Abstract
Gelatin-Zr(IV) phosphate composite (GT/ZPC) was synthesized by sol–gel method. Different techniques viz. Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), X-ray powdered diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used for the characterisation of GT/ZPC composite ion exchanger. The ion exchange capacity (IEC) of GT/ZPC was observed to be better (1.04 meq g−1) than its inorganic counterpart (0.64 meq g−1). The pH studies revealed the monofunctional nature of GT/ZPC with one inflection point. The distribution studies showed that the GT/ZPC was highly selective for Cd2+ as compare to other metal ions. The environmental applicability of ion exchanger has been analysed for binary separations of metal ions using column method. Cd2+ was effectively removed from synthetic mixture of metal ions (Zn2+, Pb2+, Ni2+, Co2+ and Cu2+).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid industrialization extensively increased the release of different pollutants from numerous industries such as paint, paper, textile etc. A large number of pollutants such as phenols, pesticides, dyes and heavy metals are continuously polluting the aquatic system [1,2,3,4,5,6,7,8,9,10]. Among all of these pollutants, heavy metals are considered to be the most common constituent present in polluted water. If the level of heavy metal ions exceeded a certain limit, it causes serious health hazard to living being [11,12,13,14]. The hazardous metals include cadmium, copper, lead, aluminium, chromium, thorium, cobalt and zinc etc. causing several diseases in the living beings [15]. Therefore, these metals must be eliminated before discharged into aquatic system. A number of methodologies such as ion exchange process, adsorption, photocatalysis, membrane filtration and chemical precipitation have been exploited for the remediation of contaminants from the surroundings [15,16,17,18,19,20]. The ion exchange technique is most promising for waste water treatment due to cheap, easily operated and handled, highly efficient and sludge-free in nature.
In recent years, the synthetic inorganic ion exchangers have been extensively used for the treatment of pollutants due to their unique properties. The salts of polyacids with multivalent metals have gained increased importance due to their ease of synthesis and subsequent utilization in column separation [21,22,23,24,25,26,27]. The ion exchangers which are zirconium based have gained more consideration due to their outstanding ion exchange properties and analytical applications as in ion exchange membranes, solid state electrochemistry and phase transitions etc. Zirconium phosphate has a layered structure which enables exchange between protons and outer ions. It has advanced properties such as high thermal and chemical stability, solid state ion conductivity, resistance to ionizing radiation etc. [28,29,30,31,32,33].
The inorganic ion exchangers enhance the thermal stability, ion-exchange behaviour and also increased the electrical conductivity while the organic ion exchangers provide the mechanical and chemical stability. But these materials have some disadvantages which restrict their applicability. The synthetic inorganic ion exchange materials are expensive, non-reproducible, and unable to handle huge volume of waste discharges while organic ion exchanger materials possess low thermal and chemical stability [34, 35]. By the blending of organic units in inorganic matrix, numerous inorganic–organic composite ion exchanger materials have been established recently. These materials provide better chemical, thermal, mechanical stabilities and possessing good selectivity for heavy metals which are very difficult to remove due to their non-degradable nature.
The growing interest in composite ion exchangers is due to their multifunctionality, selectivity and specificity in different fields [36,37,38,39,40,41]. Due to unique chemical, mechanical, electrochemical and magnetic properties composite ion exchangers have been received huge attention [42,43,44,45,46,47,48]. They have been used as ion selective electrode, adsorbent, catalyst, antimicrobial activity, chromatography and environmental science. At nano level, composite materials have many properties superior to the bulk materials due to their specificity, selectivity and widespread applicability [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66].
Recently, bio-based composites have been synthesized because off their renewable nature. The biopolymers such as gelatin, alginates, cellulose, proteins, lignin, starch, pectin and chitin have been utilized for the preparation of various composites. Gelatin is an amphoteric polyelectrolyte which is a denatured derivative of collagen [67]. It was broadly used in pharmaceutical, nutrients and photographic areas [68]. However, their deprived mechanical properties restrict their applications as structural biomaterial. Consequently, many attempts have been made to improve their mechanical properties [69, 70].
In the present investigation, an attempt has been made to explore the biopolymer based composite (GT/ZPC) ion exchanger for the separation of toxic heavy metal ions. The instrumental analysis of GT/ZPC has been attempted using various techniques such as FTIR, TGA, XRD, SEM and TEM. On the basis of Kd values, the quantitative separations of Cd2+ ions was attained from the synthetic mixtures of different metal ions by using GT/ZPC.
Materials and Methods
Reagents and Instruments
Zirconium oxcychloride (CDH, India), orthophosphoric acid (CDH, India), gelatin (CDH, India) and nitric acid (SD Fine Pvt. Ltd., India) were used. Also, metal nitrates such as zinc nitrate, cadmium nitrate, cuprous nitrate, aluminium nitrate, lead nitrate, nickel nitrate, cobalt nitrate and magnesium nitrate have been purchased from CDH, India. FTIR(Perkin Elmer Spectrum-BX USA), digital pH meter (Elico LI-10, India), UV–Visible spectrophotometer (Systeronics), X-ray diffractrometer (Perkin Elmer Series II CHNS/O 2400), SEM (Quanta 250, FEI Make and Mode No. D9393), and TEM (FEI Tecnai F 20) were used.
Synthesis of Zr(IV) Phosphate
Zr(IV) phosphate was synthesized by mixing of 0.1 M zirconium oxcychloride and 0.1 M H3PO4 solutions drop wise in a fixed volume ratio (1:2) with continuous stirring at 65 °C. The pH of reaction mixture was kept between 0 and 1 with the addition of 0.1 N HNO3. The resulting mixture was agitated for 2 h continuously on a magnetic stirrer [70]. Then the resulting precipitates were filtered, washed thoroughly with double distilled water and oven dried at 60 °C for 24 h.
Synthesis of GT/ZPC
GT/ZPC was synthesized by sol–gel method [71,72,73]. At the starting, Zr(IV) phosphate was synthesized by the above method discussed in “Synthesis of GT/ZPC”. Then, the gel of gelatin was prepared in hot water and added to above mixture with constant stirring. The resulting reaction mixture was kept for digestion for 24 h with infrequent shaking. The precipitates of gelatin-Zr(IV) phosphate so obtained were washed and dried at 50 °C in a hot air oven. In a similar way, different samples of GT/ZPC were synthesized by changing the percentage of gelatin and IEC of each sample was studied.
Ion Exchange Capacity (IEC)
The ion exchange capacity of gelatin-Zr(IV) phosphate composite (GT/ZPC) was determined by the well-known standard column method [72].
Thermal Effect on IEC
The effect of temperature onto the IEC of GT/ZPC was studied in the range of 100–700 °C. 1.0 g of GT/ZPC in H+ form was heated in a muffle furnace at different temperature for 1 h. After cooling at room temperature, the IEC was examined by column method as explained in “Ion exchange capacity (IEC)”.
pH Titration Study
The pH titration studies were performed as defined by Top and Pepper method [73]. In this method, 0.5 g of GT/ZPC in H+ form was placed in a number of conical flasks of 250 mL. Then, the solutions of metal chlorides and their hydroxides of same concentration was added in different volume ratio. The final volume of each flask was maintained to 50 mL. The pH of each flask was examined for every 24 h until the equilibrium was reached.
Characterization
FTIR spectrum of gelatin-Zr(IV) phosphate composite was performed by Fourier transform infrared spectrophotometer (Perkin Elmer Spectrum- BX USA) using KBr disk method. Scanning electron microphotographs of GT/ZPC were recorded at different magnifications using QUANTA250 FEI D9393 scanning electron microscope (SEM). GT/ZPC and ZP were placed on a carbon tape with silicon adhesive and mounted on an aluminum stub. The instrument was operated at 5–10 kV and 30,000 magnifications.
The TEM result of GT/ZPC ion exchanger was analyzed under transmission electron microscope FEI Tecnai F 20. The particle size and morphology was determined by preparing the suspensions of GT/ZPC in ethanol solution and placed on carbon copper grid.
X-ray diffraction of GT/ZPC ion exchanger was noted with an analytical X-ray diffractometer (XRD, Philips model X’PERT PRO) using CuKa radiation (l = 1.5418 A˚).
Distribution Coefficient (Kd) Studies
Batch process was applied to find the Kd values of various metals onto GT/ZPC. In this method, 200 mg of GT/ZPC in H+ ion form was taken in 20 mL solutions of diverse metal ions and preserved for 24 h with constant shaking at 25 ± 2 °C until equilibrium was attained. The concentrations of metal ions were evaluated by EDTA titration [74]. The Kd values were calculated as [74]:
Binary Separations of Metal Ions
In this process, 1.0 g of GT/ZPC in H+ form was taken in column and the mixture of two metal ions (Al3+–Zn2+, Mg2+–Zn2+, Cu2+–Cd2+, Pb2+–Cd2+, Pb2+–Mg2+, Al3+–Cu2+, Cd2+–Al3+ and Ni2+–Co2+) was loaded in the column. The above mixture was permitted to pass gradually by adjusted flow rate to 2–3 drops/min. The column was rinsed with double distilled water to remove the metal ions which were not exchanged. The adsorbed metal ions get eluted on the exchanger via suitable eluting reagents [75,76,77,78,79,80,81]. In 10 mL divisions, the effluent was collected and titrated against EDTA solution.
Selective Separation of Metal Ions
Selective separation of Cd2+ ions from the synthetic mixture having metal ions such as Zn2+, Pb2+, Ni2+, Co2+, Cu2+ was achieved on column of GT/ZPC. In this, the quantity of the Cd2+ ions was varied and quantity of the rest metal ions in synthetic mixture was kept constant. The determination of metal ions in the effluent was achieved by titrating against standard solution of 0.01 M EDTA.
Results and Discussion
The samples of GT/ZPC were prepared by adding a fixed ratio of gelatin into the inorganic material ZP. The varying concentration of biopolymer affects the IEC. It was recorded that the addition of gelatin to Zr(IV) phosphate improved the IEC. The IEC of GT/ZPC (1.04 meq g−1) was better than its inorganic counterpart (0.64 meq g−1) Table 1. The better IEC of GT/ZPC was due to the bonding of gelatin with inorganic part (ZP) as gelatin provides more number of active sites to be attached of replaceable hydrogen ions [82,83,84,85]. GT/ZPC has comparative IEC in comparison to other ion exchangers as described in Table 2.
Temperature variation and mixing amount of reagents has been affected the IEC and yield percentage of GT/ZPC. Effect of heating on the ion exchange capacity was shown in Table 3. It was observed that at ambient temperature, mass, color and IEC of GT/ZPC were changed with increase in temperature. The ion exchanger has high thermal stability because it retained its IEC upto 300 °C which might due to the binding of gelatin moiety with inorganic ZP. The decrease in IEC beyond 300 °C may be due to complete degradation of gelatin from the composite.
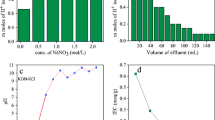
The effect of elution behaviour and eluent concentration for the elution of H+ ions from GT/ZPC is shown in Fig. 1a,b which demonstrated that 250 mL of 0.5 M sodium nitrate solution was the best eluant. The pH titration curve showed one inflection point in the curve confirming the monofunctional behaviour of GT/ZPC ion exchanger (Fig. 1c). The composite material was found to be strong cation exchanger as inferred from itsintial low pH values, when no OH− ions were added. The weak acidic groups remain undissociated at low pH and the solution was neutralized by the addition of NaOH at higher pH. Hence weak group has been dissociated and ion exchange process moves toward completion. The exchange level was noted fast for H+–Na+ in contrast to H+–K+ system as shown in Fig. 1c.
XRD pattern of GT/ZPC ion exchanger was presented in Fig. 1d. The low intensity peaks supported the amorphous nature of GT/ZPC.
FTIR spectra of ZP and GT/ZPC ion exchanger are shown in Fig. 2a, b. A strong and broad peaks at 3399 cm−1 for –OH stretching which shows the presence of lattice water [86], 1649 cm−1 for N–H stretching frequency of amide group, 1545 cm−1 for COO− asymmetric stretching, 1456 cm−1 was due to CH3 asymmetric bending [87, 88] and peak at 1407 cm−1 was due to C–H bending of methylene group of gelatin.The additional bands observed at 1054 and 605 cm−1 was may be due to adsorption peak of C–O and phosphate peaks [89]. The diverse variation in the intensities of bands and appearance of new bands clearly indicated the assimilation of gelatin in the inorganic moiety.
Thermal analysis of GT/ZPC is shown in Fig. 3. The initial loss of about 10% was observed up to 194 °C which was due to loss of surface water molecules of GT/ZPC composite [90]. The weight loss of 21.8, 37.5% between 353 and 647 °C might be due to the breakdown of organic part of the composite.
Figure 4a, b shows the scanning electron microscope images of ZP and GT/ZPC ion exchangers. Figure 3b confirmed the incorporation of the inorganic material with the organic polymer. It was found that after binding of organic part the morphology was completely changed. The rough surface of inorganic part was completely reformed into irregular surface with large surface area.
TEM images of GT/ZPC were shown in Fig. 3c, d. TEM images indicated homogeneous distribution of gelatin and ZP particle with two different morphologies. The darker portion in TEM images specified the wrapped gelatin in ZP particles while grey portion corresponds to gelatin. TEM results confirmed the particle size ranged from 50 to 100 nm with the average particle size 80 nm.
Distributions coefficient studies of GT/ZPC for separations of heavy metal ions were accomplished in various solvents as given in Table 4. It has been found that the Kd values depends upon the solvent nature. The highest Kd values for Cd2+ metal ions showed that this metal was strongly adsorbed on the GT/ZPC. The lowest Kd values for all metal ions in nitric acid was due to the presence of high concentration of H+ ions in acidic medium which inverted the adsorption/ion exchange process [91,92,93,94,95]. The elution of various metal ions viz. Al3+–Zn2+, Mg2+–Zn2+, Cu2+–Cd2+, Pb2+–Cd2+, Pb2+–Mg2+, Al3+–Cu2+, Cd2+–Al3+ and Ni2+–Co2+ is shown in Fig. 5. The metal–ligand stability through the column plays vital role for the consecutive elution of metal ions [96, 97]. It has been observed that weakly adsorbed metal ions were eluted first and strongly eluted at last (Table 5). The Cd2+ metal ion was selectively separated from a synthetic mixture of metal ions containing Zn2+, Pb2+, Ni2+, Co2+, Cu2+ and Cd2+ on the column of GT/ZPC. The amount of Cd2+ ion was varied and other metal ions remained constant in the synthetic mixtures. Table 6 showed the separation of Cd2+ was efficient and the recovery was more than 96%.
Conclusion
GT/ZPC was characterized by various techniques. The material had better IEC as compared to its inorganic counterpart (ZP). Due to the differences in Kd values of metal ions, a few binary separations have been performed on the columns of GT/ZPC. Sorption studies revealed that GT/ZPC was selective for Cd2+ metal ion. This material was favourably used for the separation of Cd2+ ions from binary and synthetic mixtures of metal ions. GT/ZPC was selectively used for the separation of Cd2+ ions, one of the most noxious metal ions existing in environment.
References
Saravanan R, Sacari E, Gracia F, Khan MM, Mosquera E, Gupta VK (2016) J MolLiq 221:1029–1033
Rajendran S, Khan MM, Gracia F, Qin J, Gupta VK (2016) Sci Rep 6:31641–31652
Saravanan R, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015) J Saudi Chem Soc 19:521–527
Saravanan R, Gracia F, Khan MM, Poornima V, Gupta VK, Narayanan V, Stephen A (2015) J Mol Liq 29:374–380
Saravanan R, Khan MM, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015) RSC Adv 5:34645–34651
Saravanan R, Khan MM, Gupta VK, Mosquera E, Gracia F, Narayanan V, Stephen A (2015) J Colloid Interface Sci 452:126–133
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Mater Sci Eng 32:12–17
Gupta VK, Saleh TA, Pathania D, Rathore BS, Sharma G (2015) Ionics 21:1787–1793
Sharma G, Naushad M, Kumar A, Rana S, Sharma S, Bhatnagar A, Stadler FJ, Ghfar AA, Khan MR (2017) Process Saf Environ Prot 109:301–310
Rathore BS, Sharma G, Pathania D, Gupta VK (2014) Carbohydr Polym 103:221–227
Sharma G, Gupta VK, Agarwal S, Kumar A, Thakur S, Pathania D (2016) J Mol Liq 219:1137–1143
Sharma G, Naushad M, Pathania D, Kumar A (2016) Desalin Water Treat 57:19443–19455
Naushad M, ALOthman ZA, Sharma G, Inamuddin (2015) Ionics 21:1453–1459
Devaraj M, Saravanan R, Deivasigamani R, Gupta VK, Gracia F, Jayadevan S (2016) J Mol Liq 221:930–941
Ersahin ME, Ozgun H, Dereli RK, Ozturk I, Roest K (2012) Bioresour Technol 122:196–206
Mittal A, Naushad M, Sharma G, ALothman ZA, Wabaidur SM, Alam M (2016) Desal Water Treat 57:21863–21869
Shahat A, Awual MR, Naushad M (2015) Chem Eng J 271:155–163
Gupta VK, Agarwal S, Pathania D, Kothiyal NC, Sharma G (2013) Carbohyd Polym 96:277–283
Albadarin AB, Collins MN, Naushad M, Shirazian S(2017) Chem Eng J 307:264–272
Akar ST, Akar T, Kaynak Z, Anilan B, Cabuk A, Tabak AZ, Demir TA, Gedikbey T (2009) Hydrometallurgy 97:98–104
Christensen AN, Andersen EK, Andersen IK, Alberti G, Nielsen M, Lehmann MS (1990) Acta Chem Scand 44:865–872
Alberti G, Constantino U (1974) J Chromatogr A 102:5–29
Alberti G, Costantino U (1984) J Mol Catal 27:235–250
Alberti G, Costantino U, Környei J, Giovagnotti ML (1985) React Funct Polym 4:1–10
Alberti G, Costantino U, Millini R, Perego G, Vivani R (1994) J Solid State Chem 113:289–295
Alberti G, Costantino U, Allulli S, Tomassini N (1978) J Inorg Nucl Chem 40:1113–1117
Clayden NJ (1987) J Chem Soc Dalton Trans 8:1877–1881
Alberti G, Costantino U, Marmottini F, Vivani R, Zappelli P (1993) Angew Chem Int Ed 32(9):1357–1359
Clearfield A, Wang JD, Tian Y, Stein E, Bhardwaj C (1995) J Solid State Chem 117:275–289
Yokomori Y, Idaka S (1998) Microporous Mesoporous Mater 21:365–370
Varshney KG, Pandith AH (2001) J Indian Chem Soc 78:250–253
Clearfield A, Bortun AI, Bortun LN, García J (1998) Inorg Chem Commun 1:206–208
Clearfield A, Smith GD (1969) Adv Inorg Chem 8:431–436
Siddiqui WA, Khan SA, Inamuddin (2007) Coll Surfaces A: Phys Eng Aspects 295:193–199
Al-Othman ZA, Alam MM, Naushad M (2013) J Ind Eng Chem 19:956–960
Rehman SU, Islam N, Ahad S, Fatima SZ, Pandith AH (2013) J Hazard Mater 260:313–322
Ngah WW, Teong LC, Hanafiah MA (2011) Carbohydr Polym 83:1446–1456
Ahad S, Islam N, Bashir A, Rehman SU, Pandith AH (2015) RSC Adv 5:92788–92798
Yu JG, Zhao XH, Yang H, Chen XH, Yang Q, Yu LY, Jiang JH, Chen XQ (2014) Sci Total Environ 482:241–251
Yu JG, Yu LY, Yang H, Liu Q, Chen XH, Jiang XY, Chen XQ, Jiao FP (2015) Sci Total Environ 502:70–79
Ahad S, Bashir A, Manzoor T, Pandith AH (2016) RSC Adv 6:35914–35927
Gupta VK, Pathania D, Kothiyal NC, Sharma G (2014) J Mol Liq 190:139–145
Alqadami AA, Naushad M, Abdalla MA, Ahmad T, ALOthman ZA, ALShehri SM, Ghfar AA (2017) J Cleaner Production 10:426–436
ALOthman ZA, Inamuddin, Naushad M (2011) Chem Eng J 169:38–42
Fopah-Lele A, Rohde C, Neumann K, Tietjen T, Rönnebeck T, N’Tsoukpoe KE, Osterland T, Opel O, Ruck WKL (2016) Energy 114:225–238
Gupta VK, Sharma G, Pathania D, Kothiyal NC (2015) J Ind Eng Chem 21:957–964
Hassan SSM, Marei SA, Badr IH, Arida HA (2001) Anal Chim Acta 427:21–28
Dzyazko Y, Rozhdestveskaya L, Zmievskii Y, Volfkovich Y, Sosenkin V, Nikolskaya N, Vasilyuk S, Myronchuk V, Belyakov V (2015) Mater Today 2:3864–3873
Arrad O, Sasson Y (1989) J Org Chem 54:4993–4998
Sharma G, Kumar A, Naushad M, Pathania D, Sillanpää M (2016) J Ind Eng Chem 33:201–208
Gupta VK, Jain R, Varshney S (2007) J Colloid Interface Sci 312:292–296
Sharma G, Naushad M, Kumar A, Devi S, Khan MR (2015) Iran Polym J 24:1003–1013
Nabi SA, Naushad M, Bushra R (2009) Adsorpt Sci Technol 27:423–437
Nabi SA, Naushad M, Bushra R (2009) Chem Eng J 152:80–87
Nabi SA, Shahadat M, Bushra R, Shalla AH, Ahmed F (2010) Chem Eng J 165:405–412
Sanghavi BJ, Mobin SM, Mathur P, Lahiri GK, Srivastava AK (2013) Biosens Bioelectron 39:124–132
Sanghavi BJ, Sitaula S, Griep M, Karna S, Ali M, Swami N (2013) Anal Chem 85:8158–8165
Sanghavi BJ, Srivastava AK (2013) Analyst 138:1395–1404
Mittal A, Gupta VK, Malviya A, Mittal J (2008) J Hazard Mater 151:821–832
Mittal A, Mittal J, Malviya A, Gupta VK (2009) J Colloid Interface Sci 340:16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010) J Colloid Interface Sci 344:497–507
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) J Colloid Interface Sci 343:463–473
Awual MR, Hasan MM, Naushad M, Shiwaku H, Yaita T (2015) Sen Actuat B 209:790–797
Kumar A, Guo C, Sharma G, Pathania D, Naushad M, Kalia S, Dhiman P RSC Adv 6(16):13251–13263
Sharma G, Thakur B, Naushad M, Al-Muhtaseb AH, Kumar A, Sillanpaa M, Mola GT (2017) Mat Chem Phys 193:129–139
Crespo J, Satorre MA, Quintana JA, Ania F (1995) J Mater Sci30:6145–6150
Digenis GA, Gold TB, Shah VP (1994) J Pharm Sci 83:915–921
Zhao W, Kloczwski A, Mark JE, Erman B, Bahar I (1996) Chem Tech26:32–38
Bigi A, Panzavolta S, Roveri N (1998) Biomaterials 19:739–744
Sharma G, Naushad M, Al-Muhtaseb AH, Kumar A, Khan MR, Kalia S, Shweta, Bala M, Sharma A (2017) Int J Bio Macromol 95:484–493
ALOthman ZA, Naushad M, Inamuddin (2011) Chem Eng J 172:369–375
Pathania D, Sharma G, Naushad M, Kumar A (2014) J Ind Eng Chem 20:3596–3603
Pathania D, Sharma G, Thakur R (2015) Chem Eng J 267:235–244
Reilley CN, Schmid RW, Sadek FS (1959) J Chem Educ 36:555–564
Nabi SA, Naushad M (2008) Coll Surf A 316:217–225
Saravanan R, Gupta VK, Mosquera E, Gracia F (2014) J Mol Liq 198:409–412
Saravanan R, Prakash T, Gupta VK, Stephen A (2014) J Mol Liq 193:160–165
Saravanan R, Gupta VK, Narayanan V, Stephen A (2013) J Mol Liq 181:133–141
Saravanan R, Karthikeyan S, Gupta VK, Sekaran G, Narayanan V, Stephen A (2013) Mater Sci Eng 33(1):91–98
Saravanan R, Thirumal E, Gupta VK, Narayanan V, Stephen A (2013) J Mol Liq 177:394–401
Saravanan R, Gupta VK, Prakash T, Narayanan V, Stephen A (2013) J Mol Liq 178:88–93
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Waste manage 17:517–522
Gupta VK, Saleh TA (2013) Environ Sci Pollut Res 20:2828–2843
Pathania D, Sharma G, Naushad M, PriyaV (2016) Desalin Water Treat 57:468–475
Gupta VK, Agarwal S, Asif M, Fakhri A, Sadeghi N (2017) J Colloid Interface Sci 497:193–200
Sharma G, Pathania D, Naushad M (2015) Ionics 21:1045–1055
Pathania D, Thakur M, Sharma A, Agarwal S, Gupta VK (2016) Ionics 1–8
Jacinth MK, Muthulakshmi R, Prathees CK, Subramaniam P, Murugesan R (2017) Polym Plast Technol 26:55–70
Kaushal S, Badru R, Kumar S, Sharma PK, Mittal SK, Singh P (2016) RSC Adv 6:111606–111615
Gershevitz O, Sukenik CNJ (2004) Am Chem Soc 126:482–483
Inamuddin, Khan SA, Siddiqui WA, Khan AA(2007) Talanta 71:841–847
Kumar S, Rai SB (2010) Indian J Pure Appl Phys 48:251–255
Mani P, Suresh S (2009) Rasayan J Chem 2:307–311
Sharma G, Naushad M, Pathania D, Kumar A (2016) Desalin Water Treat 57:19443–19455
Sharma G, Pathania D, Naushad M, Kothiyal NC (2014) Chem Eng J 251:413–421
Sharma G, Pathania D, Naushad M (2014) J Ind Eng Chem 20:4482–4490
Pathania D, Thakur M, Mishra AK (2017) J Alloys Compd 701:153–162
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no RG-1436-034. The authors are also thankful to the Department of Chemistry, Shoolini University, Solan, India for research facilities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Thakur, M., Pathania, D., Sharma, G. et al. Synthesis, Characterization and Environmental Applications of a New Bio-Composite Gelatin-Zr(IV) Phosphate. J Polym Environ 26, 1415–1424 (2018). https://doi.org/10.1007/s10924-017-1043-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1043-0