Abstract
A tegotheriid docodontan Sibirotherium rossicum from the Early Cretaceous of Western Siberia, Russia, was considered to have six lower premolars, as in the tegotheriids Agilodocodon and Microdocodon from the Middle Jurassic of China. The micro-computed tomography of two dentary specimens with a supposed submolariform ultimate deciduous premolar (dp6) revealed absence of a replacing tooth germ in this locus. Also, the morphology of the roots of this tooth is more consistent with that of molariform teeth. Based on the new data, we interpret Sibirotherium to possess five lower premolars rather than six and that the supposed dp6 might in fact be better interpreted as the first molariform (m1). The results of our phylogenetic analysis suggest that this is a plesiomorphic condition for Docodonta and, under slow character optimization, for Tegotheriidae, with the number of premolars reduced to three to four in Docodontidae (Haldanodon, Docodon, and Docofossor).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Docodonta is an early-diverging branch of mammaliaforms that achieved a considerable taxonomic and ecomorphological diversity in the Middle Jurassic – Early Cretaceous of Laurasia (Kielan-Jaworowska et al. 2004; Martin 2018) and possibly persisted into the early Late Cretaceous of Gondwana (Martin et al. 2022). The clade includes taxa with ecomorphological specializations including for semiaquatic, fossorial, and arboreal lifestyles (Martin 2005; Ji et al. 2006; Luo et al. 2015; Meng et al. 2015). The molariform teeth of docodontans are strikingly similar to those of tribosphenic mammals in having the enlarged lingual cusps on the upper teeth grinding against the basins on the lower teeth. The docodontan molariform teeth were capable of more complex shearing and crushing functions compared with other contemporaneous Mesozoic mammaliaforms, suggesting more efficient food processing (Schultz et al. 2019). The middle ear bones in docodontans were still attached to the dentary, as in other stem mammals (Kielan-Jaworowska et al. 2004).
The decline of docodontans in the Early Cretaceous may have been related to the emergence and dispersal of tribosphenic mammals, which have (among other features) similar shearing and crushing dentition, but their middle ear bones detached from the dentary, providing a wider range of hearing capabilities. However, to date, the fossils of tribosphenic mammals have not been found in the Early Cretaceous of Siberia, and docodontans appear to have persisted. The docodontans Khorotherium yakutense and Sibirotherium rossicum are among the most commonly found mammals in the Early Cretaceous faunas of Yakutia and Western Siberia, respectively (Maschenko et al. 2003; Lopatin et al. 2009, 2020; Averianov et al. 2018).
Lopatin et al. (2009) suggested Sibirotherium rossicum possessed six lower premolars, similar to other docodontans such as Agilodocodon scansorius and Microdocodon gracilis from the Middle Jurassic Tiaojishan Formation of Inner Mongolia, China (Meng et al. 2015; Zhou et al. 2019). In Castorocauda lutrasimilis, also from the same formation (Ji et al. 2006), and in Borealestes serendipitus from the Middle Jurassic Kilmaluag Formation of Scotland, United Kingdom (Waldman and Savage 1972; Panciroli et al. 2019, 2021), there are five lower premolars, and the remaining docodontan taxa for which the tooth row is known, Docodon, Haldanodon, Dsungarodon, and Docofossor, have three to four lower premolars (Kielan-Jaworowska et al. 2004; Schultz et al. 2019). The dental formula count in S. rossicum was based on the interpretation of the first lower molariform tooth as a partially molarized ultimate deciduous premolar (dp6). Here we present results of the micro-computed tomography of two specimens of S. rossicum indicating absence of the replacing permanent premolar in this locus, and discuss implications of this discovery for the interpretation of dental formula and phylogenetic affinities of this docodontan.
Institutional abbreviations
LMCCE, Laboratory of Mesozoic and Cenozoic Continental Ecosystems, Tomsk State University, Tomsk, Russia; PM TGU, Paleontological Museum of Tomsk State University, Tomsk, Russia.
Materials and methods
The cusp nomenclature of docodontan teeth follows that of Averianov et al. (2010: fig. 1), which was modified from Luo and Martin (2007). The nomenclature differs from Luo and Martin (2007) in the addition of cusp bb on the lower molariform teeth and that the cusp df on lower molariform teeth is labelled as cusp dd.
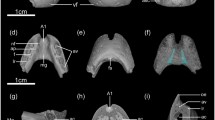
PM TGU 16/5–22, holotype of Sibirotherium rossicum, left dentary fragment with p5, m1-2, and alveoli for p3-4. a. Segmented surface visualization of permanent teeth (blue) and dentary (translucent yellow), lingual view. b, c. Surface rendering based on high-resolution X-ray computed tomography scans, lingual (b) and labial (c) views. Shestakovo 1, Kemerovo oblast, Russia; Ilek Formation, Lower Cretaceous (Aptian). Abbreviations: m, molar; Mg, Meckelian groove; p, premolar. Scale bar equals 1 mm
Two specimens of Sibirotherium rossicum, the holotype PM TGU 16/5–22 and PM TGU 120/9–34, were CT scanned at the Saint Petersburg State University Research Centre for X-ray Diffraction Studies (Saint Petersburg, Russia) using the CT model SkyScan 1172. They were imaged at 100 kV and 0.1 mA, generating a resolution of 2.07 μm and 3.45 μm of isotropic voxel size and output of 3024 × 2436 and 1928 × 1928 pixels per slice. The CT scan data were imported to the software Avizo Lite 2019.1 (FEI-VSG Company) for segmentation and reconstruction of three-dimensional surface files.
For the phylogenetic analysis, we used a data matrix focused on Docodonta presented by Zhou et al. (2019) with some modifications. First, we excluded Tikitherium and Gondtherium from the matrix because they are not docodontans (Averianov et al. 2010; Zhou et al. 2019). Second, we changed the scorings for Borealestes and Sibirotherium on the number of premolar or premolar positions (character 49) from unknown to five premolars (character state 2) following Panciroli et al. (2019) and this study. Third, we changed scorings for Haldanodon and Docofossor for the same character (number of premolars or premolar positions) from four premolars (state 1) to three premolars (state 0) following Krusat (1980), Martin and Nowotny (2000), and Luo et al. (2015). All characters were treated as unordered. The parsimony analysis was run on the TNT software (v. 1.5) (Goloboff and Catalano 2016). The implicit enumeration search produced six most parsimonious trees of 143 steps with a consistency index of 0.55 and a retention index of 0.83. The strict consensus tree, tree statistics, and character distribution and optimization were obtained using WinClada v. 1.00.08 (Nixon 2002). The fast and slow character optimizations of Winclada correspond to ACCTRAN and DELTRAN optimizations, respectively, of PAUP (Swofford 2002).
Systematic paleontology
Mammaliaformes Rowe, 1988
Docodonta Kretzoi, 1946
Tegotheriidae Tatarinov, 1994
Sibirotherium rossicum Maschenko et al., 2003
Sibirotherium rossicus Maschenko et al., 2003: p. 77, figs. 1–4 (original description).
Sibirotherium rossicus Maschenko et al., 2003: Averianov 2004: fig. 1D.
Sibirotherium rossicum Maschenko et al., 2003: Lopatin et al. 2009: p. 454, figs. 2–6, pl. 12 (emended spelling).
PM TGU 120/9–34, Sibirotherium rossicum, right dentary fragment with c, p1-5, m1-3, and root of ix. a. Segmented surface visualization of permanent teeth (blue) and dentary (translucent yellow), lingual view. b, c. Surface rendering based on high-resolution X-ray computed tomography scans, lingual (b) and labial (c) views. Shestakovo 1, Kemerovo oblast, Russia; Ilek Formation, Lower Cretaceous (Aptian). Abbreviations: c, canine; i, incisor; m, molar; Mg, Meckelian groove; p, premolar. Scale bar equals 1 mm
Holotype. PM TGU 16/5–22, left dentary fragment with p5, m1-2, and alveoli for p3-4.
Type locality and horizon. Shestakovo 1 locality (55° 54′ 36.4" N, 87° 56′ 53.6" E), Chebula raion, Kemerovo oblast, Russia. Ilek Formation, Lower Cretaceous (Aptian).
Referred specimens. Maxillary and dentary fragments, isolated upper and lower teeth; see Lopatin et al. (2009) for the list of specimens. A lower molar LMCCE 005/127 from the Ilek Formation (Barremian?) at Bol’shoi Kemchug 3 locality, Krasnoyarsk krai, Russia may belong to a different species and was identified as Sibirotherium sp. (Lopatin et al. 2020).
Description. See Lopatin et al. (2009).
Results
The micro-computed tomography of the holotype of S. rossicum and dentary fragment PM TGU 120/9–34 reveal no tooth germs for replacing permanent teeth in these specimens (Figs. 1a, 2a). This suggests that what Lopatin et al. (2009) identified as an ultimate deciduous premolar lacking the cusp g is more likely to be the first molariform tooth (m1), and the rest of the premolars represent the permanent generation (Fig. 3). This re-interpreted m1 has long and robust roots similar to the rest of the molars, in contrast with slightly shorter and slender roots of the premolars (Fig. 2a), a feature also seen in the holotype PM TGU 16/5–22 (Fig. 1a). We therefore reinterpret the lower dental formula of S. rossicum as including five premolars (Figs. 3 and 4).
PM TGU 120/9–34, Sibirotherium rossicum, right dentary fragment with c, p1-5, m1-3, and alveolus for ix. Surface rendering based on high-resolution X-ray computed tomography scans, occlusal view (stereopair). Shestakovo 1, Kemerovo oblast, Russia; Ilek Formation, Lower Cretaceous (Aptian). Abbreviations: c, canine; i, incisor; m, molar; Mg, Meckelian groove; p, premolar. Cusps designated on m1-2. Scale bar equals 1 mm
PM TGU 16/5–22, holotype of Sibirotherium rossicum, left dentary fragment with p5, m1-2, and alveoli for p3-4. Surface rendering based on high-resolution X-ray computed tomography scans, occlusal view (stereopair). Shestakovo 1, Kemerovo oblast, Russia; Ilek Formation, Lower Cretaceous (Aptian). Abbreviations: m, molar; p, premolar. Cusps designated on m1-2. Scale bar equals 1 mm
On the strict consensus tree obtained in the TNT analysis (Fig. 5), the family Docodontidae (Haldanodon, Docodon, and Docofossor) is the basalmost clade of Docodonta. The most deeply nested clade is the family Tegotheriidae, including Krusatodon, Agilodocodon, Sibirotherium, Tegotherium, Microdocodon, and Hutegotherium. Tegotheriidae is defined here as a stem clade including all docodontans more closely related to Tegotherium than Docodon or Simpsonodon. This analysis does not support Simpsonodontidae sensu Averianov et al. (2010) (including Simpsonodon and Dsungarodon). Simpsonodon is a sister taxon to the Tegotheriidae and Dsungarodon is clustered with Tashkumyrodon and Castorocauda.
The resulting consensus tree differs in a number of details from the single most parsimonious tree produced by the PAUP analysis in Zhou et al. (2019). Tashkumyrodon, Dsungarodon, and Borealestes are not included in the Docodontidae but are more deeply nested within the cladogram and a position closer to Tegotheriidae. Castorocauda is not the basalmost docodontan but clusters with Tashkumyrodon and Dsungarodon outside of Docodontidae. Within the Tegotheriidae, Microdocodon is the sister taxon to Hutegotherium rather than to Tegotherium.
Discussion
The holotype of S. rossicum was interpreted as having two deciduous premolars (dp3-4?) and first molariform tooth (m1) in the original description (Maschenko et al. 2003). The alveoli of two more anterior teeth were attributed to the anterior deciduous teeth (dp1-2?). The dental formula of S. rossicum was estimated as including four lower premolars and at least six lower molariforms. The interpretation of the anterior teeth in this specimen as deciduous was based on their similarity with the teeth on the single known specimen of Peraiocynodon inexpectatus Simpson, 1928 from the Lower Cretaceous (Berriasian) Purbeck Limestone Group of England (Simpson 1928), proposed to be deciduous teeth by Butler (1939) and synonymized with Docodon (later supported by Kielan-Jaworowska et al. 2004, though maintained as a distinct taxon by Sigoneau-Russell 2003). The latter is certainly a juvenile specimen, with a tooth erupting posterior to the fourth preserved tooth. The presumed ultimate deciduous premolar in P. inexpectatus and S. rossicum has only one lingual cusp (c), while molariform teeth have two lingual cusps (c and g). According to Averianov (2004), all known deciduous lower premolars of docodontans lack the cusp g. Butler’s interpretation of the teeth on the holotype of P. inexpectatus was accepted by a number of authors (see review in Averianov 2004), but alternatively, these teeth have been interpreted as m1-4 (Simpson 1928) or p1, dp2-3, and m1 (Krusat 1980). According to Sigogneau-Russell and Kielan-Jaworowska (2002) and Sigogneau-Russell (2003), the last preserved molariform tooth on the holotype of P. inexpectatus is m1 whereas as some of the anterior teeth could be deciduous premolars.
Our tomographic data reveal that in PM TGU 120/9–34, there are five premolars with simple crowns between the large two-rooted canine and a molariform tooth with a single lingual cusp (c) (Figs. 2 and 3). The latter tooth was considered a deciduous premolar (dp6) by analogy with the holotype, while all the anterior premolars are permanent. Thus, the dental formula of S. rossicum was estimated as including six lower premolars. Such a high number of lower premolars is known for Agilodocodon and Microdocodon (Meng et al. 2015; Zhou et al. 2019), as well as stem mammal Kuehneotherium and australosphenidan Bishops (Kermack et al. 1968; Rich et al. 2001; Martin 2018). Therefore, a dental formula with six premolars of Sibirotherium did not seem unusual. Nevertheless, the micro-computed analysis of the relevant specimens of Sibirotherium presented herein suggests that this taxon had only five lower premolars based on the absence of any developing replacement teeth. Among docodontans, five lower premolars are also present in Borealestes and Castorocauda (Fig. 5). Under fast (ACCTRAN) optimization, having five premolars is plesiomorphic for docodontans (Fig. 5a). In this model the number of premolars would be reduced to three-four in Docodontidae and increased to six in the clade Simpsonodon + Tegotheriidae with a reversal to five in Sibirotherium. Under slow optimization (DELTRAN), having five premolars is also plesiomorphic for docodontans, with a parallel increase in the number of premolars to six in Agilodocodon and Microdocodon (Fig. 5b).
There is some morphological variation between the ultimate permanent lower premolars (p5) in the holotype of S. rossicum and PM TGU 120/9–34 (Figs. 1, 2, 3 and 4). On the holotype, the p5 has more pronounced mesial and distal accessory cusps and complete lingual cingulid in contrast with PM TGU 120/9–34 where the accessory cusps are smaller and the lingual cingulid is incomplete. In absence of a larger sample, we do not know the significance of this variation. The deciduous premolars are currently unknown for S. rossicum. In Haldanodon expectatus from the Upper Jurassic (Kimmeridgian) Guimarota Beds of Portugal, the ultimate deciduous premolar (dp4) is submolariform with a lingual cusp c and without a lingual cusp g, and is replaced by a p3 with a simple crown (Krusat 1980; Martin and Schultz 2023). A similar pattern is observed in the last premolar locus (p4) in Docodon spp. from the Upper Jurassic (Kimmeridgian-Tithonian) of the USA (Schultz et al. 2019: fig. 8). Schultz et al. (2019) interpreted the anterior cusp in the ultimate deciduous premolar in Docodon as a cusp g, which would not fit the pattern suggested for molariform ultimate premolars in docodontans, so we reinterpret this as variation in the development of the lingual cusp c. In Agilodocodon the ultimate lower permanent premolar (p6) has a submolariform crown, with lingual cusp c and lacking a cusp g (Meng et al. 2015: figs. 2F, J and S3), which is congruent with the ultimate deciduous premolar of Haldanodon and our interpretation of Docodon (above), or in the first molariform of Sibirotherium (m1). The similarity of the first molariform tooth of Sibirotherium with the ultimate deciduous premolar in some docodontans in lacking the cusp g could be explained by loss of replacement at the distal premolar position. This implies an ancestral condition with six premolars for Sibirotherium, which is postulated under the accelerated character transformation on our phylogenetic tree (Fig. 5a). However, this hypothesis is weakened by the fact that the first molariform tooth lacks cusp g in Agilodocodon, which have six lower premolars (Meng et al. 2015). Applying the same mechanism to Agilodocodon suggests that its ancestors had seven lower premolars, which is unknown for any of the docodontans. More data is needed to reconstruct the dental replacement pattern and tooth homology in Docodonta.
Availability of data and materials
The fossil specimens have been accessioned into a recognized public collection, where it is available for study. Virtual models of the specimens available upon request.
References
Averianov AO (2004) Interpretation of the Early Cretaceous mammal Peraiocynodon (Docodonta) and taxonomy of some British Mesozoic docodonts. Russ J Theriol 3:1–4. https://doi.org/10.31610/trudyzin/2010.314.2.121
Averianov AO, Lopatin AV, Krasnolutskii SA, Ivantsov SV (2010) New docodontans from the Middle Jurassic of Siberia and reanalysis of Docodonta interrelationships. Proc Zool Inst RAS 314:121–148
Averianov AO, Martin T, Lopatin AV, Skutschas PP, Schellhorn R, Kolosov PN, Vitenko DD (2018) A high-latitude fauna of mid-Mesozoic mammals from Yakutia, Russia. PLoS One 13:e0199983. https://doi.org/10.1371/journal.pone.0199983
Butler PM (1939) The teeth of the Jurassic mammals. Proc Zool Soc Lond 109:329–356. https://doi.org/10.1111/j.1096-3642.1939.tb00719.x
Goloboff PA, Catalano SA (2016) TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32:221–238. https://doi.org/10.1111/cla.12160
Ji Q, Luo Z-X, Yuan C-X, Tabrum AR (2006) A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311:1123–1127. https://doi.org/10.1126/science.112302
Kermack DM, Kermack KA, Mussett F (1968) The Welsh pantothere Kuehneotherium praecursoris. J Linn Soc Lond Zool 47:407–423. https://doi.org/10.1111/j.1096-3642.1968.tb00519.x
Kielan-Jaworowska Z, Cifelli RL, Luo Z-X (2004) Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York
Kretzoi M (1946) On Docodonta, a new order of Jurassic Mammalia. Ann Hist-Nat Mus Nat Hung 39:108–111
Krusat G (1980) Contribução para o conhecimento da fauna do Kimeridgiano da mina de lignito Guimarota (Leiria, Portugal). IV Parte. Haldanodon exspectatus Kühne & Krusat 1972 (Mammalia, Docodonta). Mem Serv Geol Port 27:1–79
Lopatin AV, Averianov AO, Kuzmin IT, Boitsova EA, Saburov PG, Ivantsov AY, Skutschas PP (2020) A new finding of a docodontan (Mammaliaformes, Docodonta) in the Lower Cretaceous of Western Siberia. Doklady Earth Sci 494:667–669. https://doi.org/10.1134/S1028334X20090123
Lopatin AV, Averianov AO, Maschenko EN, Leshchinskiy SV (2009) Early Cretaceous mammals from Western Siberia: 2. Tegotheriidae. Paleontol J 43:453–462. https://doi.org/10.1134/S0031030109040157
Luo Z-X, Martin T (2007) Analysis of molar structure and phylogeny of docodont genera. Bull Carnegie Mus Nat Hist 39:27–47
Luo Z-X, Meng Q-J, Ji Q, Liu D, Zhang Y-G, Neander AI (2015) Evolutionary development in basal mammaliaforms as revealed by a docodontan. Science 347:760–764. https://doi.org/10.1126/science.1260880
Martin T (2005) Postcranial anatomy of Haldanodon exspectatus (Mammalia, Docodonta) from the Late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zool J Linn Soc 145:219–248. https://doi.org/10.1111/j.1096-3642.2005.00187.x
Martin T (2018) Mesozoic mammals – early mammalian diversity and ecomorphological adaptations. In: Zachos FE, Asher RJ (eds) Mammalian Evolution, Diversity and Systematics. De Gruyter, Berlin, Boston, pp 199–299
Martin T, Goin FJ, Schultz JA, Gelfo JN (2022) Early Late Cretaceous mammals from southern Patagonia (Santa Cruz Province, Argentina). Cret Res 133:105127. https://doi.org/10.1016/j.cretres.2021.105127
Martin T, Nowotny M (2000) The docodont Haldanodon from the Guimarota mine. In: Martin T, Krebs B (eds) Guimarota – a Jurassic Ecosystem. Verlag Dr. Friedrich Pfeil, Munich, pp 91–96
Martin T, Schultz JA (2023) Deciduous dentition, tooth replacement, and mandibular growth in the Late Jurassic docodontan Haldanodon exspectatus (Mammaliaformes). J Mamm Evol. https://doi.org/10.1007/s10914-023-09668-2
Maschenko EN, Lopatin AV, Voronkevich AV (2003) A new genus of the tegotheriid docodonts (Docodonta, Tegotheriidae) from the Early Cretaceous of West Siberia. Russ J Theriol 1:75–81. https://doi.org/10.15298/rusjtheriol.01.2.01
Meng Q-J, Ji Q, Zhang Y-G, Liu D, Grossnickle DM, Luo Z-X (2015) An arboreal docodont from the Jurassic and mammaliaform ecological diversification. Science 347:764–768. https://doi.org/10.1126/science.1260879
Nixon KC (2002) WinClada version 1.00.08. Software published by the author, Ithaca, NY. Available on-line at www.cladistics.org
Panciroli E, Benson RBJ, Fernandez V, Butler RJ, Fraser NC, Luo Z-X, Walsh SL (2021) New species of mammaliaform and the cranium of Borealestes (Mammaliformes: Docodonta) from the Middle Jurassic of the British Isles. Zool J Linn Soc 192(4):1323–1362. https://doi.org/10.1093/zoolinnean/zlaa144
Panciroli E, Benson RBJ, Luo Z-X (2019) The mandible and dentition of Borealestes serendipitus (Docodonta) from the Middle Jurassic of Skye, Scotland. J Vert Paleontol 39:e1621884. https://doi.org/10.1080/02724634.2019.1621884
Rich THV, Flannery TF, Trusler P, Constantine A, Kool L, Klaveren NA, van, Vickers-Rich P (2001) A second tribosphenic mammal from the Mesozoic of Australia. Rec Queen Victoria Mus 110:1–9
Rowe TB (1988) Definition, diagnosis, and origin of Mammalia. J Vert Paleontol 8:241–264. https://doi.org/10.1080/02724634.1988.10011708
Schultz JA, Bhullar B-AS, Luo Z-X (2019) Re-examination of the Jurassic mammaliaform Docodon victor by computed tomography and occlusal functional analysis. J Mamm Evol 26:9–38. https://doi.org/10.1007/s10914-017-9418-5
Sigogneau-Russell D (2003) Docodonts from the British Mesozoic. Acta Palaeontol Pol 48:357–374
Sigogneau-Russell D, Kielan-Jaworowska Z (2002) Mammals from the Purbeck Limestone Group of Dorset, southern England. Spec Pap Palaeontol 68:241–255
Simpson GG (1928) A Catalogue of the Mesozoic Mammalia in the Geological Department of the British Museum. British Museum (Natural History), London
Swofford DL (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0. Sunderland, Sinauer Associates.
Tatarinov LP (1994) On an unusual mammalian tooth from the Mongolian Jurassic. Paleontol Zh 2:97–105 [In Russian]
Waldman M, Savage RJG (1972) The first Jurassic mammal from Scotland. J Geol Soc 128:119–125. https://doi.org/10.1144/gsjgs.128.2.0119
Zhou C-F, Bhullar B-AS, Neander AI, Martin T, Luo Z-X (2019) New Jurassic mammaliaform sheds light on early evolution of mammal-like hyoid bones. Science 365:276–279. https://doi.org/10.1126/science.aau934
Acknowledgements
We thank Thomas Martin for providing the data matrix from Zhou et al. (2019) and two anonymous reviewers for reading the paper and useful comments. We are grateful to the staff of the Saint Petersburg State University Research Centre for X-ray Diffraction Studies (Saint Petersburg, Russia) for CT scanning of PM TGU 16/5–22 and 120/9–34.
Funding
This work was supported by the Russian Science Foundation (project 19–14–00020–P) and the Zoological Institute, Russian Academy of Sciences (project 122031100282–2).
Author information
Authors and Affiliations
Contributions
AA designed the research, acquired funding, segmented and reconstructed 3D surface files from the CT data, and prepared Figs. 1, 2, 3, 4 and 5. AA and AL wrote the main manuscript text. SL organized the fieldwork and curated the specimens. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Averianov, A.O., Lopatin, A.V. & Leshchinskiy, S.V. New interpretation of dentition in Early Cretaceous docodontan Sibirotherium based on micro-computed tomography. J Mammal Evol 30, 811–817 (2023). https://doi.org/10.1007/s10914-023-09682-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-023-09682-4