Abstract
In this study, 10 wt% sugarcane bagasse fiber-reinforced starch foam composites were prepared with different oregano essential oil (OEO) contents in the range of 2–8 wt% to improve the antimicrobial effectiveness of bioactive packaging. FT-IR analysis confirms that OEO penetrates into starch foam composite. From the result of antimicrobial activity, the incorporation of 8 wt% OEO in starch foam composite achieves the minimum inhibitory concentration against Escherichia-coli (E. coli) and Staphylococcus-aureus (S. aureus). OEO is more effective against S. aureus (Gram-positive bacteria) than E. coli (Gram-negative bacteria). The water absorption capacity and hygroscopicity of foam composite decrease with increasing OEO content. The lowest monolayer value is observed in starch foam composite containing 8 wt% OEO. The soil biodegradation rate and flexural strength of samples slightly decrease with increasing OEO content. As a result, starch foam composite containing OEO can be applied as bioactive packaging to preserve foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of materials from renewable resources has attracted a lot of attention. The use of bio-based material is one alternative to solve a critical environmental problem from petroleum-based products consumption [1, 2]. These products are non-biodegradable and require several years for degradation [3–5]. Various methods such as incineration and recycling have been used to manage the plastic waste. However these methods are insufficient for solving the global environmental pollution [6, 7].
Starch is one of the alternative materials that have been widely used to produce biodegradable packaging due to its abundant and environmentally friendly [8, 9]. The common starch used for producing foam packaging includes cassava [10], corn [11], potato [12], and rice [13]. Novel sugar palm starch has lately been reported to be a good thin film-forming material for food packaging application due to its high amylose content (~37%) as compared to conventional starch polymers [14–16]. Previous research reported that degree of polymerization efficiency was dependent on amylose content of the starch. Starch with different amylose/amylopectin ratio have different phase transition behaviors and rheological properties [17]. However, native starch has some disadvantages i.e. high water absorption and poor mechanical properties [18]. Recently, natural fibers such as sugarcane bagasse [19], cassava bagasse [20, 21], kraft pulp [22], sugar palm fiber [16] and oil palm fiber [13] have been used as reinforcing phase to improve the mechanical properties. Water absorption of starch foam was also enhanced by the addition of hydrophobic materials such as palm oil [22], essential oil [20, 23–25], beeswax [26] and agar [14]. Essential oils are interesting hydrophobic natural additives with antimicrobial and antioxidant properties [20]. They are classified as generally recognized as safe (GRAS) compound and extensively used in the food industry [27, 28]. Essential oils have been incorporated into various types of bioactive packaging in order to extend the shelf life of food products and inhibit the growth of food-borne bacteria [20, 23–25]. The addition of essential oils into edible films have been reported by many researchers whereas few studies have been focused on the effect of essential oils on the properties of biodegradable foams [29–33]. Debiagi et al. [20] studied foam trays from synthetic biopolymer such a polyvinyl alcohol composite containing clove and oregano essential oils. The addition of essential oils improved the water adsorption and flexibility of specimens. The foam trays incorporated with oregano essential oil was the most effective on antimicrobial activity to inhibit the growth of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).
Currently, there is no information about the effect of oregano essential oil (OEO) on the overall properties of natural biopolymer foam composite. Therefore, the purpose of this research is to investigate the properties of starch foam composite containing OEO in terms of physical, mechanical and biodegradable characteristics. The antimicrobial properties of the samples against gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli) are observed.
Materials and Methods
Materials
Cassava starch was purchased from Bangkok Interfood Ltd., Thailand. Sugarcane fiber with particle size of 180–250 µm was used. The OEO (Origanum vulgare) was obtained from Sigma-Aldrich Co. LLC.
Microbial Culture
Gram-positive bacteria (S. aureus ATCC25923) and gram-negative bacteria (E. coli ATCC25922) were obtained from Department of Microbiology, Faculty of Science, Khon Kaen University, Khon Kaen, Thailand.
Preparation of Starch Foam Composite Containing OEO
The starch foam composite was prepared by adding 30 ml of water to 30 g of cassava starch and mixed for 5 min using kitchen aid mixer (Electrolux EHM 2000). The sugarcane fiber at 10 wt% of starch were added and mixed for another 5 min at 80 °C. The OEO at the dosages of 2, 4, 6 and 8 wt% of starch was added and mixed for 3 min. The obtained compound was pressed in 100 mm × 100 mm × 6.5 mm mold with hydraulic press using a pressure of 4.0 MPa at 230 °C for 5 min. The high temperature in the range of 190–250 °C was recommended to achieve the minimum baking time and maximum venting of water from batter which causes the starch to foam out and take up the shape of the mold [22, 26, 34, 35]. The samples of starch-based foam specimens are demonstrated in Fig. 1.
Characterization of the Starch Foam Composite Containing OEO
Attenuated total reflection infrared (FTIR–ATR) spectra of sample were tested by Bruker Tensor 27 spectrometer. All spectra were taken as a function of time with 64 scans at a resolution of 4 cm−1 and a spectral range of 4000–650 cm−1.
The surface of sample was investigated using a scanning electron microscope (SEM) (Hitachi model S-3000N) at an acceleration voltage of 20 kV. All samples were cut to 3 mm and coated with gold using an ion sputtering device.
Water absorption capacity (WAC) was tested in accordance with Cobb test [36]. The dimension of specimen was 50 mm × 25 mm × 6.5 mm. All samples were weighted and submerged in 100 ml of distilled water for 1, 5, 10, 15 and 30 min and then removed, wiped and weighted. WAC was calculated according to the weight difference and expressed as the mass of absorbed water per mass of the original sample. The reported values are the average of five samples.
Water sorption isotherm of starch foam composites containing OEO were determined by GAB (Guggenheim, Anderson, de Boer) model. The GAB model was used to calculate the isotherm data and monolayer values of various types of starch foam composites. The GAB isotherm is shown in Eq. (1) [20, 37].
where M is the equilibrium moisture content at a given water activity (\(a_{w}\)), \(a_{w}\) is RH/100, \(m_{o}\) is the monolayer value (g water/g solid) and C and K are the GAB constants.
The 0.5 g samples were weighted and pre-dried for 15 days over anhydrous calcium chloride before the moisture sorption isotherm test. The samples were then placed over saturated salt solution in separate desiccators at specific relative humidity (10, 38, 43, 79 and 84) and held at 25 °C. The equilibrium moisture was calculated from the dry sample mass gain at each relative humidity. At each relative humidity condition, the weight of sample was recorded at the initial and final incubation times (14 days).
Antimicrobial activity of the samples was studied using the agar diffusion method according to a modified version of Hosseini et al. [38]. Two different foodborne pathogens viz., E. coli and S. aureus were used as representative strains of pathogens. The bacterial were cultured overnight in brain heart fusion broth at 37 °C, then bacterial culture was diluted in medium until turbidity equal to McFarland scale 0.5 (approximately 108 CFU/ml). The Muller Hinton Agar plates were seeded with bacterial inoculums by swab plate technique. The sterile samples were cut into 6 mm disk and placed plates. The diameter of inhibition zone (mm) around the disc was measured after 24 h of incubation at 37 °C.
Flexural strength of starch foam composites containing OEO was tested by universal testing machine (Instron, Model 5567) equipped with a 10 kN load cell in accordance with ASTM D 790-10 [39]. The test was performed in a 3-point bending mode with a support span of 100 mm at the crosshead speed of 2.67 mm/min.
Soil burial degradation test was performed using the method suggested by Ibrahim et al. [40]. The dimension of specimen was 40 mm × 6.5 mm × 2 mm. All specimens were buried at 10 cm depth in a mixture of 50% sand and 50% soil with temperature of 30 ± 2 °C and water content of 35 ± 5%. After 30 days, the specimens were collected and washed with distilled water and dried in the oven at 105 °C for 24 h. The weight loss was calculated. The reported results were the average of three samples.
Results and Discussion
Attenuated Total Reflection Infrared Analysis of Starch Foam Composite Containing OEO
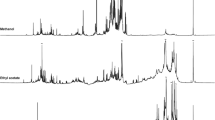
The interactions of starch matrix and additives viz., sugarcane bagasse and OEO were analyzed by FTIR spectroscopy. ATR–FTIR spectra of starch, sugarcane bagasse and OEO are shown in Fig. 2. Figure 2a showed the characteristic FTIR graph of starch. The peaks at 3280 and 1645 cm−1 were attributed to O–H stretching and bending of water, respectively. The adsorption band at 2918 cm−1 was assigned to C–H stretching whereas the peak at 1443 cm−1 was related to C–H2 stretching. The peaks at 986 and 1158 cm−1 were attributed to C–O stretching [41, 42]. The characteristic peaks of sugarcane bagasse are shown in Fig. 2b. The main characteristic peaks were attributed to lignin, cellulose and hemicellulose. The broad bands at 3336 and 2926 cm−1 were assigned to O–H group and C–H group, respectively. The peaks at 1727 cm−1 was related to C=O of hemicellulose. The peaks at 1427 and 1236 cm−1 were assigned to C–H2 and C–O–C of cellulose chain. The band at 1145 cm−1 corresponded to the asymmetric deformation of C–O–C of cellulose and hemicellulose [43]. Figure 2c showed the characteristic of adsorption bands of OEO. The broad band in the range of 3300–3600 cm−1 was related to O–H group of phenolic compounds. The peak at 1627 cm−1 was assigned to C=C vinyl ester stretching from terpenic derivatives such a beta-farnesene in OEO. The peaks at 1581 and 1518 cm−1 were attributed to benzene ring of the main phenolic compounds i.e. carvacol and thymaol. The peak at 1427 cm−1 was also related to O–H bending of the phenolic compounds. The sharp peak at 812 cm−1 was assigned to O–H bending out of plane in di- and tri-substituted aromatic compounds such as carvacol, thymol and derivative terpenes [33, 44].
Generally, the shift of characteristic spectra peak indicated the chemical interactions of polymer blend due to hydrogen bond or dipolar interaction [10, 45]. Figure 3 depicted the adsorption bands of starch foam reinforced with sugarcane bagasse (SCB) and blended SCB–OEO. The presence of characteristic peaks of OEO in starch foam composite was observed as shown in Fig. 3b. This result confirmed that the major components of OEO penetrated into the starch foam composite after processing. Furthermore, the shift of spectra at 3200–3400 and 1443 cm−1 for SCB and blended SCB–OEO was also observed as illustrated in Fig. 3a, b. This phenomenon indicated the intermolecular hydrogen bond among starch matrix, sugarcane bagasse and OEO.
Antimicrobial Activity
The results of antimicrobial activity of samples containing 2, 4, 6 and 8 wt% OEO against E. coli and S. aureus are shown in Fig. 4 and summarized in Table 1. The addition of 8 wt% OEO was required for starch foam composite to achieve the minimum inhibitory concentration against E. coli and S. aureus. The lower concentration of OEO had no observed the growth inhibition. The antimicrobial activity of OEO was attributed to oregano’s terpenoid fraction consisted of carvacrol, thymol and p-cymene [46, 47]. These components were hydrophobic which enabled them to partition in the lipid of bacterial cell membrane and mitochondria, resulting in the leakage of ions from the cytoplasm and cell death [48]. Moreover, the starch foam incorporated with 8 wt% OEO showed the largest clear zone for S. aureus, as clearly depicted in Fig. 4. This observation indicated that OEO was more effective against S. aureus (Gram-positive bacteria) than E. coli (Gram-negative bacteria). It is hypothesized that the presence of outer membrane surrounding the cell wall in gram negative bacteria restricts the diffusion of hydrophobic compounds via its lipopolysaccharide covering [48, 49]. Similar observations were also observed in biodegradable polymer incorporated with OEO such as cassava bagasse/polyvinyl alcohol [20], fish gelatin/chitosan film [46], biogenic gelatin film [50] and cassava starch/chitosan film [33]. Based on this result, it can be concluded that the starch foam composite incorporated OEO could be applied as bioactive packaging to preserve foods.
Water Absorption Capacity (WAC)
The WAC of starch foam composite containing 2, 4, 6 and 8 wt% OEO is depicted in Fig. 5. The plot of percentage of WAC against time for all samples showed similar behavior. The sample rapidly absorbed water during first stage (0–5 min). The WAC values of samples decreased with increasing OEO content and ranged between 257 and 324% after 30 min of immersion. The starch foam composite containing 8 wt% OEO was saturated after 20 min of water immersion while the other samples presented the increase of WAC values. This was due to the incorporation of OEO improved the water resistant of samples by increasing the hydrophobicity of composite [41]. This observation is in good agreement with the previous reports. Debiagi et al. [20] showed that the addition of OEO can reduce the WAC of polyvinyl alcohol foam containing cassava bagasse. Kaisangsri et al. [22] reported that the incorporation of palm oil and natural fiber improved both water solubility and water absorption indices of starch foams. The synergistic effect for water resistance of starch foam containing combined additives such as kaolin, beewax and natural fiber were also observed [26].
Water Sorption Isotherms
The water sorption isotherms of the starch foam composites containing 0, 4 and 8 wt% OEO are shown in Fig. 6 and GAB model parameters are summarized in Table 2. All samples showed similar trends of isotherm sorption i.e. the equilibrium moisture content value increased with increasing relative humidity. This observation is in good agreement with the previous works [20, 51].
The monolayer value is a parameter obtained from GAB model. This parameter indicates the maximum amount of absorbed water in a single layer per gram of dry material. It is a measure of number of sorbing sites in the water sample [52]. Table 2 showed that the monolayer value of samples was in range of 0.027–0.037 g/g. The monolayer value tended to decrease with increasing OEO content. The lowest monolayer value was observed with 8 wt% OEO in starch foam composite. This result indicated that the addition of OEO in starch foam composite reduced the hygroscopicity of starch foam.
From Table 2, parameter C corresponded to the difference of water sorption in the upper layers and in monolayer. This parameter was affected by the addition of OEO. Parameter C of composite without OEO was 3.848 and increased to 8.685 and 7.0582 with 4 and 8% additions of OEO, respectively. Parameter K was, however, independent of the composition of starch foam composite. The result is in a good agreement with previous works [53, 54].
Biodegradation in Soil
The weight loss of sample during soil burial test indicates the biodegradation process by the moisture and microorganism in soil [40, 55]. Figure 7 presents the weight loss of starch foam composites containing 2, 4, 6 and 8 wt% OEO after 30 days of soil burial. The weight loss values were 28.80–57.09%. The weight loss of samples decreased with increasing OEO content. As shown in Fig. 8a–e, the surface morphology of all samples changed after the soil burial test. Some cracks and holes were clearly observed on the surface of starch foam composites due to high biodegradation of starch, especially starch foam composite without OEO as shown in Fig. 8a. For the starch foam composites containing OEO as depicted in Fig. 8b–e, the existence of cracks and holes reduced as the content of OEO increased. This indicated that the OEO hindered the penetration of water and microorganism due to its hydrophobicity. The biodegradation rate decreased when the hydrophobicity of polymer matrix increased [55]. It should be noted here that the components of essential oil also affected the microorganism in soil. The essential oil can inhibit spore germination and mycelia growth of fungi whereas some microorganism can use the essential oil as substrate of growth [56].
Flexural Strength of Starch Foam Composite Containing OEO
Bending is the most common deformation of starch-based foam packing. The results from the bending mode fracture can indicate the failure of expanded particle of foam and thus reveals the strength of inter-particle-fusion. Therefore, this study focused on the flexural properties of starch foam composites [9, 10]. The flexural properties are necessary starch foam composite. Another type of packaging material such a starch film, it is a thin film and does not have the flexural strength. Figure 9 shows the flexural strength of starch foam composites containing OEO. The flexural strength values ranged from 0.86 to 1.74 iMPa with the addition of 0–8 wt% OEO. The flexural strength of samples slightly decreased with increasing OEO content. The decrease of flexural strength in composite was due to the plasticizing effect of the OEO on starch foam composite. Similar result was also observed in polyvinyl alcohol foam composite modified with essential oil [20]. However, the flexural strength of starch foam composites containing OEO was comparable to those of previous reported composites of starch foam blended with palm oil (0.25–1.10 MPa) [22], soy protein (0.5–0.8 MPa) [22], gluten (0.28–0.34 MPa) [22], oil palm fiber/chitosan/palm oil (1.04–2.21 MPa) [10], latex rubber/calcium carbonate (1.05–3.7 MPa) [57], and polylactic acid (0.4–1.5 MPa) [58]. However, with the high content of 8 wt% OEO, the flexural strength was 0.86 MPa which was comparable with commercial foam packaging based on expanded polystyrene (0.85–0.97 MPa) [20].
Conclusions
The properties of starch foam composites with 10 wt% of sugarcane bagasse and different OEO contents were studied in this research. The antimicrobial effectiveness test revealed that the addition of 8 wt% OEO was required for starch foam composite to achieve the minimum inhibitory concentration against E. coli and S. aureus. The OEO was more effective against S. aureus (Gram-positive bacteria) than E. coli (Gram-negative bacteria). The addition of 8 wt% OEO exhibited the largest clear zone for S. aureus. The WAC values of samples after 30 min of immersion in water decreased with increasing OEO content. The starch foam composite containing 8 wt% OEO was saturated after 20 min of immersion in water. The water absorption capacity and hygroscopicity of starch foam decreased. The maximum amount of absorbed water in a single layer tended to decrease with increasing OEO content. The presence of 8 wt% OEO in composite provided the lowest monolayer value. The soil biodegradation test indicated that the weight loss of samples decreased with increasing OEO content and the biodegradation rate also decreased. The flexural strength of sample was comparable with that of commercial foam. These results suggested that the starch foam composite containing OEO has potential to be used as a green bioactive composite.
References
Edhirej A, Sapuan SM, Jawaid M, Zahari NI (2016) Polym Compos. doi:10.1002/pc.24121
Rodrigues CA, Tofanello A, Nantes IL, Rosa DS (2015) ACS Sustain Chem Eng 3:2756–2766
Carmona VB, Corrêa AC, Marconcini JM, Mattoso LHC (2015) J Polym Environ 23:83–89
Sun Q, Mekonnen T, Misra M, Mohanty AK (2016) J Polym Environ 24:23–36
Trujillo-de Santiago G, Rojas-de Gante C, García-Lara S, Verdolotti L, Maio ED, Iannace S (2015) J Polym Environ 23:72–82
Pornsuksomboon K, Holló BB, Szécsényi KM, Kaewtatip K (2016) Carbohydr Polym 136:107–112
Guarás MP, Alvarez VA, Ludueña LN (2016) J Appl Polym Sci 133:44163
Trujillo-de Santiago G, Rojas-de Gante C, García-Lara S, Verdolotti L, Maio ED, Iannace S (2014) J Polym Environ 22:508–524
Aygün A, Uslu MK, Polat S (2016) J Polym Environ. doi:10.1007/s10924-016-0886-0
Kasemsiri P, Dulsang N, Pongsa U, Hiziroglu S, Chindaprasirt P (2016) J Polym Environ. doi:10.1007/s10924-016-0818-z
Ramesh S, Shanti R, Morris E (2012) Carbohydr Polym 87:701–706
Beilvert A, Chaubet F, Chaunier L, Guilois S, Pavon-Djavid G, Letourneur D (2014) Carbohydr Polym 99:242–248
Prachayawarakorn J, Limsiriwong N, Kongjindamunee R, Surakit S (2011) J Polym Environ 20:88–95
Jumaidin R, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Int J Biol Macromol 89:575–581
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Carbohydr Polym 146:36–45
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) BioResources 11:4134–4145
Zou W, Yu L, Liu X, Chen L, Zhang X, Qiao D et al (2012) Carbohydr Polym 87:1583–1588
Ago M, Ferrer A, Rojas OJ (2016) ACS Sustain Chem Eng 4:5546–5552
Vercelheze AE, Fakhouri FM, Dall’Antônia LH, Urbano A, Youssef EY, Yamashita F, Mali S (2012) Carbohydr Polym 87:1302–1310
Debiagi F, Kobayashi RK, Nakazato G, Panagio LA, Mali S (2014) Ind Crops Prod 52:664–670
Debiagi F, Marim BM, Mali S (2015) J Polym Environ 23:269–276
Kaisangsri N, Kerdchoechuen O, Laohakunjit N (2014) Carbohydr Polym 110:70–77
Petchwattana N, Covavisaruch S, Wibooranawong S, Naknaen P (2016) Measurement 93:442–448
Petchwattana N, Naknaen P (2015) Mater Chem Phys 163:369–375
Bhullar SK, Kaya B, Jun M-BG (2015) J Polym Environ 23:416–423
Polat S, Uslu M-K, Aygün A, Certel M (2013) J Food Eng 116:267–276
Rojas-Graü MA, Avena-Bustillos RJ, Friedman M, Henika PR, Martín-Belloso O, McHugh TH (2006) J Agric Food Chem 54:9262–9267
Desai MA, Parikh J (2015) ACS Sustain Chem Eng 3:421–431
Souza A, Goto G, Mainardi J, Coelho ACV, Tadini CC (2013) LWT Food Sci Technol 54:346–352
Jemaa BM, Falleh H, Neves MA, Isoda H, Nakajima M, Ksouri R (2017) Food Chem 217:726–734
Bonilla J, Sobral PJA (2016) Food Biosci 16:17–25
Klangmuang P, Sothornvit R (2016) Food Hydrocoll 61:609–616
Pelissari FM, Grossmann MV, Yamashita F, Pineda EAG (2009) J Agric Food Chem 57:7499–7504
Kaisangsri N, Kerdchoechuen O, Laohakunjit N (2012) Ind Crops Prod 37:542–546
Soykeabkaew N, Supaphol P, Rujiravanit R (2004) Carbohydr Polym 58:53–63
Método de Cobb (1999) NBR NM-ISO 535:1999 Papel E Cartao-Determinacao Da Capacidade De Absorcao De Agua In: ABNT-Associac¸ ão Brasileira de normas Técnicas
Bizot H (1984) Using the GAB model to construct sorption isotherms. In: Jowitt R, Escher F, Hallistrom B, Meffert HFT, Spiess WEL, Vos G (eds) Physical properties of foods. Applied Science Publishers, London, pp 27–41
Hosseini M, Razavi S, Mousavi M (2009) J Food Process Preserv 33:727–743
ASTM D 790-10 (2010) Standard test methods for flexural properties of unreinforced and reinforced plastics and electrical insulating materials, ASTM International, West Conshohocken
Ibrahim H, Farag M, Megahed H, Mehanny S (2014) Carbohydr Polym 101:11–19
Matsuda DK, Verceheze AE, Carvalho GM, Yamashita F, Mali S (2013) Ind Crops Prod 44:705–711
Prachayawarakorn J, Chaiwatyothin S, Mueangta S, Hanchana A (2013) Mater Des 47:309–315
Mothé CG, de Miranda IC (2009) J Therm Anal Calorim 97:661–665
Salmieri S, Islam F, Khan RA, Hossain FM, Ibrahim HM, Miao C, Hamad WY, Lacroix M (2014) Cellulose 21:4271–4285
Prachayawarakorn J, Limsiriwong N, Kongjindamunee R, Surakit S (2012) J Polym Environ 20:88–95
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2015) Ind Crops Prod 67:403–413
Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F (2013) Carbohydr Polym 95:50–56
Burt S (2004) Int J Food Microbiol 94:223–253
Kahrilas GA, Haggren W, Read RL, Wally LM, Fredrick SJ, Hiskey M, Prieto AL, Owens JE (2014) ACS Sustain Chem Eng 2:590–598
Martucci JF, Gende LB, Neira L, Ruseckaite RA (2015) Ind Crops Prod 71:205–213
Mello LR, Mali S (2014) Ind Crops Prod 55:187–193
Strauss U, Porcja R, Chen S (1991) Volume effects of starch-water interactions. In: Levine H, Slade L (eds) Water relationships in foods: advances in the 1980s and trends for the 1990s, vol 302. Springer, Boston, pp 351–363
Coupland JN, Shaw NB, Monahan FJ, O’Riordan ED, O’Sullivan M (2000) J Food Eng 43:25–30
Mali S, Debiagi F, Grossmann MVE, Yamashita F (2010) Ind Crops Prod 32:353–359
Riaz U, Ashraf SM, Sharma HO (2011) Polym Degrad Stab 96:33–42
Vokou D, Liotiri S (1999) Chemoecology 9:41–45
Shey J, Imam SH, Glenn GM, Orts WJ (2006) Ind Crops Prod 24:34–40
Preechawong D, Peesan M, Supaphol P, Rujiravanit R (2005) Carbohydr Polym 59:329–337
Acknowledgements
This work was financial supported by Khon Kaen University (Synchrotron Light Research). The authors also would like to acknowledge the support of Thailand Research Fund (TRF) under the TRF Senior Research Scholar, Grant No. RTA5780004 and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Advanced Functional Materials Cluster of Khon Kaen University. Support from Applied Engineering for Important Crops of the North East Research group, Khon Kaen University, Thailand is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ketkaew, S., Kasemsiri, P., Hiziroglu, S. et al. Effect of Oregano Essential Oil Content on Properties of Green Biocomposites Based on Cassava Starch and Sugarcane Bagasse for Bioactive Packaging. J Polym Environ 26, 311–318 (2018). https://doi.org/10.1007/s10924-017-0957-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-0957-x