Abstract
In the present study, an adsorbent named chitosan/rice husk ash/nano-γ alumina was prepared and used for the removal of lead ions from aqueous solutions. It was characterized by FESEM, FTIR, XRD, and BET. The important and interactive effects of some considered parameters, such as the initial lead ion concentration, pH, adsorbent mass and contact time, were estimated and optimized by using the central composite design (CCD). The Maximum adsorption efficiency of lead ions under optimum conditions, including the pH of 5, adsorbent mass of 0.01 g, the initial lead ion concentration of 30 ppm, and the contact time of 105 min, was achieved to be 90.98%. The adsorption kinetic was consistent with the pseudo-second-order model (R2 = 0.9967). The lead adsorption isotherms were well fitted to the Langmuir model, indicating the maximum adsorption capacity of 181.82 mg g−1. Thermodynamic parameters (R2: 0.9200, ΔH°: − 22871.81 kJ mol−1, ΔG°: − 10.63 kJ mol−1, and ΔS°: 118.37 kJ mol−1 K−1) were calculated and indicating adsorption process was exothermic and spontaneous in the temperature range of 283.15–313.15 k. The maintenance above 70% capacity of the adsorbent was observed by the regeneration study of chitosan/rice husk ash/nano-γ alumina after six cycles. Comparison of adsorption efficiency of the studied adsorbent and other adsorbents in the removal of lead ions was also done in this paper. Consequently, the excellent properties of the presented nanocomposite could make it an ideal adsorbent for the removal of lead ions from aqueous solutions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, with the expansion of the economy, the number of heavy metal ions, such as cadmium, chromium, cobalt, lead, and mercury, that are applied in various industrial activities is growing rapidly in chemical manufacturing. Heavy metal contamination is an extremely serious environmental problem around the world [1,2,3,4,5,6]. The non-biodegradable, heavy metal ions can enter the human body by the food chain when they gather in animals and crops through the soil, water, and air. The important damage would emerge once they are swallowed by human [7]. Among these heavy metals, lead is vastly utilized in the industrial processes including electroplating, painting and dying, explosive manufacturing, and lead batteries manufacturing [8]. Meanwhile, a large amount of sewage consists of lead ions are discharged into the environment [9]. It has been shown that lead ions can have successive toxicity, leading to such serious illnesses such as anemia, sterility, hypertension, learning disabilities, abortion, kidney harm, and mental retardation [10, 11]. The permissible amount of lead in drinking water is 0.010 ppm [12]. Hence, it is compulsory to find a desirable material to remove and decrease lead ions to provide a safe living environment for the human.
To date, various methods have been studied to eliminate heavy metals, such as ion exchange, electrolysis, chemical precipitation, flocculation, reverse osmosis, membrane separation, and adsorption [13,14,15,16,17]. Among them, adsorption is more efficient, in comparison with others, owing to its simplicity, sludge free operation, ease in handling, availability of different adsorbents, and the regenerative and more impressive removal of heavy metals at lower concentration levels [18,19,20,21,22,23]. Therefore, it is apparently important to choose appropriate adsorbents [20, 24,25,26,27,28,29,30,31,32,33,34,35,36].
In recent years, different kinds of adsorbents have been explored for the removal of lead ions from wastewater; these include Zeolites [37], Kaoline [38], Manganese oxides [39], activated carbon [40], ion exchange resins [41], and various biosorbents [9, 42]. Lately, the application of biomaterials for the environmental clean-up has received much attention. For instance, chitosan based material has been the focus of much research owing to its desirable characteristics and versatility in treating different contamination [22, 43,44,45,46,47,48,49,50,51,52,53].
Chitosan is a plentiful biopolymer produced from alkaline N-deacetylation of chitin [54]; it is regarded as an eco-friendly adsorbent with low cost, non-toxicity, biodegradability, biocompatibility, and large accessibility in nature [55]. Chitosan is able to make complexes with nearly all metal ions due to the attendance of massive amino and hydroxyl functional groups. So, it is vastly utilized for removal of heavy metal ions. Nevertheless, chitosan has serious disadvantages in terms of mechanical strength and solubility in acidic media, acidic media, restricting its performance.
Lately, attentions have been focused on the application of nano particles to improve the removal efficiency of pollutants [56]. This is because nanoadsorbents provide a high surface-to-volume ratio and a great number of active sites for the interaction with adsorbate species. Some nano particles including alumina, iron-oxide, zirconia, jacobsite, maghemite, nickel oxide, copper oxide, and carbon nanotubes have been employed for the removal of contaminants from aqueous solution [57]. Alumina based adsorbent has some profits such as stability, high surface area, possible reuse, short adsorption contact time, and high mechanical characteristics [58].
In this project, a novel nanocomposite, the compound of chitosan, rice husk ash and nano-γ alumina was synthesized for removal of lead ions in aqueous solutions. Rice husk is an agricultural waste with globular structure; its main components are silica and carbon [59, 60]. Rice husk was used to anchor chitosan, and nano-γ alumina was expected to make good binding sites for them, as well as being employed as a direct adsorbent for removal of metal ions. In fact, rice husk provides a large surface area for combining chitosan and nano-γ alumina while impeding particle accumulation to enhance the adsorption capacity for the removal of pollutants. In fact, this is the first time that CTS/RHA/γ-Al2O3 composite used as an adsorbent for removal of pollutants and according to the results, this nano adsorbent can remove lead ions easily with a good adsorption capacity.
Consuming a long time and requiring a large number of experiments are disadvantages of the conventional approach for optimization of variables [61]. Central composite design (CCD) under response surface methodology (RSM) is the most utilized way that can optimize the efficient variables, as well analyze the interaction between them without suffering the expressed difficulties. In this work, the influence of various parameters such as pH of lead solutions, the initial concentration, adsorbent dosage, and contact time on the removal of lead ions was investigated by using central composite design (CCD) [62].
In continuation of our previous works in removal [63,64,65,66] and to the best of acknowledging, no reports on the adsorption of lead ions by the composite of chitosan with rice husk ash and nano-γ alumina can be found. The article has been organized in some sections, (1) synthesis of chitosan/rice husk ash/nano-γ alumina and its characterization by FTIR, FESEM, XRD, and BET; (2) optimization by CCD combined with RSM; (3) assessment of the influences of four independent variables including pH of lead solutions, the initial concentration the adsorbent dosage, and contact time, and their reciprocal actions on removal of lead ions, and (4) to study adsorption isotherm and kinetic, and thermodynamic models.
Experimental
Materials
Medium molecular weight chitosan and γ-Al2O3 powder with average particle size were purchased from Sigma-Aldrich. Rice husk was achieved from a local rice mill. The chemicals applied in this study, such as sulfuric acid, acetic acid, hydrochloric acid, sodium hydroxide, were supplied by Merck. Double distilled water was utilized to prepare all the solutions.
Preparation of rice husk ash
Rice husk was comprehensively washed well with distilled water and dried in a hot air oven at 110 °C for 24 h. The dried sample (50 g) was treated with the mixture of 10% hydrochloric acid and sulfuric acid (1 L) for 2 h. The rice husk was then rinsed several times to eliminate the remaining acid content and stirred for 1 h to neutralize. After that, the sample was kept in an oven overnight, dried at 60 °C and ground. Subsequently, it was calcined at 950 °C for 2 h. Finally, the calcined product of the husk was sieved to be 40–175 µm mesh.
Preparation of Chitosan/Rice Husk Ash/Nano Alumina
Initially, 0.3 g chitosan was dissolved in 5 mL acetic acid (1%) and stirred for 1 h to prepare chitosan solution. Next, 0.04 g nano-γ alumina was dissolved in 2 mL distilled water under stirring for 3 h. After that, it was kept in ultrasonic apparatus for 15 min. Subsequently, chitosan solution was added into alumina solution under ultrasonic vibration for 3 h. The mixture was added 0.03 g husk and stirred for 3 h. The obtained solution was neutralized by adding sodium hydroxide (0.5 M) and stirring it for some hours. The solution was filtered. Finally, the obtained precipitate was isolated, washed with distilled water several times to remove excess sodium hydroxide, oven-dried overnight at 100 °C, and then conserved in a desiccator. The synthesized composite was referred to as chitosan/rice husk ash/nano-γ alumina.
Characterization
The surface morphology of the samples was studied by field emission scanning electron microscopy (FE-SEM; VEGA//TESCAN-LMU). FTIR spectra were recorded by FT-IR spectrophotometer (Model; FT-IR JASCO 680 plus). X-ray diffraction analyses were carried out for the samples utilizing an ASENWARE AW-XDM 300, 40KV X-ray diffractometer (XRD) with Cu Kα radiation. The Brunauer–Emmett–Teller (BET) was employed to study the surface and pore structure of the samples (BEL: PHS 1020), and AASpect 203 Flame atomic absorption spectrometer (FAAS) was used for lead analysis.
Adsorption Experiments
Adsorption studies were done by batch equilibration. A standard stock solution of Pb(II) (1000 ppm) was diluted by deionized water to prepare the required concentrations. The pH of the Pb(II) solution was adjusted by applying 0.1 M HCl/NaOH. Batch experiments were carried out by adding fixed amounts of the adsorbent in the particular volume of Pb(II) solutions. Each mixture was shaken at a speed of 100 rpm at room temperature. The adsorbent was separated by centrifugation and the residual metal concentration was studied by a Flame atomic absorption spectrometer (FAAS). Removal percentage (R%) and the amount of Pb(II) adsorbed on the chitosan/rice husk ash/nano-γ alumina were achieved by following Eqs. 1 and 2:
where Ci and Ce (mg L−1) are the initial and the equilibrium concentrations of Pb(II), respectively. qe is the amount adsorbed on per unit mass of the adsorbent (mg g−1), V is the volume of the solution, and W is the mass of the adsorbent. Adsorption kinetic experiments were performed by adding fixed quantities of the adsorbent with different contact time at various Pb(II) ion concentrations such as 250, 350, 450, and 550 ppm under the same condition as adsorption isotherm experiments.
Central Composite Design
Response surface methodology (RSM) is a statistical method that can optimize and analyze the interaction between parameters by using experimental data got from particular experimental design to assess an optimum response [67]. Overall, CCD is defined by three operations viz: 2n axial runs, 2n factorial runs, and central runs. In the current study, CCD was formulated by performing 8 axial points, 16 factorial points, and 6 replicates at the center (30 experiments) based on the amount in Table 1.
where n is the number of parameters, and nc is the number of center points. Alpha (α) roughly indicates the distance of the axial from center point, which is turnable and vigorously depends on the number of factorial points; it is estimated from following equation [68]:
In the present study, four variables including pH (X1), concentration of Pb2+ ion (X2), adsorbent dosage (X3), and contact time (X4), was selected to design experiments in a randomized fashion so as to reduce the influence of the uncontrolled parameters [69].
Removal percentage of Pb(II) ions was illustrated by the following quadratic equation [70]:
where Y is the predicted response (removal percentage), xi and xj represent the coded independent variables, and b0, \({{\text{b}}_{\text{i}}}\), \({{\text{b}}_{{\text{ii}}}}\), and \({{\text{b}}_{{\text{ij}}}}\) state the model constant, the linear coefficient, the quadratic coefficients and interaction coefficients, respectively. A mathematical model in the form of a second-order polynomial was expanded to foresight the response. The analysis of variance (ANOVA) was used to estimate the importance of the quadratic regression model (Table 2) [71]. Designed experiments used by CCD to examine the influence of different parameters and optimization of the process are exhibited in Table 3.
Results and Discussion
Characterization of the Adsorbent
FTIR Analysis
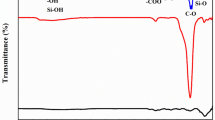
FTIR spectra of pure rice husk ash, chitosan, nano-γ alumina, and composites such as rice husk ash/alumina, chitosan/alumina, chitosan/rice husk ash, as well as chitosan/rice husk ash/nano-γ alumina, before and after adsorption with different molar ratios, are given in Fig. 1. In the spectrum of pure rice husk ash, as shown in Fig. 1A: a, a broad band between 3100 and 3700 cm−1 exhibits the presence of both hydroxyl groups and Si–OH on the rice husk ash surface. The weak and wide peak at 1623 cm−1 was assigned to the –C=O and –C–OH groups stretching. A vigorous peak at 1102 cm−1 was ascribed to Si–O–Si stretching. The peaks at 785 and 482 showed the attendance of Si–H. The small shoulders located at 2920 and 2875 cm−1 were related to the stretching of –CH2 and –CH3 [60]. The FTIR spectra of the chitosan, as given in Fig. 1A: b, depicts a wide band located at 3400 − 3200 cm−1 which corresponded to –OH and –NH. The bands at 2978 and 2865 cm−1 were related to the stretching vibration of aliphatic C–H bonds. Also, the bands at 1635 cm−1 was assigned to –NH bending vibration in –NH2 while the band appeared at 1081 cm− 1 was ascribed to –CO stretching vibration in –COH [72]. Figure 1A: c illustrates the FTIR spectra of nano-γ alumina. The characteristic vibrations of aluminum oxide emerged in the range of 500–1000 cm−1. The wide peaks at 596 and 746 cm−1 corresponded to the bending mode of O–Al–O and Al–O stretching mode, respectively [73].
A (a) Rice husk ash, (b) Chitosan, and (c) Nano-Alumina; B (a) Rice husk ash/Alumina, (b) Chitosan/Alumina, and (c) Chitosan/Rice husk ash; C (a) Chitosan (20%) /rice husk ash (40%) /nano-γ alumina (40%), (b) Chitosan (20%) /rice husk ash (50%) /nano-γ alumina (30%), (c) Chitosan (30%) /rice husk ash (30%) /nano-γ alumina (40%), and (d) Chitosan (30%) /rice husk ash (30%) /nano-γ alumina (40%) after adsorption of Pb(II)
The FTIR spectrum of two-component composites was slightly different from those of the pure rice husk ash, chitosan, and alumina but the characteristic peaks of them could be found. In fact, some new significant peaks appeared in the spectrum of two-component composites, such as the band placed at 511 cm−1 in rice husk ash/alumina and the bands at 602, as well as 533 cm−1 in Chitosan/alumina (Fig. 1B: b), all showing characteristic vibrations of aluminum oxide; also, the peak at 516 cm−1 in chitosan/rice husk ash (Fig. 1 B:c) representing the Si-H group, which all exhibiting the reaction between components and immobilization in mentioned two-component composites.
The FTIR spectra of Chitosan/rice husk ash/nano-γ alumina with different content of Chitosan, rice husk ash, and nano-γ alumina is presented in Fig. 1C before (a, b, c) and after adsorption (d). The nanocomposite with 40% nano-γ alumina was selected as an optimum adsorbent. As can be seen, the intensity of the characteristic peaks of the nanocomposite, depending on the molar ratio of the components was varied, shown in Fig. 1C: a, b, c. It was obvious that the characteristic peaks of optimum nanocomposite with 40% nano-γ alumina were much stronger. Figure 1C: d illustrates the FTIR spectra of the nanocomposite after adsorption. Comparing the spectrum of the adsorbent before and after adsorption showed that the band at 1629 was added for Pb(II) loaded on the adsorbent resulting from the adsorption of lead ions on the nanocomposite surface [74].
XRD Analysis
X-ray diffraction patterns of the two-component composites including rice husk ash/alumina (Fig. 2a), chitosan/alumina (Fig. 2b), and chitosan/rice husk (Fig. 2c), as well as those of nanocomposite chitosan/rice husk ash/nano-γ alumina with diverse molar ratios, before and after adsorption of lead ions (Fig. 3a–d) were appraised in the angle range of 2θ = 9–80. The X-ray diffraction pattern of raw rice husk ash indicates a wide hump between 2θ = 15 and 35°. A wide bump around 2θ = 23° revealed the existence of amorphous silica (disordered cristobalite) [60, 75]. The X-ray diffraction pattern of neat chitosan showed characteristic peaks around 2θ = 7.8°, 10.9°, and 19.9°. The initial two peaks were related to a hydrated crystalline structure, while the third peak gives an amorphous structure for chitosan [76, 77]. For the nano-γ alumina sample, all peaks were compatible with standard amounts of γ-alumina crystalline phase, based on the joint committee on powder diffraction standards (JCPDS) file no 29–63. The wide XRD peaks show that the size of γ-Al2O3 particles in the sample was in the nanometer scale. Two clear peaks emerged at 2θ = 45.6° and 66.9° which were according to the standard values of the γ-alumina crystalline phase in the literature [78]. For two component composites, as shown in Fig. 2a–c, other crystalline phases were also discovered, in addition the neat component phases. For the chitosan/rice husk ash/nano-γ alumina, an improvement in the intensity of characteristic peaks of nanocomposite with 40% nano-γ alumina, as optimum nanocomposite, occurred, as shown in Fig. 3. It could be concluded that the intensity of characteristic peaks of nanocomposite relied on the molar ratio of the elements in intended nanocomposite, so a reduction in the molar ratio of a component could lead to the reduction of intensity of important peaks of it. Comparing XRD patterns of nanocomposites before and after adsorption showed that the nano-composite had an amorphous structure before adsorption, but after the absorption of lead ions, the existence of sharp peaks revealed the formation of a large crystalline material with a more normal structure.
FESEM
In this work, field emission scanning electron microscopy (FE-SEM) was applied to study the surface morphology of the adsorbent. Figure 4 gives FE-SEM images of chitosan/rice husk ash/nano-γ alumina before and after adsorption of lead ions. Chitosan acted as a bed for rice husk ash and nano-γ alumina because of its broad surface. In fact, dispersing rice husk ash and nano alumina on the surface of chitosan increase the surface roughness, leading to a more porous structure and surface area for chitosan/rice husk ash/nano-γ alumina and Pb-adsorbent, as compared to the neat chitosan. Based on Fig. 4, FE-SEM images the nano adsorbent after adsorption, as compared to those of the nano-adsorbent before adsorption, had a less porous structure and grooves that could be ascribed to entrapping lead ions into the pores.
BET Analysis
Figure 5 exhibits the nitrogen adsorption–desorption isotherms and the pore size distribution. The BET surface area values of the chitosan and chitosan/rice husk ash/nano-γ alumina are also presented in Table 4. Accordingly, 14.0531 m2 g−1, 6.80 nm, and 0.0239 cm3 g−1 were obtained for the BET surface area, the average pore diameter and pore volume, respectively. The surface area of the nanocomposite was larger than the surface area of chitosan because the particles of rice husk ash and nano-γ alumina with the greater surface area were dispersed on the chitosan bed to enhance its surface. As a result, the ions can easily penetrate into the pores, given the fact that the average pore diameter of the nano-composite was greater than that of lead ions (nearly 350 pm). The adsorption–desorption isotherm was investigated by applying nitrogen as the adsorptive gas. The isotherm was a type of IV representing a mesoporous structure and an H3 hysteresis loop, based on IUPAC classification. The sort H3 loop, which doesn’t reveal any restriction adsorption at high p/p°, is often observed with the conjunction of plate-like particles, leading to slit-shaped pores. In addition, the size of pores and complete release of gas from the pores depends on the plot of adsorption–desorption. Approaching the isotherm plots represents the equality of the pores and leads the adsorbent to be more active, and does not to lose its activation soon too [79].
Response Surface Methodology (RSM)
Central composite design (CCD), Box-Behnken design, and three level factorial design, as Several sorts of RSM, have various characteristics; among them, CCD is the most famous method used to optimize variables and assessment of the main variables, as well as the interaction between them [80] (Table 3). The polynomial regression equation is the most important equation showing the relationship between variables and responses. Analysis of variance (ANOVA) was applied to examine the statistical significant factor. The significance of the response surface quadratic model was estimated by the values of F, P and predicted R2, adjusted R2 and correlation coefficient (R2). The value of the F-value parameter (621.69) and P-value parameter (< 0.001) showed that the model was highly significant for removal of lead ions. The quality of fit of the polynomial model equation was stated by the determination coefficient (R2 = 0.9985 and adjusted R2 = 0.9969), showing a good correlation between the observed and predicted values (Fig. 6). The removal percentage of lead ions as a function of independent variables was predicted by the following equation:
Figure 6 exhibits the plot of the predicted versus the observed response (R%) and the plot of the residuals versus the predicted response (removal percentage). Both the figures express the existence of a linear relationship between them with a high correlation coefficient that showing the normal contribution of error around the mean and the suitability of the model for the description of experimental data. These plots were needed to verify the normality supposition in the fitted model.
The response surface plots (Fig. 7) were prepared by changing two factors while keeping the other constant. Figure 7 indicates the most related fitted response surfaces for the design; it also shows the response surface plots of removal percentage (R%) versus significant variables. The curvatures of these plots could express the interaction between variables, as explained below:
The 3D response surface plots and contour plots for interactive effect of a pH and adsorbent dosage; time and Pb(II) concentration at the level of − 1, b pH and Pb(II) concentration; time at the level of − 1 and adsorbent dosage at the level of + 1, c adsorbent dosage and time; pH at the level of + 1and Pb(II) concentration at the level of − 1
Interactive Effects of Adsorption Variables
The combined effects and the main interactions between the independent variable and the removal percentage of lead ions could be demonstrated by 3D response surface and contour plots. Figure 7 indicates the reciprocal interactions of the combination of self-determining parameters on R% Pb(II) in the style of 3D surface contour plots. In all plots, two agents were kept at a central level and the response is plotted concerning to the other two factors.
The pH representing the acidity or basicity of the solution has a significant role in the adsorption process. The pH of the solution was studied in the acidity range because Lead hydroxide precipitation was formed in basicity pH. Based on the results shown in Fig. 7a, b, at low pH, the sorbent surface was protonated, not able to adsorb ion metals. In fact, hydrogen ions competed with analytes to take up the active sites. Therefore, a decrease occurred in the adsorption of metal ions. The adsorption efficiency was reduced at the high pH owing to the formation of metal hydroxides in the solution, which diminishes the concentration of metal ions on the surface of the adsorbent. So, the pH of 5 was chosen as the best operational pH. The contact time between the adsorbent and adsorbate is one of the most significant factors that can play a critical role in the adsorption process. Figure 7c represents the mutual action of contact time with the amount of chitosan/rice husk ash/nano-γ alumina composite. It is obvious that the removal efficiency of lead ions was increased by the growth of the contact time due to the enlarged surface area and scattering of the adsorbent into the solution. It could be seen, that the fast initial increase in lead occupation was due to the copiousness of the available adsorbent surface. Then, the rate of lead uptake was gradually reduced with the progress of adsorption (because of active sites occupation) until arriving equilibrium. Regarding adequate reaction, the optimum time of 105 min was considered for the removal of lead ions.
According to the results shown in Fig. 7a, c, it is obvious the adsorption process was enhanced with an increase in the amount of sorbent because of an improved number of active and unoccupied sites for the metal ion leading to taking up and more contact surface among the metal ions, which causes more removal efficiency by adsorbent. Therefore, the amount of 0.01 g was selected for further studies.
The influence of initial Pb(II) ion concentration on the adsorption efficiency is exhibited in Fig. 7b which indicates at lower concentrations of Pb(II) ions, adsorption performance becomes larger due to the low ratio of dissolved concentrations to the chitosan/rice husk ash/nano-γ alumina composite sites. On the contrary, at the higher concentration of Pb(II) ions removal percentage was reduced, probably because of the saturation of the nano adsorbent. Consequently, the Pb ions adsorption onto chitosan/rice husk ash/nano-γ alumina was dependent on the initial concentration of Pb(II) ions. The optimum value of 30 ppm was achieved for the initial concentration of lead ions.
Adsorption Isotherms
Adsorption isotherms depict the relationship between the amount of adsorbed material and the concentration in the equilibrium solution. Fitting the experimental data to a suitable adsorption isotherm model is an important element of the adsorption process of a metal ion. Adsorption isotherms give valuable information regarding mechanism and nature of the adsorption process. Different adsorption isotherm equations including Langmuir, Freundlich, and Temkin isotherms were employed to simulate the adsorption isotherms. The Langmuir isotherm is defined as:
where Ce is the equilibrium concentration of lead solution (mg L−1), qmax (mg g−1) and kl (L mol−1) is the theoretical maximum adsorption capacity and Langmuir equilibrium constants, respectively.
where Kf and n are Freundlich adsorption isotherm constants, indicating the extent of the adsorption. The degree of nonlinearity between Kf and 1/n can be appraised from the linear plot of lnqe against Ce.
The Temkin isotherm is described as:
where B and Kt are Temkin adsorption isotherm constants and their values can be calculated from the plot of lnCe versus qe. based on Table 5, the Langmuir isotherm had the best suitability of experimental data owing to high coefficient of determination (R2 = 0.9864) indicating the monolayer adsorption of lead ions on the rough surface of chitosan/rice husk ash/nano alumina.
Adsorption Kinetics
The rate of adsorption is one of the most important features of an adsorbent. The adsorption kinetic parameters for chitosan/rice husk ash/ nano alumina are shown in Table 6. The adsorption rate of lead ions was rapidly increased in the first phases of the process. After 2 h, the adsorption of lead ions was closely unvaried representing that the uptake rate was at equilibrium. Hence, the time of 2 h was adequate and was applied for the adsorption isotherm to be assured that the adsorption equilibrium was obtained. Different kinetic models such as pseudo-first-order, pseudo-second-order, intraparticle diffusion, and Elovichwere investigated. The rate constant can be expressed utilizing the pseudo-first-order equation given by Langergren [81]:
where k1 is pseudo-first order rate constat (min−1), and qe and qt are the amount as of adsorbed lead (mg g−1) at equilibrium ant at time t (min).
Based on equilibrium adsorption, the pseudo-second-order is described as [82]:
where k2 (g mg−1 min−1) is the rate constant of the pseudo-second-order adsorption.
The intraparticle diffusion equation is given as [83]
Where kdif is the intraparticle diffusion rate constant (mg/(g min1/2)) and C shows the boundary layer thickness.
The linear form of Elovich model is usually defined as [82]:
The properties of each kinetic model are exhibited in Table 6. According to the experimental results, k1 and qe were assessed from the slope and intercept of the plot of log (qe − qt) against t. The coefficient of determination (R2) of the pseudo-first-order model has not shown suitable fitness with experimental data to this model. In addition, the best fitness of the pseudo-second-order model to the experimental data was achieved from the plot of t/qt versus t. k2 and qe were calculated from the intercept and slope of the pseudo-second-order plot, respectively. The third kinetic model, intraparticle diffusion, was described by the plot of qt against ln(t). Based on Table 6, the plot of qt versus ln(t) presented linear relationship and given an intercept ((1/β)ln(αβ)) and slope of (1/β). Consequently, the correlation coefficient (R2) for the pseudo-second-order kinetic model was greater than that of other studied kinetic models, it was 0.9967 (given in Table 6), revealing that the adsorption process of the lead ion on the adsorbent could be better described by pseudo-second-order kinetic model and suggested that chemisorption could be the rate monitoring step.
Adsorption Thermodynamic
Thermodynamic parameters such as Gibbs free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°) are determined by following equations [84]:
where Ce (mg L−1) is the equilibrium concentration of lead ions in solution, qe (mg g−1) is the amount of lead ions adsorbed onto adsorbent, R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (in Kelvin) and ke (L g−1) is the adsorption equilibrium constant. ΔH° and ΔS°were evaluated from the slope and intercept of the plot of lnke against 1/T. Al, ΔG was determined from true values of ΔH° and ΔS°. Table 7 gives the thermodynamic parameters coming from the experimental data of isotherms at various temperatures. The negative value of ΔH° showed that the sorption of lead ions was an exothermic process. The negative values of ΔG° (− 10.6253 to − 17.1966) illustrated the physical adsorption of lead ions on the nanocomposite. In addition, the positive value of ΔS° revealed that the adsorption process was spontaneous.
Regeneration Study
Reusability is one prominent property of the adsorbent in a practical application which causes a remarkable reduction in the cost of the treatment. So, it is essential to increase the reusability of adsorbent. For the regeneration, the lead-adsorbed adsorbent was dipped in 10 mL of HNO3 solution (0.1 mol L−1) for 3 h and then washed six times with deionized water. The influence of six subsequent adsorption–desorption cycles was studied. The results are exhibited in Fig. 8. As can be seen, the removal efficiency of the adsorbent was reduced softly with the enhancement of the cycling times. The adsorption capacity was kept at above 70% after six cycles, indicating that the chitosan/rice husk ash/nano-γ alumina composite could be utilized as an outstanding reusable adsorbent for the removal of lead ions.
Comparison of Adsorbent for Removal of Pb(II)
Figure 9 compares the lead adsorption capacity of the adsorbents with different molar ratios of components. As can be seen, the removal efficiency of lead ions was flourished with the growth of the molar ratio of γ-Al2O3 to CTS/RHA. Besides, the removal performance of CTS/RHA/γ-Al2O3 nanocomposite with 40% nano-γ alumina content was higher than that of other adsorbents. Hence, the nanocomposite with 40% of nano-γ alumina was considered as an optimum composite.
Moreover, it was found that the composite adsorbent showed some intensified removal for the lead ions, as compared to other components. The relationship of the adsorbents for the lead ions was in the order: CTS/RHA/γ-Al2O3 > RHA/γ-Al2O3 > CTS/γ-Al2O3 > γ-Al2O3 > CTS/RHA > RHA > CTS, representing that combination of the components made an excellent synergistic effect which enhanced adsorption capacity of lead ions from aqueous solutions.
Removal Performance of CTS/RHA/γ-Al2O3 in Real Sample
To asses CTS/RHA/γ-Al2O3 nanocomposite efficiency in a real sample, the sorption experiments were applied with industrial wastewater spiked with Pb(II) which is fed with various concentrations of the lead ions. The results have been prepared in Table 8. For this study, in optimized conditions, different amounts of Pb(II) (20, 40 and 60 mg L−1) were spiked to the real sample. Then, spiked samples were done under the proposed method. The results indicate ideal removal percentage showing the acceptable performance of nano adsorbent proposed for the real sample.
Comparison with Other Adsorbents
There have been numerous reports on the adsorption of lead ions. Different materials have been studied as adsorbents for the removal of lead ions. Table 9 presents the comparison of the maximum adsorption capacity of various adsorbents under different experimental conditions for the removal of lead ions. The maximum adsorption capacity of chitosan/rice husk ash/nano-γ alumina was obtained to be higher than that given in Table 9 (qm = 181.82). So, it may be deduced that the nanocomposite could be used as a potential adsorbent for the removal of lead ions from aqueous solutions.
Conclusion
Finally, we would like to make the readers of this article aware of some achieved results by doing this investigation. The first one, it should be mentioned that FTIR, FE-SEM, XRD, and BET methods confirmed the characterization of the synthesized nanocomposite. CCD method was applied to optimize such experimental parameters as pH, the contact time, the initial concentration of lead ions, and adsorbent dosage, resulting 90.98% removal efficiency of the adsorbent under optimum conditions as follow: pH: 5, contact time: 105 min, initial concentration of lead ions: 30 ppm, adsorbent dosage: 0.01 g. Adsorption behavior of lead ions on the adsorbent followed the Langmuir isotherm. The adsorption data for the nano adsorbent also exhibited desirable compliance with the pseudo-second-order kinetic model. The thermodynamic studies further explain that the adsorption process of lead ions was exothermic and spontaneous. Finally, regeneration experiments confirmed that chitosan/rice husk ash/nano-γ alumina, as a new nano adsorbent, has excellent adsorption capacity for removal of lead ions from aqueous solution. Also, removal performance of CTS/RHA/γ-Al2O3 adsorbent in a real sample was investigated and the results show acceptable removal percentage confirming the good performance of nano adsorbent.
References
Chen K, He J, Li Y, Cai X, Zhang K, Liu T et al (2017) Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J Colloid Interface Sci 494:307–316
Gupta VK, Ali I (2012) Environmental water: advances in treatment, remediation and recycling, Newnes, Heidelberg
Ali I, Aboul-Enein HY (2006) Instrumental methods in metal ion speciation. CRC Press, Boca Raton
Ali I, Aboul-Enein HY, Gupta VK (2009) Nanochromatography and nanocapillary electrophoresis: pharmaceutical and environmental analyses. John Wiley & Sons, Hoboken
Basheer AA (2018) Chemical chiral pollution: impact on the society and science and need of the regulations in the 21st century. Chirality 30(4):402–406
Gupta V, Ali I (2002) Encyclopedia of surface and colloid science. Marcel Dekker, New York, pp 136–166
Wang Y, Shi L, Gao L, Wei Q, Cui L, Hu L et al (2015) The removal of lead ions from aqueous solution by using magnetic hydroxypropyl chitosan/oxidized multiwalled carbon nanotubes composites. J Colloid Interface Sci 451:7–14
Aroua MK, Leong S, Teo L, Yin CY, Daud WMAW (2008) Real-time determination of kinetics of adsorption of lead (II) onto palm shell-based activated carbon using ion selective electrode. Biores Technol 99(13):5786–5792
Blázquez G, Martín-Lara M, Tenorio G, Calero M (2011) Batch biosorption of lead (II) from aqueous solutions by olive tree pruning waste: Equilibrium, kinetics and thermodynamic study. Chem Eng J 168(1):170–177
García-Rosales G, Colín-Cruz A (2010) Biosorption of lead by maize (Zea mays) stalk sponge. J Environ Manag 91(11):2079–2086
Chen L-F, Liang H-W, Lu Y, Cui C-H, Yu S-H (2011) Synthesis of an attapulgite clay@ carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water. Langmuir 27(14):8998–9004
Luo S, Lu T, Peng L, Shao J, Zeng Q, Gu J-D (2014) Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb (II) from aqueous solution. J Mater Chem A 2(37):15463–15472
Van der Bruggen B, Koninckx A, Vandecasteele C (2004) Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res 38(5):1347–1353
Mhamdi M, Elaloui E, Trabelsi-Ayadi M (2013) Adsorption of zinc by a Tunisian Smectite through a filtration membrane. Ind Crops Prod 47:204–211
Víctor-Ortega M, Ochando-Pulido J, Hodaifa G, Martínez-Ferez A (2014) Ion exchange as an efficient pretreatment system for reduction of membrane fouling in the purification of model OMW. Desalination 343:198–207
Zhao D, Gao X, Wu C, Xie R, Feng S, Chen C (2016) Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr (VI). Appl Surf Sci 384:1–9
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X et al (2014) Synthesis of amidoxime-functionalized Fe3O4@ SiO2 core–shell magnetic microspheres for highly efficient sorption of U (VI). Chem Eng J 235:275–283
Sugashini S, Begum KMS, Ramalingam A (2015) Removal of Cr (VI) ions using Fe-loaded chitosan carbonized rice husk composite beads (Fe-CCRCB): Experiment and quantum chemical calculations. J Mol Liq 208:380–387
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271(2):321–328
Ali I, Gupta V (2006) Advances in water treatment by adsorption technology. Nat Protoc 1(6):2661
Ali I, Alharbi OM, Alothman ZA, Alwarthan A (2018) Facile and eco-friendly synthesis of functionalized iron nanoparticles for cyanazine removal in water. Colloids Surf B 171:606–613
Ali I, Alharbi OM, Alothman ZA, Badjah AY, Alwarthan A (2018) Artificial neural network modelling of amido black dye sorption on iron composite nano material: kinetics and thermodynamics studies. J Mol Liq 250:1–8
Gupta VK, Ali I (2008) Removal of endosulfan and methoxychlor from water on carbon slurry. Environ Sci Technol 42(3):766–770
Dashamiri S, Ghaedi M, Asfaram A, Zare F, Wang S (2017) Multi-response optimization of ultrasound assisted competitive adsorption of dyes onto Cu (OH)2-nanoparticle loaded activated carbon: Central composite design. Ultrason Sonochem 34:343–353
Ali I, AL-Othman ZA, Alwarthan A (2016) Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J Mol Liq 221:1168–1174
Ali I, Khan TA, Asim M (2012) Removal of arsenate from groundwater by electrocoagulation method. Environ Sci Pollut Res 19(5):1668–1676
Ali I, Aboul-Enein HY (2002) Speciation of arsenic and chromium metal ions by reversed phase high performance liquid chromatography. Chemosphere 48(3):275–278
Ali I, Jain CK (2004) Advances in arsenic speciation techniques. Int J Environ Anal Chem 84(12):947–964
Ali I, ALOthman ZA, Sanagi MM (2015) Green synthesis of iron nano-impregnated adsorbent for fast removal of fluoride from water. J Mol Liq 211:457–465
Ali I, Gupta VK, Khan TA, Asim M (2012) Removal of arsenate from aqueous solution by electro-coagulation method using Al-Fe electrodes. Int J Electrochem Sci 7:1898–1907
Ali I, AL-Othman ZA, Alwarthan A (2016) Molecular uptake of congo red dye from water on iron composite nano particles. J Mol Liq 224:171–176
Ali I, AL-Othman ZA, Alwarthan A (2016) Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J Mol Liq 219:858–864
Ali I (2012) New generation adsorbents for water treatment. Chemical reviews 112(10):5073–5091
Ali I (2014) Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev 43(3):175–205
Alharbi OM, Khattab RA, Ali I (2018) Health and environmental effects of persistent organic pollutants. J Mol Liq 263:442–453
Ali I, Alharbi OML, Alothman ZA, Al-Mohaimeed AM, Alwarthan A (2019) Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ Res 170:389–397
Kragović M, Daković A, Sekulić Ž, Trgo M, Ugrina M, Perić J et al (2012) Removal of lead from aqueous solutions by using the natural and Fe (III)-modified zeolite. Appl Surf Sci 258(8):3667–3673
Jiang M-q, Jin X-y, Lu X-Q, Chen Z-l (2010) Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto natural kaolinite clay. Desalination 252(1):33–39
Su Q, Pan B, Pan B, Zhang Q, Zhang W, Lv L et al (2009) Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci Total Environ 407(21):5471–5477
Momčilović M, Purenović M, Bojić A, Zarubica A, Ranđelović M (2011) Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 276(1):53–59
Misra R, Jain S, Khatri P (2011) Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr (VI), Cd (II), Ni (II) and Pb (II) from their aqueous solutions. J Hazard Mater 185(2):1508–1512
Montazer-Rahmati MM, Rabbani P, Abdolali A, Keshtkar AR (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J Hazard Mater 185(1):401–407
Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat M (2013) Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv Coll Interface Sci 193:24–34
Bée A, Obeid L, Mbolantenaina R, Welschbillig M, Talbot D (2017) Magnetic chitosan/clay beads: a magsorbent for the removal of cationic dye from water. J Magn Magn Mater 421:59–64
Dil EA, Ghaedi M, Asfaram A (2017) The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: application of ultrasound wave, optimization and modeling. Ultrason Sonochem 34:792–802
Burakova EA, Dyachkova TP, Rukhov AV, Tugolukov EN, Galunin EV, Tkachev AG et al (2018) Novel and economic method of carbon nanotubes synthesis on a nickel magnesium oxide catalyst using microwave radiation. J Mol Liq 253:340–346
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep Purif Rev 40(1):25–42
Ali I, Alothman ZA, Alwarthan A (2017) Supra molecular mechanism of the removal of 17-β-estradiol endocrine disturbing pollutant from water on functionalized iron nano particles. J Mol Liq 241:123–129
Ali I, Alothman Z, Al-Warthan A (2016) Sorption, kinetics and thermodynamics studies of atrazine herbicide removal from water using iron nano-composite material. Int J Environ Sci Technol 13(2):733–742
Ali I, Al-Othman ZA, Al-Warthan A (2016) Removal of secbumeton herbicide from water on composite nanoadsorbent. Desalin Water Treat 57(22):10409–10421
Ali I, Al-Othman ZA, Alharbi OM (2016) Uptake of pantoprazole drug residue from water using novel synthesized composite iron nano adsorbent. J Mol Liq 218:465–472
Ali I, Asim M, Khan T (2013) Arsenite removal from water by electro-coagulation on zinc–zinc and copper–copper electrodes. Int J Environ Sci Technol 10(2):377–384
Cho D-W, Jeon B-H, Chon C-M, Kim Y, Schwartz FW, Lee E-S et al (2012) A novel chitosan/clay/magnetite composite for adsorption of Cu (II) and As (V). Chem Eng J 200:654–662
Xie J, Li C, Chi L, Wu D (2013) Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel 103:480–485
Xiao C, Liu X, Mao S, Zhang L, Lu J (2017) Sub-micron-sized polyethylenimine-modified polystyrene/Fe3O4/chitosan magnetic composites for the efficient and recyclable adsorption of Cu (II) ions. Appl Surf Sci 394:378–385
Siahkamari M, Jamali A, Sabzevari A, Shakeri A (2017) Removal of Lead (II) ions from aqueous solutions using biocompatible polymeric nano-adsorbents: A comparative study. Carbohydr Polym 157:1180–1189
Srivastava V, Sharma Y, Sillanpää M (2015) Response surface methodological approach for the optimization of adsorption process in the removal of Cr (VI) ions by Cu2(OH)2CO3 nanoparticles. Appl Surf Sci 326:257–270
Hassanzadeh-Tabrizi S, Motlagh MM, Salahshour S (2016) Synthesis of ZnO/CuO nanocomposite immobilized on γ-Al2O3 and application for removal of methyl orange. Appl Surf Sci 384:237–243
Foo K, Hameed B (2009) Utilization of rice husk ash as novel adsorbent: a judicious recycling of the colloidal agricultural waste. Adv Coll Interface Sci 152(1):39–47
Imyim A, Prapalimrungsi E (2010) Humic acids removal from water by aminopropyl functionalized rice husk ash. J Hazard Mater 184(1):775–781
Pavlović MD, Buntić AV, Mihajlovski KR, Šiler-Marinković SS, Antonović DG, Radovanović Ž et al (2014) Rapid cationic dye adsorption on polyphenol-extracted coffee grounds—A response surface methodology approach. J Taiwan Inst Chem Eng 45(4):1691–1699
Hajati S, Ghaedi M, Yaghoubi S (2015) Local, cheep and nontoxic activated carbon as efficient adsorbent for the simultaneous removal of cadmium ions and malachite green: Optimization by surface response methodology. J Ind Eng Chem 21:760–767
Teimouri A, Nasab SG, Vahdatpoor N, Habibollahi S, Salavati H, Chermahini AN (2016) Chitosan /Zeolite Y/Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution. Int J Biol Macromols 93(Part A):254–266
Teimouri A, Ghanavati Nasab S, Habibollahi S, Fazel-Najafabadi M, Chermahini AN (2015) Synthesis and characterization of a chitosan/montmorillonite/ZrO2 nanocomposite and its application as an adsorbent for removal of fluoride. RSC Adv 5(9):6771–6781
Nasab SG, Semnani A, Teimouri A, Kahkesh H, Isfahani TM, Habibollahi S (2018) Removal of Congo red from aqueous solution by hydroxyapatite nanoparticles loaded on zein as an efficient and green adsorbent: response surface methodology and artificial neural network-genetic algorithm. J Polym Environ 26:1–21
Nasab SG, Semnani A, Teimouri A, Yazd MJ, Isfahani TM, Habibollahi S (2018) Decolorization of crystal violet from aqueous solutions by a novel adsorbent chitosan/nanodiopside using response surface methodology and artificial neural network-genetic algorithm. Int J Biol Macromol 124:429–443
Sahu J, Acharya J, Meikap B (2009) Response surface modeling and optimization of chromium (VI) removal from aqueous solution using Tamarind wood activated carbon in batch process. J Hazard Mater 172(2):818–825
Khodadoust S, Ghaedi M (2013) Optimization of dispersive liquid–liquid microextraction with central composite design for preconcentration of chlordiazepoxide drug and its determination by HPLC-UV. J Sep Sci 36(11):1734–1742
Roosta M, Ghaedi M, Daneshfar A, Sahraei R, Asghari A (2014) Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason Sonochem 21(1):242–252
Madhavi V, Prasad T, Reddy AVB, Reddy BR, Madhavi G (2013) Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim Acta Part A Mol Biomol Spectrosc 116:17–25
Gusain D, Bux F, Sharma YC (2014) Abatement of chromium by adsorption on nanocrystalline zirconia using response surface methodology. J Mol Liq 197:131–141
Zavareh S, Zarei M, Darvishi F, Azizi H (2015) As (III) adsorption and antimicrobial properties of Cu–chitosan/alumina nanocomposite. Chem Eng J 273:610–621
Teimouri A, Ebrahimi R, Chermahini AN, Emadi R (2015) Fabrication and characterization of silk fibroin/chitosan/Nano γ-alumina composite scaffolds for tissue engineering applications. RSC Adv 5(35):27558–27570
Liu C, Bai R, San Ly Q (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: behaviors and mechanisms. Water Res 42(6):1511–1522
Genieva S, Turmanova S, Dimitrova A, Vlaev L (2008) Characterization of rice husks and the products of its thermal degradation in air or nitrogen atmosphere. J Therm Anal Calorim 93(2):387–396
Ogawa K, Hirano S, Miyanishi T, Yui T, Watanabe T (1984) A new polymorph of chitosan. Macromolecules 17(4):973–975
Wang S-F, Shen L, Zhang W-D, Tong Y-J (2005) Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromol 6(6):3067–3072
Hosseini SY, Nikou MRK (2012) Investigation the effect of temperature and weight hourly space velocity in dimethyl ether synthesis from methanol over the nano-sized acidic gamma-alumina catalyst. J Am Sci 8(3)
Teimouri A, Nasab SG, Vahdatpoor N, Habibollahi S, Salavati H, Chermahini AN (2016) Chitosan/Zeolite Y/Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution. Int J Biol Macromol 93:254–266
Saleh TA, Sarı A, Tuzen M (2017) Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J Environ Chem Eng 5(1):1079–1088
Asfaram A, Ghaedi M, Hajati S, Rezaeinejad M, Goudarzi A, Purkait MK (2015) Rapid removal of Auramine-O and Methylene blue by ZnS: Cu nanoparticles loaded on activated carbon: a response surface methodology approach. J Taiwan Inst Chem Eng 53:80–91
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div 89(2):31–60
Mohammadzadeh A, Ramezani M, Ghaedi A (2016) Synthesis and characterization of Fe2O3–ZnO–ZnFe2O4/carbon nanocomposite and its application to removal of bromophenol blue dye using ultrasonic assisted method: Optimization by response surface methodology and genetic algorithm. J Taiwan Inst Chem Eng 59:275–284
Bingöl D, Hercan M, Elevli S, Kılıç E (2012) Comparison of the results of response surface methodology and artificial neural network for the biosorption of lead using black cumin. Bioresour Technol 112:111–115
Anwar J, Shafique U, Salman M, Dar A, Anwar S (2010) Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour Technol 101(6):1752–1755
Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Biores Technol 101(15):5808–5814
Issabayeva G, Aroua MK, Sulaiman NMN (2006) Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour Technol 97(18):2350–2355
Jahangiri M, Kiani F, Tahermansouri H, Rajabalinezhad A (2015) The removal of lead ions from aqueous solutions by modified multi-walled carbon nanotubes with 1-isatin-3-thiosemicarbazone. J Mol Liq 212:219–226
Bartczak P, Norman M, Klapiszewski Ł, Karwańska N, Kawalec M, Baczyńska M et al (2015) Removal of nickel (II) and lead (II) ions from aqueous solution using peat as a low-cost adsorbent: a kinetic and equilibrium study. Arabian J Chem 11:1209–1222
Acknowledgements
We gratefully acknowledge the financial assistance provided by the Payame Noor University in Isfahan Research council. Also, the authors appreciate Shahrekord University and the Center of Excellence for Mathematics, Shahrekord University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fooladgar, S., Teimouri, A. & Ghanavati Nasab, S. Highly Efficient Removal of Lead Ions from Aqueous Solutions Using Chitosan/Rice Husk Ash/Nano Alumina with a Focus on Optimization by Response Surface Methodology: Isotherm, Kinetic, and Thermodynamic Studies. J Polym Environ 27, 1025–1042 (2019). https://doi.org/10.1007/s10924-019-01385-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01385-3