Abstract
The adhesion properties of magnesium oxide filled epoxidized natural rubber (ENR 25)/acrylonitrile-butadiene rubber (NBR) blend adhesives were studied using petro resin and gum rosin as tackifiers. Toluene was used as the solvent throughout the experiment. Five different loadings, i.e. 10, 20, 30, 40 and 50 phr magnesium oxide was used in the adhesive formulation. The SHEEN hand coater was used to coat the adhesive on polyethylene terephthalate at 30 and 120 µm coating thickness. The tack, peel strength and shear strength were determined by a Lloyd adhesion tester operating at 30 cm min−1. Results shows that all the adhesion properties of the ENR 25/NBR adhesives show a maximum value at 10 phr filler loading. Loop tack and peel strength pass through a maximum, an observation which is associated to the optimum wettability of adhesive on the substrate. For the shear test, maximum shear strength occurs due to the optimum cohesive strength of the adhesive. Results also show that all petro resin based adhesives have higher adhesion properties than gum rosin based adhesive. In all cases, the adhesion properties of adhesives also increase with increasing coating thickness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The adhesion property of pressure sensitive adhesive (PSA) depends on the filler content loading and type of tackifier. To achieve viscoelastic behaviour, the rubber (elastic component) have to be mixed with a tackifier (viscous component). Tackifier is the raw material used in PSA formulation. The addition of tackifier to the adhesive formulation can improve the adhesion properties of the adhesive system [1, 2]. From our previous study, we carried out a systematic study of the addition of tackifier into a single rubber component adhesive system. Poh et al. [3] have studied viscosity and shear strength of natural rubber based adhesives in the presence of gum rosin and petro resin. Meanwhile, effect of molecular weight of rubber on tack and peel strength of SMR-L based pressure adhesives using gum rosin and petro resin as tackifiers were investigated by Poh and Yong [4]. Poh and Firdaus [5] have studied the effect of hybrid tackifiers on adhesion properties of epoxidized natural rubber based PSAs. Besides that, Poh and Firdaus [6] also investigated the viscosity, shear strength, and peel strength of (natural rubber) based adhesives containing hybrid tackifiers. On the other hand, Poh and Yong [7] have studied the effect of molecular weight of epoxidized natural rubber on viscosity and tack of PSAs by using coumarone indene resin, gum rosin and petro resin. In view of the scarcity of data reported on the effect of different tackifiers on the adhesion properties with filled PSAs, it is thus the aim of the present study to investigate the effect of petro resin and gum rosin on adhesion property of magnesium oxide filled epoxidized natural rubber (ENR 25)/acrylonitrile-butadiene rubber (NBR) blend adhesive. The work is novel as no author has studied the effect of tackifier on the adhesion property of filled rubber blend adhesive. Our previous study focuses on the dependence of adhesion property on tackifier in a single rubber component adhesive system.
Experimental
Materials
Epoxidized Natural rubber (ENR 25, having 25 mol% of epoxidation) and acrylonitrile-butadiene rubber (NBR, acrylonitrile content of 33 %) were used as the elastomer for the preparation of PSA, The rubbers were supplied by Rubber Research Institute of Malaysia (Kuala Lumpur, Malaysia) and Bayer Company (Penang, Malaysia) respectively. Petro resin and gum rosin were used as the tackifier. It was freshly supplied by Euro Chemo-Pharma Company (Penang, Malaysia). Magnesium oxide and toluene were used as the filler and solvent respectively. Polyethylene terephthalate (PET) film was chosen as the coating substrate throughout the experiment.
Characterization of Magnesium Oxide
Figure 1 shows a SEM micrograph of the magnesium oxide filler used in this study. The photo indicates that agglomeration of magnesium oxide particles with sizes ranging from 2 to 10 μm. Therefore, magnesium oxide needs to be ground before the addition into the rubber adhesive. FTIR spectrum of magnesium oxide is shown in Fig. 2. The presence of small quantity of hydroxyl group is illustrated by the weak absorption peak at 3299.20 cm−1 whereas other oxygenated groups are indicated by absorption peaks at 1028.74 and 1075.86 cm−1. Figure 3 shows the thermogravimetric analysis (TGA) of magnesium oxide filler. From the thermograph, a slight decrease in weight at about 100 °C is attributed to the evaporation of moisture. There is a drop in about 4 wt% in the temperature range of 250–350 °C, an observation which is associated to the loss of organic volatile matter.
Preparation of Adhesive
ENR 25 and NBR were masticated on a two-roll mill for 10 min. 5 g of masticated rubber were shredded into small pieces. Blends ratios in grams of ENR 25/NBR rubber blend is fixed at 1/4, corresponding to 20 % ENR 25 was prepared. The rubber blend was dissolved in 30 ml of toluene and then left for 24 h to ensure complete dissolution. With constant stirring, 2 g of tackifier which corresponded to 40 phr of resin was added slowly to the rubber solution. This was followed by the addition of five different weights of magnesium oxide, i.e., 0.5, 1, 1.5, 2, 2.5 g corresponding to 10, 20, 30, 40 and 50 phr filler were added separately to the rubber solution. For comparison purpose, one control sample without magnesium oxide was used to prepare the adhesive.
Testing
Loop Tack
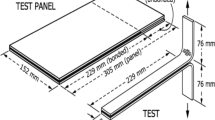
A PET film with dimension of 4 cm × 25 cm was coated with adhesive by using a SHEEN Hand Coater. The coated area was 4 cm × 4 cm at the center of the PET film at a coating thickness of 30 and 120 μm respectively. The coated sample was conditioned at room temperature for 24 h before testing. The PET film strip was formed into a loop and the outer surface with adhesive area was brought into contact with a glass plate. A Lloyd Adhesion Tester operating at 30 cm min−1 was used to measure the debonding force to detach the loop from the glass plate. The loop tack value is expressed as the average debonding force per area of contact [N m−2].
Peel Strength
The peel adhesion test was carried out by using PET film as the base stock and face stock. T-peel test, 90°-peel and 180°-peel tests were the three modes of peeling test that was carried out. The dimensions of PET film for the T- peel test were 20 cm × 4 cm. For the 90° peel test, the dimensions were 20 cm × 4 cm and 15 cm × 7 cm for the base stock and face stock respectively. The respective dimensions of the substrate were 25 cm × 4 cm and 10 cm × 10 cm for the 180° peel test. The adhesive was coated from the end of the PET film at a coating area of 10 cm × 4 cm film at a coating thickness of 30 and 120 μm respectively by using a SHEEN Hand Coater. The face stock was then placed on the coated PET film (base stock). The testing sample was conditioned for 24 h before testing by a Lloyd Adhesion Tester operating at a testing rate of 30 cm min−1. The average peeling force was determined from the three highest peaks recorded from the load-propagation graph. Peel strength is defined as the average load per width of the bond line required to separate progressively a flexible member from a rigid member or another flexible member (ASTM D 907).
Shear Strength
The dimension of the PET film was 20 cm × 4 cm. A SHEEN hand coater was used to coat the adhesive from the end of the film at a coated area of 10 cm × 4 cm for a coating thickness of 30 and 120 μm respectively. One end of the face stock (10 cm × 4 cm) was gently placed on the coated area of the base stock. The sample was conditioned for 24 h before testing by a Lloyd Adhesion Tester operating at a testing rate of 30 cm min−1. The testing distance was 10 cm which corresponded to the length of the coated area. Shear strength was defined as the shear force per unit area of testing (N m−2).
Results and Discussion
Tack
Tack may be defined as the property of a material that enables it to form a bond with low applied pressure [8, 9] Fig. 4 shows the dependence of tack on magnesium oxide loading at 30 and 120 µm coating thickness of petro resin based ENR 25/NBR blend adhesive. The plot shows that loop tack of adhesive increased steadily with increase in magnesium oxide loading up to 10 phr for both coating thicknesses. The increasing tack value with increasing magnesium oxide loading is attributed to the adhesive attains optimum wettability and compatibility on the substrate. At this maximum tack value, the adhesive conforms to the irregularities of the substrate, i.e., low surface energy condition is observed [10]. Further increase in the magnesium oxide loading will cause a dilution effect which lowers the tack value after 10 phr magnesium oxide loading. This phenomenon is attributed to the decreasing wettability and miscibility of adhesive. A similar behavior is also exhibited by the gum rosin based adhesive as shown in Fig. 5. The graph shows that tack value also passes through a maximum value at 10 phr magnesium oxide for both coating thickness. For both tackifiers used, 120 µm coated sample consistently exhibits the higher tack value than that of 30 µm coated sample at the fixed filler loading. This phenomenon is attributed to the higher amount of adhesive in the 120 µm coated sample. This means that more adhesive is available to effect wettability on the substrate, thus shows the higher tack value in the system [11].
Peel Strength
The effect of magnesium oxide on peel strength of petro resin based ENR 25/NBR blend adhesive at 30 and 120 µm coating thickness is shown in Fig. 6a–c for the T-, 90° and 180° peel test respectively. For all the three modes of peel tests, peel strength increases with increasing magnesium oxide loading up to 10 phr filler content for both coating thickness. This observation is ascribed to the reducing of surface tension by magnesium oxide and hence improve wettability of adhesive on the substrate. At this optimum filler loading content, maximum wettability of the adhesive on the substrate which enhances mechanical interlocking and anchorage of the adhesive in pores and irregularities in the adherent [12, 13]. Also, at this optimum filler loading content, enhancement of peel strength of adhesive is due to the maximum compatibility between magnesium oxide and the adhesive system. After the optimum loading, peel strength decrease with further addition of filler because of the dilution effect of magnesium oxide as a filler loading increased. The drop in peel strength is associated to the reducing miscibility and wettability of adhesive. Figure 7 shows a SEM micrograph of adhesive containing 10 phr magnesium oxide after 90° peel test. The SEM micrograph indicates that magnesium oxide is well dispersed in the petro resin based ENR 25/NBR blend adhesive. Besides, it clearly demonstrates the occurrence of cohesive and adhesion failure modes of the filled rubber adhesive. The cohesive failure mode is indicated by the partial adhesive remains on the substrate whereas the clean PET surface detached by the adhesive is attributed to the adhesion failure mode after the peel test. Figure 8 displays the FTIR spectrum of the magnesium oxide filled adhesive. The absorption band at 3696 cm−1 indicates the presence of magnesium oxide as confirmed by the FTIR spectrum for the filler in Fig. 2. The absorptions at 2915 and 1028 cm−1 are attributed to the C–H and C–O stretching vibration respectively [14, 15] due to the presence of NBR and ENR. The TGA of petro resin based ENR 25/NBR adhesive containing various loadings of magnesium oxide is shown in Fig. 9. From the thermograph, it is observed that the onset temperature of degradation of the adhesive system increases with the increase of magnesium oxide loading, suggesting that magnesium oxide enhances the thermal stability of the adhesive. This observation is similar to that reported by Ghanbari et al. [14] and Gholamian et al. [15] on the effect of magnesium hydroxide on the thermal stability of polymers. Figure 9 also shows that ash content increases with magnesium oxide loading, an observation which is attributed to the increasing amount of inorganic matter. In the case of gum rosin study, the peel strength of ENR 25/NBR blend adhesive at 30 and 120 µm coating thickness show similar behavior as petro resin based adhesive as shown in Fig. 10a–c for the T-, 90° and 180° peel test respectively. For both tackifiers used, the higher peel strength is exhibited by 120 µm coating thickness. Again, this observation is similar to that obtained for tack as discussed previously. A SEM micrograph on the peeling surface of magnesium oxide filled adhesive using gum rosin as the tackifier is shown in Fig. 11. It shows that magnesium oxide is well dispersed in the gum rosin based ENR 25/NBR blend adhesive. The diagram also indicates that the occurrence of cohesive and adhesion failure mode similar to that observed in the case of petro resin based adhesive. The undetached adhesive on the substrate is associated with cohesive failure. On the contrary, the detached portion of adhesive on the PET film is ascribed to adhesion failure. Figure 12 illustrates the FTIR spectrum of gum rosin based ENR 25/NBR adhesive containing 10 phr of magnesium oxide. The occurrence of 3695 cm−1 absorption band indicates the presence of magnesium oxide [14]. Meanwhile, the absorptions at 2918 and 1031 cm−1 are associated to the C–H and C–O stretching vibration [15] in NBR and ENR. Figure 13 shows the TGA of gum rosin based ENR 25/NBR adhesive containing various loadings of magnesium oxide. The thermograph demonstrates similar thermal behavior as that of petro resin based adhesive. Again, the curve shifts towards higher degradation temperature as magnesium loading is increased, implying that the filler enhances the thermal stability of gum rosin based adhesive. The ash content also increases with magnesium oxide loading due to the increased presence of the inorganic matter. Figure 14 compares the peel strength between petro resin based adhesive with that of gum rosin. From the graph, all three modes of peel tests of petro resin adhesives show higher peel strength than gum rosin based adhesives suggesting that better compatibility exists between petro resin and rubber. Our previous results show that gum rosin, which is naturally occurring tackifying resin, gives better peel strength compared to petro resin in a single rubber component adhesive system, an observation which is attributed to better compatibility and cohesiveness between the gum rosin and natural rubber, both of which are naturally occurring materials [4]. The present study suggests that the dependence of peel strength on type of tackifying resin is different in rubber blend adhesive system compared to single rubber component adhesive system. Figure 14 also shows that 90° peel test exhibits the highest peel strength than T- and 180° peel tests. This observation is attributed to the angle of testing, where higher strain–induced crystallization of rubber chains occurs at 90º peel test followed by 180° and T- peel tests. In all cases, the failure mode is essentially adhesive in nature [13, 16, 17].
Shear Strength
Figure 15 shows the dependence of shear strength on magnesium oxide loading at 30 and 120 µm coating thickness for petro resin based ENR 25/NBR blend adhesive. As in the case of tack and peel strength, shear strength indicates a maximum value at 10 phr magnesium oxide for both coating thicknesses. This observation is attributed to the occurrence of maximum cohesive strength due to the excellent intermolecular interaction between rubber adhesive system and magnesium oxide at 10 phr loading. However, further addition of magnesium oxide beyond 10 phr will decrease the shear strength of the system for both coating thicknesses. This phenomenon is ascribed to the dilution effect of the filler, i.e., decreasing amount of rubber content which acts as a binder in the system. A similar result is also obtained when gum rosin is used as the tackifying resin as shown in Fig. 16. For a fixed loading of filler, the 120 µm coating thickness sample shows higher shear strength value than 30 µm coated sample. This observation is associated to the higher volume of rubber adhesive in 120 µm coating thickness that gives higher cohesive strength than 30 µm coated sample with lower coating thickness as shown in Figs. 15 and 16.
Comparison of Results
Table 1 summarizes the adhesion properties of petro resin and gum rosin based ENR25/NBR blend adhesives at 10 phr magnesium oxide loading. From this study, petro resin based adhesive indicates higher tack, peel strength and shear strength value than gum rosin based adhesive. Petro resin which is a synthetic resin derived from the polymerization of petroleum cracked products, whereas gum rosin is a natural occurring rosin which is derived from vegetable sources in the forms of exudates. Magnesium oxide filled ENR 25/NBR based adhesive—which contains higher amount of synthetic rubber—showed better compatibility with synthetic resin (petro resin). On the other hand, gum rosin which is natural rosin, shows poorer compatibility between the rosin and ENR 25/NBR rubber blend [8, 18, 19]. Also, the phenomenon is ascribed to the difference in softening point of the tackifier. Petro resin which has higher softening temperature than that of gum rosin shows the higher adhesion properties to the magnesium oxide filled ENR 25/NBR based adhesive.
Conclusion
For both petro resin and gum rosin based ENR25/NBR blend adhesive, loop tack and peel strength increases with magnesium oxide loading up to 10 phr, after which it decreases with further increase in magnesium oxide loading. This phenomenon is attributed to the increasing wettability and compatibility of adhesive which culminates at 10 phr of filler content for maximum wettability for both coating thickness. The drop in tack and peel strength after 10 phr filler loading is due to the dilution effect of magnesium oxide in the adhesive system. The 90° peel test consistently gives the higher peel strength, a phenomenon which is associated to the angle of testing. Shear strength also exhibits a maximum value at 10 phr magnesium oxide loading, an observation which is ascribed to the optimum cohesive and adhesive strength during the shearing action. Petro resin based magnesium oxide filled ENR25/NBR blend adhesive consistently exhibits higher adhesion properties than gum rosin based adhesive. This observation is attributed to the better compatibility of the petro resin with the adhesive system. At a fixed filler loading, adhesion properties increase with coating thickness due to the higher amount of adhesive which enhances the viscoelastic response of the rubber blend adhesive.
References
Benedek I (2005) Developments in pressure-sensitive products. CRC Press, New York, pp 192–194
Kohjiya S, Ikeda Y (2014) Chemistry, manufacture and applications of natural rubber. Woodhead, Oxford, pp 353–370
Poh BT, Yee KW, Lim HB (2008) Viscosity and shear strength of natural-rubber-based adhesives in the presence of gum rosin and petro resin. J Appl Polym Sci 110:4079–4083
Poh BT, Yong AT (2009) Effect of molecular weight of rubber on tack and peel strength of SMR L-based pressure-sensitive adhesives using gum rosin and petro resin as tackifiers. J Macromol Sci Part A 46:97–103
Poh BT, Firdaus SZ (2010) Effect of hybrid tackifiers on adhesion properties of epoxidized natural rubber-based pressure-sensitive adhesives. J Polym Environ 18:335–338
Poh BT, Firdaus SZ (2011) Viscosity, shear strength, and peel strength on poly (ethylene terephthalate) of (natural rubber)-based adhesives containing hybrid tackifiers. J Vinyl Addit Technol 17:209–212
Poh BT, Yong AT (2010) Effect of molecular weight of epoxidized-natural rubber on viscosity and tack of pressure-sensitive adhesives. J Appl Polym Sci 115:1120–1124
Benedek I, Feldstein MM (2008) Technology of pressure-sensitive adhesives and products. CRC Press, New York, p 32
Skeist I (ed) (1990) Handbook of adhesives, 3rd ed. Van Nostrand Reinhold, New York, pp 179, 563, 565
Satas D (ed) (1982) Handbook of pressure sensitive adhesive technology. Van Nostrand Reinhold, New York 43
Poh BT, Chee CL (2007) Effect of coumarone-indene resin on adhesion property of SMR 20-based pressure-sensitive adhesives. Int J Polym Mater Polym Biomater 56:247–255
Gierenz G, Karmann W (eds) (2001) Adhesives and adhesive tapes. Wiley-VCH, New York, pp 103–104
Lee LH (ed) (1991) Adhesive bonding. Plenum Press, New York 19
Ghanbari D, Salavati-Niasari M, Sabet M (2013) Preparation of flower-like magnesium hydroxide nanostructure and its influence on the thermal stability of polyvinyl acetate and polyvinyl alcohol. Compos Part B-Eng 45:550–555
Gholamian F, Salavati-Niasari M, Ghanbari D, Sabet M (2013) The effect of flower-like magnesium hydroxide nanostructure on the thermal stability of cellulose acetate and acrylonitrile-butadiene styrene. J Clust Sci 24:73–84
Poh BT, Lee PG, Chuah SC (2008) Adhesion property of epoxidized natural rubber (ENR)-based adhesives containing calcium carbonate. Express Polym Lett 2:398–403
Poh BT, Cheong SK (2012) Adhesion behavior of natural rubber-based adhesives crosslinked by benzoyl peroxide. J Appl Polym Sci 124:1031–1035
Simpson RB (2002) Rubber basics. Rapra Technology Limited, Shawbury, U.K., pp 151–153
Benedek I (2000) Pressure-sensitive formulation. VSP, Utrecht, pp 153–201
Acknowledgments
The authors acknowledge the support of Fundamental Research Grant Scheme (FRGS) and Universiti Sains Malaysia that has resulted in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soo, K.W., Azahari, B. & Poh, B.T. Effect of Magnesium Oxide Loading on Adhesion Properties of ENR 25/NBR Blend Adhesives in the Presence of Petro Resin and Gum Rosin Tackifiers. J Polym Environ 24, 334–342 (2016). https://doi.org/10.1007/s10924-016-0778-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-016-0778-3