Abstract
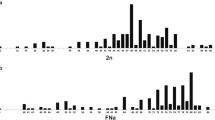
The Neotropical monkey genus Aotus (owl or night monkeys) are among the most karyological diverse primates of the world. Their diploid numbers range from 2n = 46 to 58, but even owl monkeys with the same diploid number may have radically different karyotypes. This karyotypic variability has provided precious information for taxonomists and has a potential for aiding phylogenetic analysis of these primates. However, up to now only three out of 11 species have been analyzed with molecular cytogenetic methods. Here, we report on a fourth species, A. infulatus. Females have a diploid number of 2n = 50 while males, due to a Y/autosome translocation, have 49 chromosomes. We provide a complete map of chromosome homology between humans and A. infulatus. Comparisons with previous reports allowed us to propose a putative ancestral karyotype of the genus (2n = 52) and to deduce the rearrangements that were involved in the origin of each species chromosome complement. Integration of chromosome painting and banding analysis suggests at least three chromosomes have evolutionary new centromeres that appeared during the divergence of these four owl monkey species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytogenetic data, especially comparative chromosome painting, have proved to be very useful for elucidating essential aspect of genome organization and evolution in New World monkeys (NWM; de Oliveira et al. 2012). These studies, together with morphological and molecular data, can also contribute to conservation programs through species identification, including the recognition of otherwise cryptic species within taxa (Stanyon et al. 2004). An excellent example is the genus Aotus, known as owl or night monkeys, originally believed to be a single species, A. trivirgatus (Hershkovitz 1949). Based on karyotype, phenotypic characters, and geographical distribution, Hershkovitz (1983) recognized nine species of owl monkeys and more recently, Menezes et al. (2010), using mitochondrial and nuclear DNA sequence data allied with karyotypic and biogeographic data, proposed the division of Aotus into 11 species.
Since the 1970’s extensive cytogenetic studies have shown that Aotus displays high karyotypic variability with at least 18 different karyotypes and diploid numbers ranging from 2n = 46 to 58 (Ma 1981; Ma et al. 1985; Pieczarka et al. 1993; Torres et al. 1998). Centric fusions/fissions and pericentric inversions have been described as the predominant events of chromosomal reorganization in the genus (Ma 1981; Ruiz-Herrera et al. 2005). In addition, uneven diploid numbers due to translocations between the Y chromosome and an autosome have also been found in some species (Ma et al. 1976; Ma 1981; Pieczarka and Nagamachi 1988; Pieczarka et al. 1993). The Y chromosome was found translocated onto the short arm (as in A. nigriceps) or interstitially into the long arm (in A. azarae) of autosomes (Ma 1981).
Up until now, only three Aotus species were analyzed by molecular cytogenetic methods: an unidentified species Aotus sp. (2n = 50), A. nancymaae (2n = 54), and A. griseimembra (2n = 54) (Stanyon et al. 2004, 2011; Ruiz-Herrera et al. 2005). These species shared the associations of the homologues of human chromosomes (HSA) 1/3, 1/16, 2/20, 3/21, 4/15, 5/7, 7/11, 10/11, 16/22, and the inverted synteny HSA 14/15/14/15 in addition to the disruptions of the syntenic associations HSA 2/16 and 10/16, both present in the supposed ancestral Platyrrhini karyotype (APLK, Table 1). Chromosome paints of Lagothrix lagothrica were also hybridized to A. nancymaae metaphases and the results showed that this karyotype was highly shuffled with at least 14 fissions and 13 fusions required to derive it from the APLK (Stanyon et al. 2004).
The extensive karyological variability of the genus Aotus suggests that more detailed molecular cytogenetic data, including sampling still unstudied species, may yield important data on its phylogeny and taxonomy, both between Aotus species and between Aotus and other NWM taxa. In this research, we used human chromosome-specific probes to map the karyotype of A. infulatus, aiming to contribute to the knowledge of Aotus chromosome diversification.
Material and Methods
We analyzed the karyotypes of a male and a female Aotus infulatus housed at the Fundação Zoo-Botânica de Belo Horizonte, Minas Gerais state, Brazil. Cytogenetic analyses were performed on chromosome preparations obtained from fibroblast cultures, following standard procedures (Stanyon and Galleni 1991). GTG-, CBG-banding patterns, and silver-staining of the nucleolar organizer regions (Ag-NORs) were carried out according to Seabright (1971), Sumner (1972), and Howell and Black (1980), respectively.
Fluorescence in situ hybridizations (FISH) were performed with human chromosome-specific probes prepared by DOP-PCR from flow sorted chromosomes by PCR amplification and labeling, as previously described by Dumas et al. (2005). FISH using a synthesized biotinylated telomeric sequence (TTAGGG)4 (Invitrogen) was performed in conditions similar to those described in Araújo et al. (2014). Digital images were captured under a Zeiss Axioimager 2 epifluorescence microscope coupled with a CCD camera.
All data generated or analyzed during this study are included in this published article.
Results
The diploid numbers of the specimens analyzed were 2n = 49 (male) and 2n = 50 (female). The karyotype was composed of ten pairs of biarmed chromosomes and 14 acrocentric pairs; the X was submetacentric and in the male, the Y chromosome was apparently translocated to the short arm of chromosome 16 (Fig. 1a). After comparing the GTG-banding pattern with the literature, we confirmed that the specimens were Aotus infulatus.
CBG-banding revealed pericentromeric constitutive heterochromatin blocks in all chromosomes (Fig. 1b). Additionally, chromosome pairs 10–24 had heterochromatic short arms, pair 7 presented heterochromatin in the telomeric region, and the translocated Y/16 chromosome showed interstitial bands on the short and long arms. Pair 9 had a large AgNOR-bearing interstitial secondary constriction in its long arm (Fig. 2a). The telomeric probe produced signals at the termini of all chromosome arms and additional interstitial signals were also found at the centromeric region of chromosome pairs 6 and 7 (Fig. 2b).
All the human chromosome-specific probes, except the Y, produced bright signals on the A. infulatus metaphases. We were able to produce a complete map of homology with human chromosomes (Figs. 1a and 3). A total of 41 conserved segments were found on the haploid set of A. infulatus.
Eleven human chromosomes were conserved in A. infulatus. Seven painted only one A. infulatus counterpart (HSA 6, 9, 12, 13, 17, 19, and X), and four (HSA 18, 20, 21, and 22) were conserved but were associated with other autosomes. The HSA 14 paint hybridized to a single chromosome, in association with HSA 15, but it was divided into two blocks due to an inversion. Multiple hybridization signals were observed with the probes of the other human autosomes: HSA 4, 8, 10, 11, and 16 labeled two pairs of A. infulatus each; and HSA 1, 2, 3, 5, 7, and 15 were split into three or more segments. The following syntenic associations of human chromosomes were found: HSA 1/3, 1/16, 2/7, 2/20, 3/21, 4/15, 5/7, 5/15 (twice), 7/11, 8/18, 10/11, 10/22, 15/14/15/14, and 16/22 (Table 1, Fig. 1a). Hybridization signals were not detected in the constitutive heterochromatin regions revealed after CBG-banding (Fig. 1).
Discussion
Centric fusions/fissions and pericentric inversions proposed based primarily on banding were described as important mechanisms of chromosome reorganization in the night monkeys (Ma 1981; Ruiz-Herrera et al. 2005). Our FISH experiment with a telomeric probe showed signals in the centromeric region of A. infulatus pairs 6 and 7. The interstitial labeling on pair 6 may indicate a chromosome rearrangement, probably a fusion between HSA 10 and HSA 11. The signal on pair 7 may be related to a pericentric inversion of the conserved segment homologous to HSA 20. The absence of interstitial telomeric sequences in the remaining chromosomes may be due to the loss of these sequences during rearrangements or to the small number of (TTAGGG)n repetitions, which could not be detected by FISH. Mudry et al. (2007) analyzed the karyotype of A. azarae after FISH with a telomeric sequence and observed a strong signal at the pericentromeric region of pair 5, which they related to a fusion.
As previously shown for other Aotus, our specimens had only one pair bearing NORs in its long arms. This metacentric pair is considered a marker chromosome characteristic of the genus Aotus (Torres et al. 1998).
The CBG-banding patterns reported for Aotus species, including A. infulatus, revealed heterochromatin located at pericentromeric regions of all biarmed pairs and also composing the short arms of acrocentrics (Torres et al. 1998; Prakhongcheep et al. 2013). These heterochromatic portions are rich in at least four different families of satellite DNAs, which would be involved in Aotus chromosome diversification (our unpublished data).
The Y-Translocations in Owl Monkeys
The male analyzed had 2n = 49 due to a translocation of the Y with an autosome, identified as chromosome 16, which resulted in radically different morphologies between homologs in the male. Chromosome 16 is homologous to part of HSA 3q (unpublished data). This segment, called HSA 3a, is believed to be present in the ancestral Platyrrhini karyotype (APLK, Fig. 4). Y-autosome translocations have been previously described based on GTG- and CBG-banding in A. azarae boliviensis, A. A. azarae, A. infulatus, and an unidentified “Aotus from Rondônia” (all with 2n = 49 in males and 2n = 50 in females), and in A. nigriceps (2n = 51 M/52F) (Ma et al. 1976; Ma 1981; Pieczarka and Nagamachi 1988; Pieczarka et al. 1993). After comparing the banding patterns of the Y/16 of our A. infulatus with these previous accounts, we concluded that the same autosome seems to be involved in the rearrangement, which can thus be hypothesized to have occurred in a common ancestor before the divergence of these species (Pieczarka and Nagamachi 1988; Pieczarka et al. 1993). This hypothesis will need to be tested using other molecular cytogenetic methods and eventually sequencing.
Hypothetical series of transformations showing the karyotype evolution of Aotus sp., A. nancymaae, A. infulatus, and A. griseimembra from the proposed ancestral karyotype of night monkeys (2n = 52) and Platyrrhini (2n = 54), based on the correspondence with human chromosomes. A color code for each human chromosome is shown on the bottom right
Implications for Chromosome Evolution in Aotus
We then compared our banding and hybridization results with those previously published for Aotus and other New World monkeys. The proposed APLK has 2n = 54 (Stanyon et al. 2003) and is composed by eleven conserved homologues of human chromosomes (HSA 4, 6, 9, 11, 12, 13, 17, 19, 20, 22, and X), from which only six (HSA 6, 9, 12, 13, 17, and 19) were found undisrupted in A. infulatus (Fig. 1a). In this species, HSA 4 and 11 were split into two segments, whereas HSA 20 was found in association with HSA 2, and HSA 22 with HSA 16 and 10. The presumed APLK associations 3/21, 5/7, 8/18, and 14/15 were found in A. infulatus, but HSA 2/16 and 10/16 were absent (Table 1, Fig. 1a). Instead, A. infulatus has the association HSA 10/22/16, which may indicate an insertion or a fusion of the NWM ancestral HSA 10/16 with HSA 22, followed by an inversion.
After comparisons of the four Aotus painted karyotypes, we confirmed that they share seven derived associations, which are absent in the APLK: HSA 1/3, 1/16, 2/20, 4/15, 7/11, 10/11, 16/22, and an inversion of HSA 14/15 resulting in HSA 14/15/14/15 (Table 1). Additionally, A. sp., A. infulatus and A. nancymaae share two derived associations (HSA 5/15 and 10/22), whereas A. sp. and A. nancymaae both have in common HSA 2/12 and 9/15. These cytogenetic data show that while A. griseimembra has the complement that most likely resembles that of the common ancestor of the four analyzed species, the karyotypes of A. sp. and A. nancymaae are more closely related to each other than either is to that of A. infulatus. Thus, the karyotype of an ancestor of these three species probably incorporated further rearrangements after the divergence of A. griseimembra. Furthermore, the association HSA 2/7 was found exclusively in A. infulatus, whereas HSA 3/14 and 9/17 were restricted to A. nancymaae and HSA 11/19 was exclusive to A. griseimembra (Table 1; Fig. 4).
The four Aotus species analyzed by chromosome painting shared the following features: (a) the conservation of HSA 6, 12, 13, 18, 19, 20, 21, 22, and X; (b) the presence of the APLK associations HSA 3/21, 5/7, 8/18, 14/15, and the fission of HSA 2/16; (c) and the common associations HSA 1/3/21, 1/16, 2/20, 4/15, 5/7/11/10, 10/22/16, and the inverted HSA 14/15/14/15. Based on this comparison among Aotus karyotypes and with the APLK, we suggest that the putative ancestral Aotus karyotype may have had 2n = 52 chromosomes and would be composed of: (a) the conserved chromosomes 6, 9, 12, 13, 17, 18, 19, 20, 21, 22, and X; (b) the associations HSA 1/3/21, 1/16, 2/20, 4/15, 5/7/11/10, 8/18, 10/22/16, and 14/15/14/15; (c) two pairs homologous to HSA 4, 5, 8, 10, 15, and 16 each; and (d) three pairs homologous to HSA 1, 2, 3 and 7 each (Fig. 4).
Aotus griseimembra apparently had the least derived karyotype. The differences between the A. griseimembra karyotype and the putative ancestral karyotype of Aotus was a fusion between the homologues of HSA 11 and 19 and two fissions: HSA 8/18, causing a loss of the association, and HSA 10/22/16, producing segments homologous to HSA 10 and HSA 16/22 (Fig. 4).
The other three species of Aotus are hypothesized to have karyotypes derived from a common ancestral karyotype after the divergence of A. griseimembra. The homologous associations HSA 4/15/5 and 5/15 may be explained by a fusion of the ancestral HSA 4/15 and 5, followed by the disruption of HSA 4/15/5 originating the HSA 5/15 (Fig. 4). Moreover, in the A. infulatus karyotype two fusions (HSA 2 and 7 and HSA 3 and Y) explain the main differences detected by chromosome painting, suggesting a possible chromosome marker.
The common ancestor of A. sp. and A. nancymaae probably had the synteny HSA 2/12. Complex rearrangements involving a segment homologous to HSA 15 and HSA 9 probably occurred, giving rise to the two HSA 9/15 associations. Only A. sp. had the HSA 7/11 association, indicating a possible phylogenetic marker. A fission of a segment of HSA 17 followed by a fusion to the presumed association HSA 9/15 apparently gave rise to the association HSA 15/9/17 in A. nancymaae (Fig. 4). In addition, fusions/fissions between the homologous segments of HSA 3 and HSA 14/15/14/15 would explain the origin of A. nancymaae chromosome 15 (HSA 3/14/15/14), 22 (HSA 3) and 24 (HSA 14/15) (Fig. 4).
Ma (1981) on the basis of banding proposed an ancestral Aotus karyotype with 2n = 54, presumably that found in A. nancymaae. However, our analysis shows that A. nancymaae has the most derived karyotype, with only three human homologues conserved without disruption or association (HSA 6, 13, and 19) and 17 derived associations absent from the APLK (Stanyon et al. 2004; Ruiz-Herrera et al. 2005). Instead, we hypothesize that of the four species studied with chromosome painting A. griseimembra has the karyotype that is closest to the ancestral karyotype.
Chromosomal Link between Aotus and Other NWM
The phylogenetic position of Aotus has long been controversial. Recently based on Alu insertions and nuclear DNA information, Aotus was placed in the family Cebidae (Osterholz et al. 2009; Perelman et al. 2011; Springer et al. 2012; Kiesling et al. 2014). However, our data do not show any derived chromosome characters linking Aotus to any Cebidae. Nevertheless, a recent description of the organization of human chromosomes HSA 14 and 15 (found associated in NWM) based on FISH experiments with BACs corroborated this link (Capozzi et al. 2016). The authors of this study showed that A. lemurinus and Callithrix jacchus share a pericentric inversion, which gave rise to a biarmed chromosome, corresponding to the homologous HSA 14/15. These data provide a weak link between Aotus and other Cebidae.
Possible Evolutionary New Centromeres in Aotus
From this comparison, it could be hypothesized that in addition to traditional chromosome rearrangements such as fissions, translocations, and inversions, centromere repositioning (neocentromere formation) may have also played an important role in Aotus chromosome evolution. For example, the counterpart of the submetacentric HSA 12 is acrocentric in A. infulatus and in A. griseimembra. The metacentric HSA 19 has an acrocentric correspondent in A. sp. and is submetacentric in A. infulatus and A. nancymaae. Finally, the human syntenic association HSA 1/16 corresponds to a metacentric chromosome in A. sp., A. nancymaae, and A. griseimembra, but is acrocentric in A. infulatus. In all these cases the GTG-banding patterns seem conserved between species, suggesting the occurrence of centromere repositioning.
Although chromosome painting provides information about interchromosomal rearrangements, intrachromosomal changes are usually undetected. Therefore, high- resolution molecular cytogenetic approaches using cloned DNA probes, such as BACs, should provide a better understanding of the mechanisms involved in the changes of these chromosomes.
Conclusion
Our study showed that A. infulatus has a highly rearranged karyotype when compared to the APLK. It would be of the utmost interest to use molecular cytogenetics in analyses of additional Aotus karyotypes, which could provide important information for better understanding the phylogenomics of this complicated New World monkey.
References
Araújo NP, Loss AC, Cordeiro-Junior DA, da Silva KR, Leite YLR, Svartman M (2014) New karyotypes of Atlantic tree rats, genus Phyllomys (Rodentia: Echimyidae). Genome 57:1–8
Capozzi O, Archidiacono N, Lorusso N, Stanyon R, Rocchi M (2016) The 14/15 association as a paradigmatic example of tracing karyotype evolution in New World monkeys. Chromosoma 125:747–756
de Oliveira EHC, Neusser M, Müller S (2012) Chromosome evolution in New World monkeys (Platyrrhini). Cytogenet Genome Res 137:259–272
Dumas F, Bigoni F, Stone G, Sineo L, Stanyon R (2005) Mapping genomic rearrangements in titi monkeys by chromosome flow sorting and multidirectional in-situ hybridization. Chromosome Res 13:85–96
Hershkovitz P (1949) Mammals of northern Colombia. Preliminary report no. 4: Monkeys (primates) with taxonomic revisions of some forms. Proc US Natl Mus 98:323–427
Hershkovitz P (1983) Two new species of night monkeys, genus Aotus (Cebidae, Platyrrhini): a preliminary report on Aotus taxonomy. Am J Primatol 4:209–243
Howell WM, Black DA (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 36:1014–1015
Kiesling NMJ, Yi SV, Xu K, Sperone FG, Wildman DE (2014) The tempo and mode of New World monkey evolution and biogeography in the context of phylogenomic analysis. Mol Phylogenet Evol 82:386–399
Ma NSF (1981) Chromosome evolution in the owl monkey, Aotus. Am J Phys Anthropol 54:293–303
Ma NSF, Aquino R, Collins WE (1985) Two new karyotypes in the Peruvian owl monkey (Aotus trivirgatus). Am J Primatol 9:333–341
Ma NSF, Elliott MW, Morgan L, Miller A, Jones TC (1976) Translocation of Y chromosome to an autosome in the Bolivian owl monkey, Aotus. Am J Phys Anthropol 45:191–202
Menezes AN, Bonvicino CR, Seuánez HN (2010) Identification, classification and evolution of owl monkeys (Aotus, Illiger 1811). BMC Evol Biol 10:248
Mudry MD, Nieves M, Bolzán AD (2007) Chromosomal localization of the telomeric (TTAGGG)n sequence in eight species of New World primates (Neotropical primates, Platyrrhini). Cytogenet Genome Res 119:221–224
Osterholz M, Walter L, Roos C (2009) Retropositional events consolidate the branching order among New World monkey genera. Mol Phylogenet Evol 50:507–513
Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MAM, Kessin B, Pontius J, Roelke M, Rumpler Y, Schneider MPC, Silva A, O’Brien SJ, Pecon-Slattery J (2011) A molecular phylogeny of living primates. PLoS Genet 7(3): e1001342
Pieczarka JC, Barros RMS, Faria FM Jr, Nagamachi CY (1993) Aotus from the southwestern Amazon region is geographically and chromosomally intermediate between A. azarae boliviensis and A. infulatus. Primates 34:197–204
Pieczarka JC, Nagamachi CY (1988) Cytogenetic studies of Aotus from eastern Amazonia: Y/autosome rearrangement. Am J Primatol 14:255–263
Prakhongcheep O, Chaiprasertsri N, Terada S, Hirai Y, Srikulnath K, Hirai H, Kogo A (2013) Heterochromatin blocks constituting the entire short arms of acrocentric chromosomes of Azara’s owl monkey: formation processes inferred from chromosomal locations. DNA Res 20:461–470
Ruiz-Herrera A, García F, Aguilera M, Garcia M, Fontanals MP (2005) Comparative chromosome painting in Aotus reveals a highly derived evolution. Am J Primatol 65:73–85
Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2:971–972
Springer MS, Meredith RW, Gatesy J, Emerling CA, Park J, Rabosky DL, Stadler T, Steiner C, Ryder OA, Janecka JE, Fisher CA, Murphy WJ (2012) Macroevolutionary dynamics and historical biogeography of primate diversification inferred from a species supermatrix. PLoS One 7(11): e49521
Stanyon R, Bigoni F, Slaby T, Muller S, Stone G, Bonvicino CR, Neusser M, Seuánez HN (2004) Multi-directional chromosome painting maps homologies between species belonging to three genera of New World monkeys and humans. Chromosoma 113:305–315
Stanyon R, Bonvicino CR, Svartman M, Seuánez HN (2003) Chromosome painting in Callicebus lugens, the species with the lowest diploid number (2n=16) known in primates. Chromosoma 112:201–206
Stanyon R, Galleni L (1991) A rapid fibroblast culture technique for high resolution karyotypes. Boll Zool 58:81–83
Stanyon R, Garofalo F, Steinberg ER, Capozzi O, Di Marco S, Nieves M, Archidiacono N, Mudry MD (2011) Chromosome painting in two genera of South American monkeys: species identification, conservation, and management. Cytogenetic Genome Res 134:40–50
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:305–306
Torres OM, Enciso S, Ruiz F, Silva E, Yunis I (1998) Chromosome diversity of the genus Aotus from Colombia. Am J Primatol 44:255–275
Acknowledgements
This work was supported by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Process 4072622013-0) to MS and RS and a PRIN (Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale: 2012TZF8HL_003) to RS. NPA was the recipient of a doctoral fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Araújo, N.P., Stanyon, R., do Socorro Pereira, V. et al. Interspecific Chromosome Painting Provides Clues to the Ancestral Karyotype of the New World Monkey Genus Aotus. J Mammal Evol 26, 283–290 (2019). https://doi.org/10.1007/s10914-017-9403-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9403-z