Abstract
The property profile of thin thermoplastic starch (TPS)/poly(butylene succinate) (PBS) films was investigated and the potential improvement, which can be achieved due to the utilization of hydrophilic/hydrophobic compatibilizer systems, was assessed. The evaluation in terms of morphology exhibited a very good TPS dispersion (under optimized processing conditions) within the polyester matrix, while an average particle size of 1.5 µm was obtained. Two different raw material approaches were applied for the preparation of the compatibilizers: (a) native corn starch and (b) destructurized TPS. In the course of the compounding process 0.5 and 1.0 wt% of the two compatibilizer systems (a) and (b) were added. In comparison, the addition of the TPS-based compatibilizer resulted in improved incorporation of TPS within the polyester matrix, which was accompanied by higher tensile strength and tear resistance. Explanations for this observation could be that pre-plasticized starch provides a larger reaction surface and enables better homogenization during the course of compounding. In contrast, for native starch the reaction only can take place at the granule surface and thus, the compatibilization was less efficient. The outcome of this investigation is a compostable film material with high bio-based content, which exhibits great potential for single-use, light-weight packaging applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past two decades thermoplastic starch (TPS)-based polyester compounds have been extensively investigated since starch is used in polymer formulations for three major purposes: (1) cost-reduction, (2) increase in biodegradation speed, and (3) increase in bio-based content [1]. Different strategies for the compatibilization of TPS-polyester mixtures have been developed and characterized such as carboxylic acid- [2, 3], maleic anhydride- [4,5,6,7] and glycidyl methacrylate-based systems [8], as well as binary/ternary blend formulations [9,10,11]. Many approaches have focused on the optimization of compound formulations consisting of starch and petroleum-based polybutylene adipate-co-terephthalate (PBAT), because the polyester exhibits a market competitive price and is readily available. Polyesters, which are (completely or only partially) obtained from natural building blocks, have attracted attention in recent years because of their renewable, bio-based character. Among them, poly(butylene succinate) (PBS), which is a biodegradable polyester (already commercially available) with a bio-based carbon content between 35 and 50% (which will further increase in the near future), is of great interest [12,13,14]. Therefore, PBS exhibits potential as a compound-partner in combination with TPS, intended for the preparation of cost-effective and sustainable film materials. As in the case of TPS/PBAT mixtures, the production of high-quality compounds consisting of TPS and PBS is difficult, because the miscibility of the two polymers is restricted due to differences in viscosity and interfacial tension [15,16,17].
In this study, two approaches are addressed with respect to the compatibilization of TPS/PBS compounds during reactive extrusion: the preparation of a compatibilizer system based on (1) native starch, and (2) pre-plasticized/destructurized starch, i.e. TPS (Fig. 1).
To generate a better understanding for TPS/PBS mixtures as potential future compounding partners, three hypotheses are stated: (1) The two mentioned reactive compatibilizer approaches enable different interactions with starch (in TPS) and polyester. In case of the native starch-based compatibilizer the reaction can only take place at the granule surface, which restricts the compatibilization-efficiency as well as the production of thin film materials with high surface quality (as the starch granules to some extent are likely to act as defects inside of the compound matrix). The already plasticized TPS-based system is expected to enable a better homogenization during compounding, providing a larger interaction surface since the reaction between starch and PBS can occur throughout the compatibilizer-system. (2) The addition of the TPS-based compatibilizer improves the incorporation of TPS within the PBS matrix. (3) The mechanical properties of film materials consisting of TPS and PBS are influenced by the compatibilizer concentration. Particularly, the mechanical strength is expected to improve when the compatibilizer is added. Finally, the disintegration of the produced TPS/PBS films under home composting conditions is reported.
The overall goal of this research is the development of a flexible plastic material, which is suitable to replace single-use, petro-based and persistent packaging. To meet the market-requirements, the production of thin film materials (layer thicknesses of 50 µm and lower) must be possible, whereby a preferably high elongation as well as a mechanical strength of at least 30 MPa, and tear resistance of 50 N/mm, are considered as state of the art [2, 18, 19]. The utilization of TPS in plastic-packaging usually introduces hydrophilic characteristics due to the hydrophilic functionalities that are present in starch [19]. The influence of compatibilization on the water vapor barrier is addressed in the present investigation. A full degradation under composting conditions is targeted, since a controlled, separate waste collection cannot be granted in the case of light-weight, single-use packaging.
Experimental Part
Materials and Methods
TPS Extrusion
To obtain TPS, native corn starch (Agrana Stärke, Austria) was destructurized within a co-rotating twin screw extruder (27D, Theysohn, Switzerland) at a screw speed of 200 rpm. Glycerol (13 wt%, Brenntag, Austria), and stearic acid (2.0 wt%, Sigma Aldrich) were added as plasticizer and processing aid (applied temperature profile see Fig. 2).
Compatibilizer Preparation
The starch-based as well as the TPS-based compatibilizer were prepared in accordance with the methodology described by Suchao-in, Koombhongse & Chirachanchai [16], whereat reaction time, batch size and reactant ratio were slightly adapted. Twenty grams of PBS (film type, MCPP Germany GmbH) were dissolved in chloroform (Honeywell™, Germany) in a three-neck round-bottom 1000 ml flask. As soon as PBS had fully dissolved the starch (100 g, pre-dried) [or in case of the TPS-based compatibilizer the ground TPS powder (120 g)] was added to the solution. Afterwards, the mixture was stirred for 2 h, before the flask was flushed with nitrogen and N,N′-dicyclohexylcarbodiimide (DCC, 30 g) (Sigma Aldrich, Germany) was added. After 48 h of reaction time under constant stirring at room temperature the obtained suspension was centrifuged to remove residual, unreacted starch/TPS. The supernatant was further treated with acetone (Sigma Aldrich, Germany) to precipitate the reaction product, which was then purified by repeated washing with ethanol (Sigma Aldrich, Germany). The reaction product was dried at 40 °C for 48 h. The compatibilizer preparation was also conducted without DCC (control), in order to show the absence of an interaction between starch and PBS without the reagent. The stability of the suspended reaction products in chloroform (10 mg compatibilizer suspended in 2 ml chloroform) was taken as a qualitative measure for the compatibilizer efficiency [5].
Compounding
Compounding of TPS with PBS (1:1) was conducted via extrusion using a co-rotating twin screw extruder (27D, Theysohn, Switzerland). Initially, four different screw speeds (100, 200, 300 and 400 rpm) were applied to evaluate the optimum processing range. The incorporation of TPS within the polyester matrix was investigated by means of iodine testing. For this purpose, the prepared compound granules were exposed to iodine/potassium-iodine solution (Lugol’s solution diluted 1 wt%, Sigma Aldrich, Germany) and the evolving color effects were used to differentiate between a sufficient and a non-sufficient incorporation of TPS inside the polyester matrix (solution colored → non-sufficient incorporation; only granules colored/solution yellow → incorporation and fine distribution). The addition of the two compatibilizer systems at concentrations of 0.5 and 1.0 wt% (with respect to the TPS share) was conducted via a micro-dosing unit (Brabender, Germany) at optimum screw speed only (Table 1).
Flat Film Preparation
Flat films were prepared using a small-scale flat film extrusion line (Optical Control Systems, Germany) at 160 °C, 20 rpm, and a haul off speed of 3.5 m/min. A film thickness between 40 and 50 µm was obtained (average thickness measured over 10 points, thickness testing instrument Kaefer, Germany).
Characterization
Scanning Electron Microscopy (SEM)
The compounds were fractured in liquid nitrogen and treated with hydrochloric acid to remove the dispersed starch particles. Prior to microscopic analysis the samples were gold-coated using a sputter coater (JEOL®, JCM-1200 Fine Coater). The particle surface was then investigated via an electron microscope (JEOL®, JCM 5000 NeoScope, 10 kV, high-vacuum mode, China), which was equipped with a secondary electron detector. The film surfaces were investigated by means of a microscope camera (DigiMicro, dnt, Germany).
Fourier Transform Infrared Spectroscopy (FTIR)
Infrared spectra were recorded using a FTIR spectrometer (Bruker®, Alpha Sample Compartment RT-DLaTGS, Austria) in attenuated total reflectance (ATR) mode, at a spectral resolution of 2 cm−1 (wavenumber range 400–4000 cm−1). The shown FTIR spectra are an average of three independent measurements each, with vector normalization and baseline correction (rubberband method), computed via OPUS software, version 7.5.
Differential Scanning Calorimetry (DSC)
The evaluation of the thermal compound characteristics happened via differential scanning calorimetry, using a DSC 214 Polyma (Netzsch, Germany). Measurements were conducted under a constant nitrogen flow of 50 ml/min. The temperature cycle involved a heating up to 160 °C, with a subsequent cooling to − 50 °C. The measurements were done in triplicate. The determination of relaxation processes in TPS associated with the glass transition were monitored via the glass transition temperature (Tg, point of inflection). For the determination of the required transition energy different heating rates (10, 20, 30 and 40 K/min) were applied. A linear relationship was constituted with ln β (heating rate) and the reciprocal glass transition temperature (1/Tg). The calculation of the activation energy Ea was conducted from the slope of the obtained ln β versus (1/Tg) graphs (Eq. 1, R represents the gas constant) [20,21,22,23]:
The change in heat capacity Δcp was taken as an indicator for the molecular arrangement present in TPS, which directly relates to the presence of disordered, amorphous structures [24, 25].
Size Exclusion Chromatography (SEC)
To identify possible influences due to molecular weight related changes, the weight average molecular weight (Mw) was determined with a Thermo Fisher Scientific chromatograph (Dionex Ultimate 3000, Austria). For the characterization of the polyester-phase the chromatograph was equipped with a styrene–divinylbenzene copolymer column system (1000–1,000,000 Å, chloroform, 0.6 ml/min), and a refractive index detector (Refractomax 520), with poly(methyl methacrylate) standards used for calibration purposes. The determination of the TPS-phase was conducted with a polyhydroxymethacrylate copolymer column system (100–30,000 Å, sodium nitrate, 0.7 ml/min). For sample detection a refractive index detector (RI-101) was used. Monodisperse pullulan standards were applied for calibration purposes. Measurements were conducted in triplicate.
Mechanical Material Properties
The determination of the mechanical film properties was conducted on a Zwick Roell (Germany) universal testing machine. Measurement and sample geometry followed ÖNORM EN ISO 527-3 standard, with a clamp distance of 105 mm. A cross-head speed of 100 mm/min was applied for the measurement of stress and elongation (measured via mechanical extensometer). For each film sample at least five specimens were tested (in machine direction = longitudinal production direction).
Tear resistance was determined according to ÖNORM ISO 34-1:2005. Tests were carried out using an angle test sample (Graves) with notch (method B). For each experiment five samples were tested, using a test speed of 100 mm/min. Preload was set to 0.2 N, and a 2 kN load cell was used. The clamp distance equaled 60 mm.
Water Vapor Barrier
The water vapor transmission rate (WVTR) was obtained using a water vapor transmission tester (W3/031, Labthink, China). The tests were performed at 38 °C and 90% RH following ASTM E96. The tested area was circular shaped with a diameter of 74 mm. Prior to the test, a 1 h preheating step was performed. 10 weighting cycles were performed per sample in 30 min intervals.
Disintegration Testing
The flat films were weighed, cut to a size of 10 × 15 cm and placed on a mesh, which was then fixed within a wooden frame by using staples [26]. The samples were buried in a mixture consisting of soil and fresh compost (1:1). The temperature was kept constant at 25 °C (drying closet type Memmert, Germany). After 10, 20 and 30 days the samples were removed and evaluated with respect to their optical appearance (photo documentation). At the end of the test, the remaining particles with a particle size bigger than 2 mm were determined gravimetrically. Samples and the utilized soil/compost mixtures were sieved through a 2 mm mesh. Remaining soil particles were removed and the film-fragments were conditioned in a drying chamber prior to weighing.
Statistical Evaluation
Analysis of variance (ANOVA) was conducted, followed by Tukey multiple comparison post-hoc test. Mean differences were tested for statistical significance (p < 0.05, Minitab 17.0).
Results and Discussion
Compounding
Optimizing the Process (Without Compatibilizer)
The TPS incorporation within the polyester matrix, the morphological appearance, and particle sizes of the compounded granules were evaluated via extrusion trials at 100, 200, 300 and 400 rpm (Table 2).
It is visible that the incorporation of TPS inside of the polyester matrix has improved, at higher screw speeds. At 100 and 200 rpm the compound granules retained a light color and the iodine solution was dark blue colored. The dark blue color is evidence for a complete separation of starch from the polyester matrix (into the test solution), which indicates an insufficient TPS inclusion. At 400 rpm the obtained particle sizes were small, with the compounds remaining stable when in contact with the test solution (Fig. 3).
It was also observed that a more uniform particle size distribution was achieved at elevated screw speeds (Fig. 4).
Film materials produced with compounds prepared at low screw speed (100 and 200 rpm) exhibited instable processability, with process interruptions due to reduced melt strength, along with a noticeable film surface roughness (Fig. 5). In case of the compound materials produced at 300 rpm as a well as 400 rpm the surface quality overall improved, with the quality being still better at 400 rpm than at 300 rpm. Consequently, 400 rpm was taken as the standard processing parameter for the preparation of the TPS/PBS compounds in the present study.
Incorporation of Compatibilizers During Compounding
Structural Compatibilizer Characterization
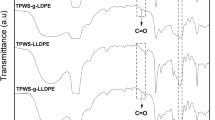
Figure 6 shows FTIR spectra of the prepared native starch- and TPS-based (i.e. starch-PBS, TPS-PBS) compatibilizer systems. Additionally, the preparation of the compatibilizers was conducted without the reagent DCC (starch without DCC: only adsorbed water visible at 1650 cm−1, TPS without DCC: adsorbed water + stearic acid visible at 1650 cm−1 and 1720 cm−1, respectively). The recorded spectra demonstrate that it′s not possible to achieve a chemical link between starch and PBS without DCC. Without DCC, the PBS is removed due to precipitation and the applied washing steps conducted during product purification. In case of the esterification with DCC a clearly defined carbonyl peak (1710 cm−1) is visible, which is representative for an interaction between starch/TPS and PBS [27, 28].
Further, the formation of hydrogen bonds (peak intensity changes at 3300 cm−1) was reduced for the esterified systems starch-PBS and for TPS-PBS.
The solubility in chloroform was influenced due to the functionalization with DCC and PBS [5]. The control samples (without DCC) precipitated immediately, while the DCC modified samples enabled a formation of a suspension with variable stability (TPS-PBS → stable, Starch-PBS → incomplete precipitation). Overall, the formation of compatibilizers with reduced hydrophilicity was verified (TPS-PBS completely, Starch-PBS partially). A method for the quantitative evaluation of the grafting efficiency is currently under development.
Compound Morphology
Table 3 shows the fractured compound surfaces after removal of the TPS phase via hydrochloric acid. The microscopic images confirm the uniform and fine distribution of TPS inside of the polyester matrix across all samples. Thus, side effects due to significant variations in particle size can be excluded.
Compound Characterization
Differential Scanning Calorimetry
Glass transition temperatures for the TPS phase within the compounded materials, and activation energies are shown in Table 4 (Figure S1).
Comparatively, a shift in Tg (endothermic hump around 70 °C) with increasing heating rate towards higher temperatures can be deduced from a demobilization of molecules due to stress [29]. Figure 7 demonstrates the investigated activation energy plots.
Apparently, slope changes and thus changes in activation energy were achieved through the addition of the compatibilizer (activation energy increased for samples 10St, 05TPS and 10TPS). This effect can be deduced from a reduced molecule mobility (starch immobilization because of increased interaction with the polyester matrix) which was caused due to an enhanced molecular entanglement, and is accompanied by changes in free volume [30]. Furthermore, it is likely that glycerol-esters (as a compatibilizer side-product in the case of 05TPS and 10TPS) were able to form interactions between starch and the polyester, and thus acted like small anchor points, which further enhanced the interaction between the two phases [31].
Table 5 shows the heat capacity changes, which were observed for the investigated compound materials. Δcp was used as indicator for the structural arrangement within TPS. The heat capacity step can be directly correlated with the presence of disordered (amorphous) regions. The observed increase in Δcp (control → 10TPS) leads to the conclusion that the compatibilizer addition promoted the growth of amorphous TPS-structures. The effect was more pronounced (statistically significant) at low heating rates (10 and 20 K/min) [32,33,34,35,36].
Size Exclusion Chromatography
The average molecular weights for TPS and polyester phases are listed in Table 6 (Figure S2).
Since the polyester constitutes the continuous phase in the investigated TPS/PBS systems (verified via scanning electron microscopy), the polyester has to fulfil essential functions such as the transmittance and distribution of stress to the dispersed particles as well as the suppression of cracks formed as a response to mechanical stress. According to the obtained results the polyester phases weight average molecular weight did not change with added compatibilizers. Thus, effects that could potentially evolve due to a matrix weakening because of molecular weight changes can be excluded [37]. In contrast, the starch phases have shown molecular weight changes (significant at the highest compatibilizer concentrations 10St as well as 10TPS). These findings correlate with torque data, as observed during the compounding experiments. According to literature there are two main mechanisms that could be responsible for the described observations: (1) the addition of the compatibilizer led to a reduced interaction between TPS and water (the used TPS contains water that potentially acts as a plasticizer), which has further provoked a slight increase in viscosity; [38] (2) the formation of clusters, which exhibited a higher susceptibility to shear-induced degradation [39, 40].
Film Characterization
Mechanical Film Characteristics
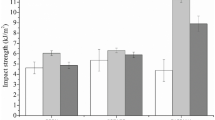
Results (Figs. 8 and S3) show a significant increase in tensile strength and tear resistance for the TPS-based compatibilizer at both concentrations 0.5 and 1.0 wt%. In case of the starch-based compatibilizer a significant increase of tensile strength and tear resistance was only visible at 1.0 wt%. The TPS dispersion has proven to be comparable for the investigated formulations and thus, cannot be the reason for the observed changes in mechanical performance (on µm scale). The observed reduction in TPS-average molecular weight has probably led to changes in viscosity, which induced an enhanced miscibility of a defined particle fraction at sub-micrometer scale (not visible on the obtained SEM micrographs). This subject needs to be further addressed. Besides that, it has been observed before that the utilization of hydrophilic/hydrophobic compabilizers in 2-phase systems provokes an improvement in interfacial adhesion, which furthermore leads to higher tensile strength. Here, literature is referring to two mechanisms: (1) changes in the maximum bearable load due to changes in adhesion between matrix and dispersed particle [41] and (2) a restriction in molecule mobility due to the formation of a structural network [42]. In this investigation, the PBS-side chains, which are present in the compatibilizer-systems, are expected to enhance the molecular entanglement within the compound system.
Water Vapor Barrier
Water vapor permeability (WVP) and water vapor transmission rates (WVTR) are listed in Table 7.
No significant changes were found for mass gain and WVTR. Apparently, the WVP only slightly increased in the case of 10TPS. In principle, the utilization of TPS in polyester formulations is reported to increase the WVP characteristics [43]. Within the compound-material even small alterations in film thickness could have led to the observed deviations in WVP. As reported in the literature, the thickness influences the structural arrangement, which could furthermore affect the mass transfer across the film [44]. Another explanation could be the improved incorporation of TPS within the polyester matrix, due to which the hydrophilic characteristics of the starch component became more dominant. Furthermore, it is likely that the compatibilization and the related interaction with the polyester has affected the formation of a close intermolecular arrangement in TPS, which additionally facilitated the WVP. Literature confirms that the introduction of hydrophobic components in compound formulations does not necessarily result in a reduced WVP. The WVP depends on versatile factors such as the formation of pores and channels, which furthermore affect the flow profile within the materials structure and thus, the permeability characteristics [45].
Disintegration
Table 8 shows the optical appearance of the various film types as a function of the disintegration-time.
The images demonstrate the disintegration of the film materials under home composting conditions. After 10 days visible cracks appeared on the surfaces of all samples, which subsequently continued with a transformation into holes (after 20 days of composting). Figure 9 shows the quantitative evaluation of the film material disintegration via (gravimetric) sieving.
Due to the beginning destruction and the associated increase in attack surface the microbial activity and hence, the disintegration started to accelerate after 20 days composting time. Figure 10 shows the formation of cracks and microbial attack, as processes appearing during composting.
The disintegration of the control sample proceeded slightly faster. To verify the significance of this observation further investigations are required.
Conclusions
The production of thin film materials based on bioplastics requires a defined polymer compound quality. Huge starch particles affect the melt strength and thus, the continuous film production as well as the film materials surface quality. Depending on the size of the dispersed particles the produced films exhibit a noticeable roughness. The present work demonstrates that the preparation of TPS/PBS compounds with a fine dispersion of starch inside of the polyester matrix is only possible under accelerated shearing conditions, which can be achieved at an increased extrusion screw speed. The synthesis of compatibilizers was conducted on the basis of TPS and native starch. Solubility tests demonstrated that the TPS-based compatibilizer formed a stable suspension in contact with chloroform. In case of the native starch-based compatibilizer only a limited stability was given. Destructurized starch in the form of TPS provided a larger interaction surface (amylose/amylopectin—PBS, and probably also glycerol—PBS) and enabled a better homogenization during the course of compounding. This is why the TPS-based compatibilizer facilitated a significant increase in tensile strength and tear resistance, while the utilization of the starch-based variant only resulted in minor effects. The investigation resulted in a film material with improved mechanical strength, which exhibits a significant potential for single-use, light-weight packaging applications. The applicability of a controlled, separate waste collection for this purpose is doubtful and hence, the compostability under ambient conditions is seen as a benefit. Future investigations will focus on a detailed evaluation and optimization of barrier properties of TPS-based film materials, along with an evaluation of mechanisms that are involved during biodegradation. As the utilization of starch in packaging always induces unwanted opacity instead of full transparency, a potential increase in transparency might be achievable with raw material functionalization, a subject important in follow-up research activities. Furthermore, the effects of varying starch contents and elevated compatibilizer concentrations are subject of ongoing research.
References
Bastioli C (2001) Global status of the production of biobased packaging materials. Starch 53:368–371
Olivato JB et al (2013) Starch/polyester films: simultaneous optimisation of the properties for the production of biodegradable plastic bags. Polímeros 23:32–36
Wang N et al (2007) Influence of citric acid on the properties of glycerol-plasticized dry starch (DTPS) and DTPS/poly(lactic acid) blends. Starch 59(9):409–417
Kalambur S, Rizvi SSH (2006) An overview of starch-based plastic blends from reactive extrusion. J Plast Film Sheeting 22(1):39–58
Ma P et al (2012) Tailoring the morphology and properties of poly(lactic acid)/poly(ethylene)-co-(vinyl acetate)/starch blends via reactive compatibilization. Polym Int 61(8):1284–1293
Wang N, Yu J, Ma X (2007) Preparation and characterization of thermoplastic starch/PLA blends by one-step reactive extrusion. Polym Int 56(11):1440–1447
Maliger RB et al (2006) Compatibilization of starch–polyester blends using reactive extrusion. Polym Eng Sci 46(3):248–263
Al-Itry R, Lamnawar K, Maazouz A (2012) Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym Degrad Stab 97(10):1898–1914
Mittal V, Akhtar T, Matsko N (2015) Mechanical, thermal, rheological and morphological properties of binary and ternary blends of PLA. TPS and PCL. Macromol Mater Eng 300(4):423–435
Shirai MA et al (2013) Thermoplastic starch/polyester films: effects of extrusion process and poly (lactic acid) addition. Mater Sci Eng C 33(7):4112–4117
Averous L, Dole P, Fringant C (2000) Properties of thermoplastic blends: starch–polycaprolactone. Polymer 41:4157–4167
Liu L et al (2009) Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym Degrad Stab 94(1):90–94
Reddy MM et al (2013) Biobased plastics and bionanocomposites: Current status and future opportunities. Prog Polym Sci 38(10–11):1653–1689
Aeschelmann F, Carus M (2017) Biobased building blocks and polymers. Michael Carus (V.i.S.d.P.)
Zeng JB et al (2011) Bio-based blends of starch and poly(butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohyd Polym 83:762–768
Kanitporn SI, Koombhongse P, Chirachanchai S (2014) Starch grafted poly(butylene succinate) via conjugating reaction and its role on enhancing the compatibility. Carbohydr Polym 102:95–102
Li J et al (2013) Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch 65(9–10):831–839
van den Oever M, Molenveld K (2017) Replacing fossil based plastic performance products by bio-based plastic products-technical feasibility. New Biotechnol 37(Pt A):48–59
Briassoulis D, Giannoulis A (2018) Evaluation of the functionality of bio-based food packaging films. Polym Testing 69:39–51
Siengchin S (2015) Thermomechanical analysis and processing of polymer blends. In: Characterization of polymer blends: miscibility, morphology and interfaces. Wiley, Weinheim, pp 393–414
Fahrngruber B et al (2017) Malic acid: a novel processing aid for thermoplastic starch/poly(butylene adipate-co-terephthalate) compounding and blown film extrusion. J Appl Polym Sci 134(48):45539
Sharma D, MacDonald JC, Iannacchione GS (2006) Thermodynamics of activated phase transitions of 8CB: DSC and MC calorimetry. J Phys Chem B 110:16679–16684
Elabbar AA (2018) Effect of thermal history on crystallization and glass transition in Se and Se90Te10 chalcogenide glasses. Chalcogenide Lett 15:515–521
Zhang Y, Rempel C, Liu Q (2014) Thermoplastic starch processing and characteristics—a review. Crit Rev Food Sci Nutr 54(10):1353–1370
Hancock BC et al (1998) A pragmatic test of a simple calorimetric method for determining the fragility of some amorphous pharmaceutical materials. Pharm Res 15(5):762–767
Seligra PG et al (2016) Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr Polym 138:66–74
Cuevas-Carballo ZB, Duarte-Aranda S, Canché-Escamilla G (2017) Properties and biodegradability of thermoplastic starch obtained from granular starches grafted with polycaprolactone. Int J Polym Sci 2017:1–13
Zuo Y et al (2019) Preparation and characterization of hydrophobically grafted starches by in situ solid phase polymerization. Polymers (Basel) 11(1):72
Abu-Bakar AS, Moinuddin KAM (2012) Effects of variation in heating rate, sample mass and nitrogen flow on chemical kinetics for pyrolysis. In: 18th Australasian fluid mechanics conference, Launceston, Australia
Khouloud JM, Sabu MC (2017) Clay-polymer nanocomposites. Elsevier, Cambridge
Bouthegourd E et al (2013) Size of the cooperative rearranging regions vs. fragility in complex glassy systems: influence of the structure and the molecular interactions. Physica B 425:83–89
Tajuddin S et al (2011) Rheological properties of thermoplastic starch studied by multipass rheometer. Carbohydr Polym 83(2):914–919
Ehrenstein GW, Riedel G, Trawiel P (2004) Thermal analysis of plastics—theory and practice. Carl Hanser Verlag, München
Mano JF, Koniarova D, Reos RL (2003) Thermal properties of thermoplastic starch/ synthetic polymer blends with potential biomedical applicability. J Mater Sci Mater Med 14:127–135
Biliaderis CG, Lazaridou A, Arvanitoyannis I (1999) Glass transition and physical properties of polyol-plasticised pullulan–starch blends at low moisture. Carbohydr Polym 40:29–47
Sreenivasan VS et al (2015) Dynamic mechanical and thermo-gravimetric analysis of Sansevieria cylindrica/polyester composite: effect of fiber length, fiber loading and chemical treatment. Composites B 69:76–86
Kulshreshtha AK, Vasile C (2002) Handbook of polymer blends and composites, vol 2. Shawbury, Rapra Technology Limited
Barbosa SE et al (2017) Starch-based materials in food packaging: processing, characterization and applications. Elsevier, New York
Liu W-C, Halley PJ, Gilbert RG (2010) Mechanism of degradation of starch, a highly branched polymer, during extrusion. Macromolecules 43(6):2855–2864
Carvalho AJFZ, Curvelo AAS, Gandini A (2003) Size exclusion chromatography characterization of thermoplastic starch composites 1. Influence of plasticizer and fibre content. Polym Degrad Stab 79:133–138
Willett JL et al (1998) Properties of starch-graft-poly(glycidyl methacrylate)–PHBV composites. J Appl Polym Sci 70:1121–1127
Mohanty S, Nayak SK (2011) Biodegradable nanocomposites of poly (butylene adipate-co-terephthalate) (PBAT) with organically modified nanoclays. Int J Plast Technol 14(2):192–212
da Silva NMC et al (2017) PBAT/TPS composite films reinforced with starch nanoparticles produced by ultrasound. Int J Polym Sci 2017:1–10
Patricia Miranda S et al (2004) Water vapor permeability and mechanical properties of chitosan composite films. J Chil Chem Soc 49(2):173–178
Dashipour A et al (2014) Physical, antioxidant and antimicrobial characteristics of carboxymethyl cellulose edible film cooperated with clove essential oil. Zahedan J Res Med Sci 16(8):34–42
Acknowledgements
The authors would like to thank the Austrian Research Promotion Agency (FFG, Project Number: 854577) for the financial support. Furthermore, the authors wish to thank Agrana Stärke for generously supplying the required starch raw material.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fahrngruber, B., Fortea-Verdejo, M., Wimmer, R. et al. Starch/Poly(butylene succinate) Compatibilizers: Effect of Different Reaction-Approaches on the Properties of Thermoplastic Starch-Based Compostable Films. J Polym Environ 28, 257–270 (2020). https://doi.org/10.1007/s10924-019-01601-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01601-0