the surface was covered with heavy metal ions. This can be proved by SEM–EDX analysis results (Fig. 4).
Figure 4 indicates the EDX spectra for TS-A before and after the biosorption of Cu(II) and Cd(II) ions. TS-A spectrum did not include the characteristic signals of Cu(II) or Cd(II) ions whereas Cu(II) and Cd(II) ions were detected by EDX analysis for Cu(II) ions loaded TS-A and Cd(II) ions loaded TS-A, respectively. While the presence of high amount of calcium is determined from the spectrum of TS-A, the decrease in the amount of calcium after biosorption suggests that ion-exchange may occur with Cu(II) or Cd(II) ions.
Figure 5 illustrates thermogravimetric (TG) curves of Ca-A, TS and TS-A. The first mass loss of 16.99% in the range of 25–185 °C was observed due to the removal of physically adsorbed water molecules onto the Ca-A surface. Then, the second and third mass losses were realized and the structure was completely decomposed at 740 °C for Ca-A. The remaining mass loss was calculated as 9.09% at 740 °C and it was assumed to be CaO.
According to Fig. 5, TS decomposed at about 260 °C. From the thermogram of TS-A, the first mass loss of 6.41% in the temperature range of 25–123 °C was considered to be the separation of water molecules. Increasing the temperature, the TG curve for TS-A illustrated the elimination of TS (melting point 167 °C) from the structure at around 270 °C. It can be said that this observation can be one of the proofs of the modification. It was also determined from the thermogram that the percentage of CaO was 6.23. The percentage of modification calculated for TS-A from the TG analysis is 22.5. This value is also consistent with the calculated value from the elemental analysis results.
The surface charge density of biosorbent changes with the pH value of the solution which affects the biosorption properties of the metal ions. Hence, the effects of pH on the biosorption capacities of TS-A for Cu(II) and Cd(II) ions were determined. The variations of pH with the zeta potential for 0.10-TS-A and 0.15-TS-A in deionized water and metal ions solutions are exhibited in Fig. 6. According to these results, 0.10-TS-A and 0.15-TS-A have no points of zero charge and the zeta potential values of TS-A in deionized water are more negative than TS-A in metal ions solutions. However, increasing the concentration of TS in the modification caused the surface charge to become more negative. The changes in the surface charges of TS-A in metal ions solution are negative until pH 3.5 and it was observed that these curve trends reverse after this pH. Moreover, the negative charge densities for Cu(II) and Cd(II) ions decrease until pH 5.5, the zeta potential value for Cd(II) ions becomes positive after pH 6.5. This can be considered that the removal of metal ions from aqueous solution by TS-A was effectively achieved due to the strong interactions between the TS-A surface and metal ions at these pHs.
The chemical compositions of Ca-A and TS-A are given in Table 1. It was found that the percentage of sulfur in Ca-A was very low. After the modification of alginate with 0.10 M and 0.15 M TS, the amounts of S sharply increased in the modified biosorbents. The percentages of TS in the structure of 0.10-TS-A and 0.15-TS-A were calculated as 20.47 and 30.78, respectively. According to the results, the modification was successfully performed by using TS as a modifying agent.
Batch Biosorption Studies
First of all, the biosorption of Cu(II) and Cd(II) ions by the prepared biosorbents namely Ca-A and TS-A was investigated. The experimental preliminary studies indicated that the amounts of biosorbed Cu(II) and Cd(II) ions by TS-A were almost 1.5 fold higher than the obtained results with unmodified Ca-A. To examine the effect of the concentration of TS on the biosorption efficiency, the modified biosorbents were prepared with various TS solutions. According to Fig. 7a, the highest biosorption amounts were obtained with 0.10 M TS for Cu(II) ions and 0.15 M TS for Cd(II) ions and these concentrations of TS were selected to prepare the modified biosorbents for these metal ions biosorption.
pH is one of the most important parameters determining the biosorption capacity of the biosorbent. The effect of pH on the biosorption of Cu(II) and Cd(II) ions is shown in Fig. 7b. It is observed from Fig. 7b that the biosorption amounts of both metal ions are very low at the pH range of 1.0–2.5. This case is due to the high hydronium ions concentration in the strong acidic medium and the repulsive force between the metal ions and the same charged surface. As the pH increases, the concentration of hydronium ions in the solution decreases and metal ions can easily interact with the surface of TS-A because of an increase in the negative charge density. According to Fig. 7b, the highest amounts of biosorption for Cu(II) and Cd(II) ions were achieved at pH 5.5 and 7.5 and these values were found to be as 191.1 and 198.5 mg g−1, respectively.
To determine the highest biosorption capacities of TS-A for Cu(II) and Cd(II) ions, the various amounts of TS-A were used and the optimum amounts were determined at pH 5.5 and 7.5, respectively. The obtained results are shown in Fig. 7c. The amounts of the biosorbed Cu(II) and Cd(II) ions increase with an increase in the biosorbent dosage. This result can be explained by the fact that the number of active sites being responsible for the biosorption increases with an increase in the biosorbent amount. From Fig. 7c, the maximum biosorption yields were assigned as 96.58% for Cu(II) and 98.66% for Cd(II) ions by using 0.5 g dm−3 of the biosorbent amount. When the amount of the biosorbent was increased by more than 0.5 g dm−3, there was no significant improvement in the percentage biosorption yields. Therefore, the optimum biosorbent dosage was selected as 0.5 g dm−3 for further batch studies.
The biosorption of Cu(II) and Cd(II) ions onto TS-A was carried out at various temperatures (from 10 to 45 °C). From Fig. 7d, the maximum amounts for the biosorbed Cu(II) and Cd(II) ions at 20 °C were found as 197.1 and 198.4 mg g−1, respectively. While the amount of biosorption for Cu(II) ions slightly increases along with the rise of temperature, the biosorption amount for Cd(II) ions was not affected by the temperature change. As a result, the temperature in the batch system for the biosorption of both metal ions was chosen as the lowest temperature, which is 20 °C.
After setting the pH, biosorbent dosage and temperature for the biosorption process, the effect of contact time on the biosorption of Cu(II) and Cd(II) ions onto TS-A was investigated at various initial concentrations (150, 175 and 200 mg dm−3). The amounts of biosorbed Cu(II) and Cd(II) ions as a function of contact time at different concentrations were illustrated in Fig. 8. The biosorption of metal ions stabilized in a relatively short period of time which is approximately 30 min and there is no significant change in the biosorption amounts after this point. This time was also selected as the biosorption equilibrium time for each metal ion. The determined biosorption amounts for Cu(II) and Cd(II) ions increased from 285.6 to 363.0 mg g−1 and from 299.0 to 396.9 mg g−1, respectively when the initial metal ion concentration was increased from 150 to 200 mg dm−3.
Biosorption Kinetics and Isotherms
To determine the equilibrium contact time in the biosorption of pollutants from aqueous solution, kinetic studies performed. In addition, kinetic and isotherm models are used to interpret the experimentally obtained data. The determination of the biosorption parameters allows for optimization of the biosorption mechanism, determination of the effect of the surface properties of TS-A on the biosorption behavior, and calculation of the biosorbent capacities.
Biosorption processes take place in four steps. First, metal ions are transported to the liquid film or boundary layer surrounding the biosorbent (Film diffusion). The second is the transport of metal ions from the boundary film to the expanding pores of the biosorbent through surface diffusion. Third, the metal ions are transferred from the surface to the intermolecular active sites of the biosorbent during pore diffusion (Particle diffusion). Fourth, metal ion attaches to active sites on the biosorbent. According to biosorption models, the slowest step in the process is considered as biosorption [30]. In order to analyze the metal biosorption mechanism, the experimental data were evaluated by kinetic models including Lagergren first-order [31, 32], pseudo second-order [33] and intra-particular diffusion [30, 34, 35]. Lagergren first-order model describes that biosorption solely occurs on localized sites and there is no interaction between biosorbed ions. The maximum biosorption results in a saturated layer of metal ions on the surface of the biosorbent. The pseudo second-order kinetic model assumes that the rate of biosorption is proportional to the square of the number of existing surface sites. The intra-particle diffusion model is also tested for the biosorption diffusion process. The equations for the kinetic models are given in Table 2. The relevant plots for these models were drawn to investigate the suitability of the models with experimental data. The kinetic parameters for the biosorption of metal ions onto TS-A at 20 °C were determined from these plots and are reported in Table 2.
According to the table, the highest correlation coefficients (R2 = 0.999) were obtained for the pseudo-second-order kinetic model and it can be emphasized that the biosorption of Cu(II) and Cd(II) ions onto TS-A obeys the pseudo-second order kinetic model. An increase in the concentration led to a rise in the amounts of biosorbed metal ions. The maximum amounts of biosorbed Cu(II) and Cd(II) ions at equilibrium in the initial concentration of 200 mg dm−3 were determined as 368.5 ± 1.5 and 398.6 ± 0.2 mg g−1, respectively. On the other hand, the intra-particle diffusion model for the biosorption of Cu(II) and Cd(II) ions onto TS-A was applied up to 25 min. The intercepts of the plots did not pass from the origin. This phenomenon indicated that the intra-particle diffusion was not the rate-determining step for the biosorption process [33, 36].
Biosorption of Cu(II) and Cd(II) ions onto TS-A was performed at various initial concentrations and the obtained data are shown in Fig. 9. Langmuir [37], Freundlich [38] and Dubinin-Radushkevich (D-R) [36] isotherm models were used to describe biosorption mechanism. Based on the Langmuir model assumptions that all the biosorption sites are energetically uniform and biosorption occurs in active sites independently [39]. The separation factor, RL, is also calculated by the Langmuir isotherm parameter (KL) to check the convenience of the biosorption process as the linear, favorable or unfavorable nature of isotherm [40, 41]. The mechanism of biosorption on multilayered or heterogeneous surfaces can be identified by the well-known Freundlich isotherm model. A measure of the deviation from the linearity of the biosorption can be determined by the Freundlich constant’s n. The numerical value of n at equilibrium is greater than unity, indicating that the biosorption is favorable. The D–R isotherm model was based on the biosorption potential theory and the physical and chemical biosorption processes can be distinguished by using this isotherm model [42, 43]. The magnitude of mean free potential energy (E) is between 8 and 16 kJ mol−1, in this case the process follows by a chemical ion-exchange biosorption, while for the values of E < 8 kJ mol−1, the biosorption process is of a physical nature. The equations and calculated parameters for the isotherm models are listed in Table 3.
As it can be seen from the table, the correlation coefficient (R2) for the Langmuir isotherm model (Fig. 9 inset) seems to be the highest in comparison with the other isotherm models. The values of RL were also found to be 2.41 × 10−2 and 4.69 × 10−2 for the biosorption of Cu(II) and Cd(II) ions onto TS-A, respectively. These values are between 0–1 and it means that the nature of isotherm for the biosorption of metal ions onto TS-A is favorable. The Langmuir constant, KL, was calculated as 0.202 and 0.406 dm3 mg−1 for Cu(II) and Cd(II) ions, respectively. These results indicate that the biosorption of these metal ions onto TS-A is favorable. The obtained maximum biosorption capacities of TS-A for Cu(II) and Cd(II) ions were 426.9 ± 5.8 and 592.9 ± 1.4 mg g–1 from the Langmuir isotherm model. The biosorption processes fit well with the Langmuir isotherm model eventually.
Column Biosorption Studies
The biosorption of Cu (II) and Cd (II) ions in a continuous system was investigated for the capability of TS-A due to its ease of application on an industrial scale. The flow rate is a very important factor that affects the performance of the biosorbent because of depending on the contact time of the biosorbate with the biosorbent in the column [44]. According to the results, although the change in flow rate from 0.5 to 4.0 cm3 min−1 did not cause a significant change in the biosorption of Cd (II) ions, it was observed that the flow rate had a very different effect for the biosorption of Cu(II) ions. Since the interaction between the TS-A and Cu(II) ions was greater at the low flow rate, the flow rate for subsequent studies was chosen as 1.0 cm3 min−1 for both ions.
To determine the optimum amount of TS-A for the biosorption of Cu(II) and Cd(II) ions in the continuous system, varying amounts of biosorbent were placed in the column to create different bed heights. The results indicated that the biosorption efficiency increases for both metal ions as the amount of TS-A rises to a certain value. When the amount of TS-A was increased from 0.2 to 1.2 g dm−3, the biosorption efficiencies for Cu(II) and Cd(II) ions were found to be from 31.15% to 86.17% and 50.70% to 81.54%, respectively. The most suitable biosorbent amounts used in the breakthrough and the reusability of TS-A studies were selected as 0.8 g dm−3 and 0.5 g dm−3 for Cu(II) ions Cd(II) ions, respectively.
In order to observe the suitability of the continuous system in the industrial scale, the breakthrough curves for the biosorption of Cu(II) and Cd(II) ions were plotted (Fig. 10). According to Fig. 10, the breaking points for Cu(II) and Cd(II) ions were found to be approximately 75 and 100 min, respectively. The saturation points of TS-A with metal ions were determined as 180 min for Cu(II) ions and 150 min for Cd(II) ions. It can be said that the biosorption of metal ions was effective up to these times and there was no more interaction between the metal ions and the biosorbent since the amounts of the biosorbed metal ions have not changed. This may also indicate that the active sites of the biosorbent reached the saturation. When the amounts of biosorbed Cu(II) and Cd(II) ions were plotted against time (Fig. 10 inset), it was observed that there was a rapid decrease in the amount of biosorbed metal ions after 90 min for Cu (II) ions and 50 min for Cd (II) ions and the obtained curves were found to be overshoot type [45]. This can be explained by the desorption of metal ions from the TS-A surface.
Biosorption from the Synthetic Wastewater
Synthetic wastewater contains various types of electrolyte ions that can compete to bind the functional groups of biosorbent surface. This leads to restricting the use of biosorbents in biosorption applications. In this study, biosorption capacities of TS-A for Cu(II) and Cd(II) ions in synthetic wastewater were investigated in the binary system. The results are compared to single system data and are shown in Fig. 11.
The obtained biosorption yields from the single system were 91.46% for Cu(II) and 96.54% for Cd(II). According to this result, it was observed that synthetic wastewater components had no significant effect on the biosorption of the metal ions. Cu(II) and Cd(II) ions were added into synthetic wastewater at the same time and the biosorption of the metal ions was investigated from this media. It was found that the biosorption efficiency results were 63.21% for Cu(II) and 43.88% for Cd(II) and these results are lower than the single system results. The reason for the obtained low biosorption efficiency for the binary system is that the metal ions compete to bind to TS-A surface. As seen in Fig. 11, the amount of biosorption capacity for Cu(II) ions is higher than Cd(II) ions in the binary system. The optimum pH’s for the highest biosorption values of Cu(II) and Cd(II) ions in the batch system were determined as 5.5 and 7.5, respectively. In terms of Cd, at pH 8, the species distribution is approximately 90% Cd(II) ions and 10% Cd(OH)+ and at around pH 9, Cd(II) ions precipitate as a Cd(OH)2. Similarly, for Cu(II) ions, the concentration of hydroxyl ions increases beyond pH 6 in the solution leading to the formation of more stable metal hydroxides like Cu(OH)2 and both cases decrease the metal ions biosorption. Consequently, the pH 5.5, which was the value where both ions were not precipitated and the highest biosorption amount was obtained for Cu(II) ions, was chosen for the binary system. At this pH value, biosorption amount of Cd(II) ions is low and also the order of electronegativities for the metals is Cu (1.90) > Cd (1.69) [46, 47]. Cu has more electronegative than Cd that leads to more electrostatic interactions and surface chelating reactions. These situations result in the high biosorption yield for Cu(II) ions in the binary system.
Comparison of Cu(II) and Cd(II) Ions Biosorption Capacities with Literature
The current biosorption capacities for TS-A were compared with the reported studies in the literature. Table 4 summarizes the maximum biosorption capacities of different alginate-based biosorbents for Cu(II) and Cd(II) ions.
The comparative data show that the amount of biosorbed metal ions by TS-A is markedly higher than most of the corresponding biopolymers reported in the literature.
Conclusions
In this study, TS-A which was prepared from the gelation of sodium alginate solution containing thiosalicylic acid with calcium ions, was used as a biosorbent for the removal of Cu(II) and Cd(II) ions. FT-IR, SEM, elemental and thermal analysis methods, and zeta potential measurements were performed to reveal the characteristic features of TS-A. The effects of pH, amount of biosorbent, temperature, contact time and initial metal ions concentration on the biosorption of the metal ions were investigated by using TS-A in the batch system. The highest biosorption values for Cu(II) and Cd(II) ions were determined to be 426.9 mg g−1 at pH 5.5 and 592.9 mg g−1 at pH 7.5, respectively. The obtained kinetic and equilibrium data were found to be consistent with the pseudo-second order and Langmuir isotherm models, respectively. The effects of flow rate and the amount of biosorbent on the biosorption of the metal ions in the continuous system were also investigated. The saturation points of TS-A for Cu(II) and Cd(II) ions were determined as 180 and 150 min, respectively. Synthetic wastewater application was also performed in the single and binary systems. It was concluded that TS-A has a very effective biosorption performance in the biosorption of Cu(II) and Cd(II) ions.
References
Amarasinghe B, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
Al-Rub FA, El-Naas M, Ashour I, Al-Marzouqi M (2006) Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem 41:457–464
USEPA (2019) National primary drinking water regulations. United States Environmental Protection Agency, Washington, D.C.
Rao RAK, Ikram S, Uddin MK (2014) Removal of Cd(II) from aqueous solution by exploring the biosorption characteristics of gaozaban (Onosma bracteatum). J Environ Chem Eng 2:1155–1164
Satarug S, Haswell-Elkins MR, Moore MR (2000) Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br J Nutr 84:791–802
Tang W-W, Zeng G-M, Gong J-L, Liang J, Xu P, Zhang C, Huang B-B (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ 468:1014–1027
Friberg L (2018) Cadmium in the environment. CRC Press, Boca Raton, Florida
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Volesky B, Holan Z (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300
Williams C, Aderhold D, Edyvean R (1998) Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res 32:216–224
Fourest E, Volesky B (1995) Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ Sci Technol 30:277–282
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents–a review of the recent literature. Bioresour Technol 101:5043–5053
Richards S, Dawson J, Stutter M (2019) The potential use of natural vs commercial biosorbent material to remediate stream waters by removing heavy metal contaminants. J Environ Manage 231:275–281
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32:762–798
Alnaief M, Alzaitoun M, García-González C, Smirnova I (2011) Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohydr Polym 84:1011–1018
Torres FG, Troncoso OP, Pisani A, Gatto F, Bardi G (2019) Natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci 20:5092
Yen TF (2008) Development of new environmental technologies using biopolymers, In: Islam MR (ed) Nature science and sustainable technology research progress. Nova Science Publishers, New York, pp 209–230
Mehta S, Gaur J (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25:113–152
Steinbüchel A, Rhee SK (2005) Polysaccharides and polyamides in the food industry: properties, production, and patents. Wiley-VCH Verlag GmbH & CO, KGaA, Weinheim
Wang S, Vincent T, Faur C, Guibal E (2016) Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int J Mol Sci 17:1453
Attar K, Demey H, Bouazza D, Sastre AM (2019) Sorption and desorption studies of Pb(II) and Ni(II) from aqueous solutions by a new composite based on alginate and magadiite materials. Polymers 11:340
Grant GT, Morris ER, Rees DA, Smith PJ, Thom D (1973) Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 32:195–198
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel(II) through adsorption. Carbohydr Polym 72:261–271
Day D (1998) Alginates. In: Kaplan DL (ed) Biopolymers from renewable resources. Springer, Berlin, pp 119–143
Chen JP, Hong L, Wu S, Wang L (2002) Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir 18:9413–9421
Papageorgiou SK, Katsaros FK, Kouvelos EP, Nolan JW, Le Deit H, Kanellopoulos NK (2006) Heavy metal sorption by calcium alginate beads from Laminaria digitata. J Hazard Mater 137:1765–1772
Cheng SY, Show P-L, Lau BF, Chang J-S, Ling TC (2019) New prospects for modified algae in heavy metal adsorption. Trends Biotechnol 37:1255–1268
Ay Ç, Özcan AS, Erdoğan Y, Özcan A (2017) Characterization and lead (II) ions removal of modified Punica granatum L. peels. Int J Phytoremediat 19:327–339
McKay G, Ho YS, Ng J (1999) Biosorption of copper from waste waters: a review. Sep Purif Methods 28:87–125
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe (Theory of adsorption substances from solution). Kungliga Svenska Vetenskapsakademiens, Handlingar 24:1–39
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Ho YS, McKay G (1998) Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf Environ Protect 76:183–191
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pigm 51:25–40
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of activated charcoal. Proc Acad Sci USSR Phys Chem Sect 55:331–337
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundam 5:212–223
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Inglezakis V (2007) Solubility-normalized Dubinin-Astakhov adsorption isotherm for ion-exchange systems. Microporous Mesoporous Mater 103:72–81
Helfferich F (1962) Ion exchange resins. McGraw Hills, New York
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Xie Y-D, Xiong W-I, Yu J-X, Tang J-Q, Chi R-A (2018) Recovery of copper from metallurgical sludge by combined method of acid leaching and biosorption. Process Saf Environ 116:340–346
Shi T, Jia S, Chen Y, Wen Y, Du C, Guo H, Wang Z (2009) Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J Hazard Mater 169:838–846
Leyva-Ramos R, Diaz-Flores PE, Aragon-Piña A, Mendoza-Barron J, Guerrero-Coronado RM (2005) Adsorption of cadmium(II) from an aqueous solution onto activated carbon cloth. Sep Sci Technol 40:2079–2094
Ahmad A, Bhat A, Buang A (2018) Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: kinetic and equilibrium modeling. J Clean Prod 171:1361–1375
Arıca MY, Bayramoǧlu G, Yılmaz M, Bektaş S, Genç Ö (2004) Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J Hazard Mater 109:191–199
Tao H-C, Li S, Zhang L-J, Chen Y-Z, Deng L-P (2019) Magnetic chitosan/sodium alginate gel bead as a novel composite adsorbent for Cu (II) removal from aqueous solution. Environ Geochem Health 41:297–308
Pandey A, Bera D, Shukla A, Ray L (2007) Studies on Cr (VI), Pb (II) and Cu (II) adsorption–desorption using calcium alginate as biopolymer. Chem Spec Bioavailab 19:17–24
Yu K, Ho J, McCandlish E, Buckley B, Patel R, Li Z, Shapley NC (2013) Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification application. Colloids Surf A 425:31–41
Cataldo S, Cavallaro G, Gianguzza A, Lazzara G, Pettignano A, Piazzese D, Villaescusa I (2013) Kinetic and equilibrium study for cadmium and copper removal from aqueous solutions by sorption onto mixed alginate/pectin gel beads. J Environ Chem Eng 1:1252–1260
Sangeetha K, Vidhya G, Vasugi G, Girija E (2018) Lead and cadmium removal from single and binary metal ion solution by novel hydroxyapatite/alginate/gelatin nanocomposites. J Environ Chem Eng 6:1118–1126
Acknowledgements
The authors would like to acknowledge to Anadolu University and Eskişehir Technical University Scientific Research Projects (1409F388) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alp Arıcı, T., Özcan, A.S. & Özcan, A. Biosorption Characteristics of Cu(II) and Cd(II) Ions by Modified Alginate. J Polym Environ 28, 3221–3234 (2020). https://doi.org/10.1007/s10924-020-01844-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01844-2