Abstract
Cellulose aerogel (CA) isolated from tea stem wastes (TSW) is a good fire retardant and heat insulator, environmentally friendly, thermally stable and highly porous material with a network structure. These outstanding properties have attracted a huge interest in the materials world. In this study, firstly, following delignification and removing hemicellulose, pure raw cellulose was isolated using TSW, hydrogel form of cellulose was prepared by regeneration of cellulose solution, and then, the final product (CA) was produced via freeze-drying. The data results showed that the aerogel had a three dimensionally network structure. Moreover, it can be deduced that thermal durability of the studied CA could be effective because of its fire retardant and heat insulating property. In addition, the production process of CA is easily available at low cost and sustainable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, the most abundant bio-polymer substance on Earth, in its various modifications is a natural macromolecule produced mostly by plants in large amounts per year, roughly a billion tons [1]. The main sources of cellulose are wood, cotton and flax [2]. Also, cellulose is one of the most commonly used natural matters and has become one of the most significant commercial raw materials [3]. An important reason why cellulosic materials are favoured is that it has a low cost, high surface area, bio-compatibility and degradability [4]. Moreover, the using of cellulosic wastes for recycling performs a considerable role in terms of decreasing in economical expenses. Functional materials with high value added, fibers, thin films and sponges can be produced from cellulose-based materials such as cellulose nanocrystals, nanofibers, hydrogels or aerogels [5,6,7,8]. Thus, this advantage can reduce costs of raw material requirements and provides an opportunity in areas where sustainable resources are limited.
In the recent times, scientists’ attention in aerogel originating from cellulose obtained from woody or vegetable wastes, which are biodegrade in a short while, has progressively increased owing to its intriguing properties such as thermal insulator, super-absorbent flame retardant and highly porous structure [9, 10]. In addition to flame retardancy and heat insulating properties, cellulose aerogels can be used in many areas in real life such as biomedical applications, adsorbent in oil/water separation [11]. Aerogel from cellulosic materials, which is one of the lightest matters and new three-dimensionally solids, is environmentally friendly and non-toxic in comparison with conventional aerogels such as silica [12,13,14]. In addition, traditional aerogel materials, including polypropylene (PP), graphene, resorcinol/formaldehyde, silica and activated carbon, are often used in the treatment of adsorption, but they suffer from disadvantages such as poor reusability, insufficiently selective material adsorption capacity, toxicity, high cost and a lack of biodegradability [11].
In this regard, when taken into consideration the diversity of present sources, the utilizing of TSW, which is found plentiful in Rize/Turkey (East Black Sea region), has a particular importance as an economical worth. With this study, a novel bio-polymer, which is a “green” product, low-cost, biodegradable, heat insulator and fire-retardant, was synthesized using a pure raw material (cellulose) isolated from pruning wastes of the tea wood, which is one of the important crops in the aforementioned area. In addition to the flame retardant and surface properties of cellulose bio-polymer aerogel, thermal and structural characteristics were examined through the instrument of BET surface analysis techniques (specific surface area pore size and volume), fourier transformed infra-red spectroscopy (FT-IR), the scanning electron microscopy (SEM), thermal analysis (TG-DTG/DTA), X-ray diffraction (XRD) spectroscopy and the tests of flame retardant property. Besides, it is predicted that it could be a potentially thermal insulating material.
Materials and Methods
Materials
All chemicals (sodium hydroxide, urea, and ethanol) were purchased from Merck and used without further purification. The studied solutions were prepared with deionized (DI) water. The isolation of raw pure cellulose from pruning tea stem wastes (TSW) was performed by an eco-friendly treatment for bleaching and extraction of cellulose.
Cellulose Production from TSW
Pure cellulose production was made same as previous study [14]. For obtaining a sawdust, TSW was ground and sieved (150–210 µm). It was then washed several times with DI water and filtrated using a cloth sieve removing of impurities such as water-soluble. Alkaline process was carried out in 4% NaOH solution at 80 °C for 3 times. Fibres (alkaline processed) were treated with H2O2 or NaClO2 bleaching of 1.7 wt% in acetic acid buffer at 80 °C for four times. The resulting fibres were filtered by a cloth strainer and rinsed with DI water until it reached neutrality. The powder of TSW sawdust was then oven dried at 105 °C.
Acid Treatment
Bleached TSW was subjected to pre-heated H2SO4 (64 wt%) in order to hydrolyze [15]. The excess acid was removed by centrifugation at 10.000 rpm for 10 min and this was repeated for a few times. The final cellulose product was washed using DI water and filtered by a cellulose membrane or glass filter up to pH 5.
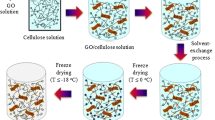
Cellulose Aerogel Production
In this study, sonicator-ultrasonic treatment was used for the first time in the preparation of aerogel. It was found that the utilization of sonicator had a positive effect on porosity and crystallinity index, which directly affected the strength of the structure. Cellulose fibres (5 wt%), dispersed into a sodium hydroxide/urea solution (1.9 wt%/10 wt%), was sonicated for 6 min via sonicator. The solution was then placed in a freezer for more than 24 h to occur gelation. After the freezing of the gel, it was thawed at room temperature, followed by submersion into 99% ethanol for coagulation (4 h). The material thickness was adjusted around 1.0 cm with a diameter of 3.8 cm using a beaker as a mold. After coagulation, solvent exchange process was made by immersing the gel in DI water for 2 days. Freeze-drying was carried out for the sample for 24 h at − 98 °C with a freeze-dryer after pre-freezing the sample at − 12 °C for 12 h.
Instrumental Characterization
For SEM studies (JEOL JSM-6610 at 10 and 20 kV), the aerogel bulk samples on carbon tapes were coated with a thin layer of gold before being inserted into the SEM chamber. FTIR spectra were recorded in the region of 3500–600 cm−1 on a Spectrum-100 FTIR spectrometer at a resolution of 4 cm−1. XRD spectra for CA was carried out on a Rigaku DMAX-3C automated diffractometer using Ni filtered CuK-beta radiation (40 kV and 30 mA). Diffractograms were recorded from 5° to 40° at a scan rate of 3°/min. Nitrogen adsorption/desorption isotherms were obtained in the relative pressure range of 0.05 < P/P0 < 1.00 with an Autosorb-iQ-2 analyser [Quantachrome Instruments, Florida, USA]. The samples were degassed at 100 °C for 5 h prior to the textural measurements at 77 K. Density studies were conducted by using a gas (helium) pycnometer (Quantachrome ULTRAPYC-1200e) and verified by Quantachrome Pore Master-60 mercury-porosimeter [Quantachrome Instruments, Florida, USA]. In the porosimeter, some mechanisms perform severally. First, the powder is pressed and condensed until the inter-particle contacts become strong enough to resist the pressure of the mercury. With further increasing pressure, the pores are filled and the density of the powder no longer increases. For all powders, the measured density is similar to the skeletal density. The thermogravimetric analyses were performed using SII A6 6300 model thermal analyzer in a dynamic nitrogen atmosphere (heating rate: 10 °C min−1, platinum crucibles, mass ~ 10 mg and temperature range: 20–800 °C).
Results and Discussions
Effect of Freeze-Drying Method on Porosity
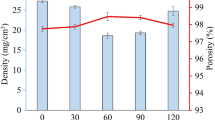
The freeze-dried sample porosity was demonstrated in Fig. 1. Freeze-drying process has an important effect on porosity. While the porosity was determined as 0.95 cm3/g at low temperatures, it decreased at higher temperatures. Along freeze-drying, negative effects may take place like the collapse upon the aerogel scaffold itself and it was stated that “the melting of ice which plasticized the matrix and decreased the porosity, as the initial sample temperature was increased” by Krokida et al. [16].
SEM Analysis
The morphological and structural features of CA were revealed in SEM images (Fig. 2a–c). It is obviously seemed that the surface of the aerogel with a widely porous and network structure is comprised of cellulosic micro-fibres (Fig. 2c). The porosity comes from removing of frozen water in the course of the freeze-drying process [17, 18]. This feature is compatible with those of nitrogen isotherm data (adsorption/desorption), which is determined in "XRD Analysis" section. Additionally, during slow freezing, it is observed that ice crystal film-like structures are formed as well as porous structures in the cellulose microfibril scaffold [19]. According to the aforementioned findings, the CA with a structure with micropore heterogenous can be formed via in situ templated production of material in the gel framework of cellulose [17, 20,21,22]. Figure 2a and b show that an irregular and complicated porous structure has fine interconnections between the cellulosic pores. Therefore, it can be said that cellulose aerogel can be used as a potential adsorbent due to tunnel-like spaces inside the network scaffold structure.
FT-IR Analysis
The IR signal related to the characteristic functional groups is demonstrated in Fig. 3. It can be observed that the various components of the CA are most likely consisted of aromatics, ketone, esters, alkenes and alcohol with different functional groups (oxygen-containing) observed, e.g., OH (3460–3320 cm−1), C=O (1777–1150 cm−1), C–O–C (1150 cm−1), and C–O–(H) (1000 cm−1) [23, 24]. The FTIR spectrum of the aerogel reveals various structural characteristics (Fig. 3) The vibration located at 3329 cm−1 can be attributed to intermolecular hydrogen bonding while the vibration at 3432 cm−1 is because of intramolecular hydrogen bonding. In addition, the FTIR absorption band at 1415 cm−1 was assigned to a symmetric CH2 bending vibration [25]. This band, which is seen in Fig. 3 at high intensity, is also known as “ the crystallinity band” for the cellulose structure [26].
Thermal Analysis
Taking into account potential applications of heat insulators, thermal characteristics of the aerogel was examined by thermogravimetric analysis (TGA). As displayed in Fig. 4, a gradual mass loss takes place from 20 to about 500 degrees. The mass loss in temperature range of 0–200 °C can be ascribed to removal of moisture in both inner and outer surface where the removal of water is more difficult relatively [23]. The grade of mass loss between 300 and 500 °C can be referred to the depolymerization of cellulose with formation of volatile hydrocarbons and CO2 [17, 27]. Finally, it is seen that the aerogel has a fine thermal durability (mass loss only 50% at around 350 °C).
XRD Analysis
XRD spectra for CA were carried out on a Rigaku DMAX-3C automated diffractometer using Ni filtered CuK-beta radiation (40 kV and 30 mA). Diffractograms were recorded from 5° to 40° at a scan rate of 4°/min. Figure 5 demonstrates typical diffraction peaks for the plane (200) at 2θ = 22.30° and (110) at 2θ = 20.7° corresponding to crystal form of cellulose [28]. In order to determine crystallinity index (CrI) of cellulosic structure of the aerogel, the intensity of the 200 peak (I200, 2θ = 22.30°) and the minimum intensity between 200 and 110 (Iam, 2θ = 20.7°) peaks was calculated using the empirical equation [12]
Considering CI data, It is clear that the cellulose aerogel has a higher crystallinity (95.71%) than the woody raw amorphous materials, probably due to the removal of lignin and hemicellulose [29]. This result also indicates that crystalline and amorphous regions of cellulose are significantly divided.
BET Specific Surface Area
In order to examine the aerogel porosity, measurement techniques of nitrogen adsorption and desorption isotherm were carried out. Morphological and porous properties including specific surface area (from N2 adsorption measurements), average pore diameter and the total pore volume of the aerogel are studied and given in Table 1. The results showed the aerogel with average pore diameter and total pore volume as 5.45 nm and 0.475 cm3/g, respectively. Besides, from Fig. 6, the nitrogen adsorption at 77 K of cellulose aerogel is similar to type II isotherm (according to IUPAC classification), which is the characteristic physical adsorption on microporous solids. This characteristic property of the isotherm is the reversible process at relatively lower pressures and the hysteresis loop at relatively higher pressures [30,31,32]. According to BET (Brunauer–Emmett–Teller) specific surface area analysis, a value of 327.42 m2 g−1 is recorded for the aerogel.
Flame Retardancy of CA
To investigate the fire (flame) retardant feature of the aerogel, the sample was enkindled and velocity of burning was recorded. During igniting of the sample, it was observed that the aerogel was taken to fire slowly and the part of only 30% was enkindled after 79 s of combustion. It has been found that the cellulose aerogel has intrinsic flame retardant properties as a result of the three dimensional arrangement of the scaffold structure [33]. The pictures before and after burning were given in Figs. 7 and 8, respectively. However, another cellulose aerogel was quickly enkindled (about 10 s) and burned completely after 10 s of combustion in the literature [17]. The velocity of aerogel combustion decreases from 80 to 79.7 mm s−1. Also, when the aerogel with the length of 80 mm was enkindled, flame propagation prominently slowed down and then the fire became get smaller and was ultimately extinguished within about 263 s. The results demonstrate that the aerogel is extremely effective for flame retardant.
Thermal Conductivity Analysis of CA
It has been revealed that aerogels, especially those which are cellulose based are widely used as effective heat insulators [33]. Within the scope of the paper, thermal conductivities of cellulose aerogel specimens obtained from TSW samples were analysed by C-THERM TCi model device. The results from using the transient plane source (TPS) method at 25 °C are given in Table 2. In addition, the thermal conductivity coefficients of some commercial materials recorded in the literature [34,35,36,37] have also been shown in the table in terms of comparison. As is seen from the table, it is obviously observed that the lowest (0.030 W/mK) coefficient of thermal conductivity belongs to the aerogel with 5.0% (w/w). It can be clearly seen that this value is almost similar to the values of products such as polyurethane, polystyrene and fiberglass which are used as heat insulation materials in many fields. Considering all these results, cellulose-based aerogel materials obtained from TSW are thought to have a potential to be used as thermal insulation materials in many areas.
Conclusion
Studied cellulose aerogel in this paper, which has a high porosity structure, was produced from pruning tea stem wastes via in situ transforming cellulose hydrogel to aerogel scaffold. This study is an influential, environmentally friendly and low-cost process for obtaining efficient porous, potential super absorbent and flame retardant material. It was clearly revealed that the obtained cellulose aerogel has a very porous structure, an excellent flame-retardant and heat insulating performance. Also, the aerogel has fine thermal stability and crystallinity feature. It is thought that this paper will provide new opportunities for the use of inexpensive cellulosic waste derivatives and “green” materials.
References
Aegerter MA, Leventis N, Koebel MA (2011) Aerogels handbook. Advances in sol-gel derived materials and technologies. Springer, New York. https://doi.org/10.1007/978-1-4419-7589-8
Leppanen K, Andersson S, Torkkeli M, Knaapila M, Kotelnikova N, Serimaa R (2009) Structure of cellulose and microcrystalline cellulose from various wood species, cotton and flax studied by X-ray scattering. Cellulose 16(6):999–1015. https://doi.org/10.1007/s10570-009-9298-9
Postek MT, Vladar A, Dagata J, Farkas N, Ming B, Wagner R, Raman A, Moon RJ, Sabo R, Wegner TH, Beecher J (2011) Development of the metrology and imaging of cellulose nanocrystals. Meas Sci Technol. https://doi.org/10.1088/0957-0233/22/2/024005
Duong HM, Nguyen ST (2016) Nanocellulose aerogels as thermal insulation materials. In: Pacheco Torgal F, Buratti C, Kalaiselvam S, Granqvist C-G, Ivanov V (eds) Nano and biotech based materials for energy building efficiency. Springer, Cham, pp 411–427. https://doi.org/10.1007/978-3-319-27505-5_15
Liu J, Cheng F, Grenman H, Spoljaric S, Seppala J, Eriksson JE, Willfor S, Xu CL (2016) Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohyd Polym 148:259–271. https://doi.org/10.1016/j.carbpol.2016.04.064
Shen XP, Shamshina JL, Berton P, Bandomir J, Wang H, Gurau G, Rogers RD (2016) Comparison of hydrogels prepared with ionic-liquid-isolated vs commercial chitin and cellulose. Acs Sustain Chem Eng 4(2):471–480. https://doi.org/10.1021/acssuschemeng.5b01400
Kwon GJ, Kim DY, Hwang JH, Kang JH (2014) Structural properties and adsorption capacity of holocellulose aerogels synthesized from an alkali hydroxide-urea solution. J Korean Phys Soc 64(10):1470–1473. https://doi.org/10.3938/jkps.64.1470
Wang ZG, Liu SL, Matsumoto Y, Kuga S (2012) Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 19(2):393–399. https://doi.org/10.1007/s10570-012-9651-2
Ahmadi M, Madadlou A, Sabouri AA (2015) Isolation of micro- and nano-crystalline cellulose particles and fabrication of crystalline particles-loaded whey protein cold-set gel. Food Chem 174:97–103. https://doi.org/10.1016/j.foodchem.2014.11.038
Awal A, Sain M, Chowdhury M (2011) Preparation of cellulose-based nano-composite fibers by electrospinning and understanding the effect of processing parameters. Compos Part B 42(5):1220–1225. https://doi.org/10.1016/j.compositesb.2011.02.011
Long LY, Weng YX, Wang YZ (2018) Cellulose aerogels: synthesis, applications, and prospects. Polymers-Basel 10(6):623
Seantier B, Bendahou D, Bendahou A, Grohens Y, Kaddami H (2016) Multi-scale cellulose based new bio-aerogel composites with thermal super-insulating and tunable mechanical properties. Carbohyd Polym 138:335–348. https://doi.org/10.1016/j.carbpol.2015.11.032
Kobayashi Y, Saito T, Isogai A (2014) Aerogels with 3D ordered nanofiber skeletons of liquid-crystalline nanocellulose derivatives as tough and transparent insulators. Angew Chem Int Ed 53(39):10394–10397. https://doi.org/10.1002/anie.201405123
Kaya M (2017) Super absorbent, light, and highly flame retardant cellulose-based aerogel crosslinked with citric acid. J Appl Polym Sci. https://doi.org/10.1002/App.45315
Beck-Candanedo S, Roman M, Gray DG (2005) Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 6(2):1048–1054. https://doi.org/10.1021/bm049300p
Krokida MK, Karathanos VT, Maroulis ZB (1998) Effect of freeze-drying conditions on shrinkage and porosity of dehydrated agricultural products. J Food Eng 35(4):369–380. https://doi.org/10.1016/S0260-8774(98)00031-4
Han YY, Zhang XX, Wu XD, Lu CH (2015) Flame retardant, heat insulating cellulose aerogels from waste cotton fabrics by in situ formation of magnesium hydroxide nanoparticles in cellulose gel nanostructures. Acs Sustain Chem Eng 3(8):1853–1859. https://doi.org/10.1021/acssuschemeng.5b00438
Nemoto J, Saito T, Isogai A (2015) Simple freeze-drying procedure for producing nanocellulose aerogel-containing, high-performance air filters. Acs Appl Mater Int 7(35):19809–19815
Jin H, Nishiyama Y, Wada M, Kuga S (2004) Nanofibrillar cellulose aerogels. Colloid Surf A 240(1–3):63–67. https://doi.org/10.1016/j.colsurfa.2004.03.007
Huang YJ, Zhou T, He S, Xiao H, Dai HM, Yuan BH, Chen XF, Yang XB (2019) Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl Surf Sci 497:143775
Mi HY, Jing X, Politowicz AL, Chen E, Huang HX, Turng LS (2018) Highly compressible ultra-light anisotropic cellulose/graphene aerogel fabricated by bidirectional freeze drying for selective oil absorption. Carbon 132:199–209
Zhang XX, Yu Y, Jiang ZH, Wang HK (2015) The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J Wood Sci 61(6):595–601
Poletto M, Ornaghi HL, Zattera AJ (2014) Native cellulose: structure, characterization and thermal properties. Materials 7(9):6105–6119. https://doi.org/10.3390/ma7096105
Zhang W, Zhang Y, Lu CH, Deng YL (2012) Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J Mater Chem 22(23):11642–11650
Zhang LN, Ruan D, Zhou JP (2001) Structure and properties of regenerated cellulose films prepared from cotton linters in NaOH/Urea aqueous solution. Ind Eng Chem Res 40(25):5923–5928
Kataoka Y, Kondo T (1998) FT-IR microscopic analysis of changing cellulose crystalline structure during wood cell wall formation. Macromolecules 31(3):760–764. https://doi.org/10.1021/ma970768c
Zhou ZH, Zhang XX, Lu CH, Lan LD, Yuan GP (2014) Polyaniline-decorated cellulose aerogel nanocomposite with strong interfacial adhesion and enhanced photocatalytic activity. RSC Adv 4(18):8966–8972
Xiao S, Gao R, Lu Y, Li J, Sun Q (2015) Fabrication and characterization of nanofibrillated cellulose and its aerogels from natural pine needles. Carbohyd Polym 119:202–209. https://doi.org/10.1016/j.carbpol.2014.11.041
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729. https://doi.org/10.1021/ie801542g
Zhang SQ, Wang J, Shen J, Deng ZS, Lai ZQ, Zhou B, Attia SM, Chen LY (1999) The investigation of the adsorption character of carbon aerogels. Nanostruct Mater 11(3):375–381. https://doi.org/10.1016/S0965-9773(99)00054-9
Zhang XT, Jing SS, Chen ZH, Zhong LX, Liu QZ, Peng XW, Sun RC (2017) Fabricating 3D hierarchical porous TiO2 and SiO2 with high specific surface area by using nanofibril-interconnected cellulose aerogel as a new biotemplate. Ind Crop Prod 109:790–802
Zhao HB, Zhou XC, Fu ZB, Mi R, Wang CY (2017) Freestanding monolithic Ni aerogel with large surface areas from cellulose aerogel templates. Mater Lett 196:296–299
Kandola BK, Horrocks AR, Price D, Coleman GV (1996) Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J Macromol Sci R M C 36(4):721–794
Wu JW, Sung WF, Chu HS (1999) Thermal conductivity of polyurethane foams. Int J Heat Mass Tran 42(12):2211–2217
Gu JW, Zhang QY, Zhang JP, Wang WW (2010) Studies on the preparation of polystyrene thermal conductivity composites. Polym-Plast Technol 49(13):1385–1389
Golovanevskii VA (1993) Prediction of the thermal-conductivity of oriented fiberglass plastics. Mech Compos Mater 29(3):289–293
Flaifel MH, Ahmad SH, Hassan A, Bahri S, Tarawneh MA, Yu LJ (2013) Thermal conductivity and dynamic mechanical analysis of NiZn ferrite nanoparticles filled thermoplastic natural rubber nanocomposite. Compos Part B-Eng 52:334–339
Acknowledgements
The financial support by “Recep Tayyip Erdoğan University Scientific Research Project Council (Project No: FBA-2016-550)” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaya, M., Tabak, A. Recycling of an Agricultural Bio-waste as a Novel Cellulose Aerogel: A Green Chemistry Study. J Polym Environ 28, 323–330 (2020). https://doi.org/10.1007/s10924-019-01609-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01609-6