Abstract
The study of convergences in mammals is crucial to understand the evolutionary processes underlying the origin of shared traits. A classic example is the independent evolution in the pygmy rock-wallaby, the silvery mole-rat, and manatees of continuous dental replacement to compensate for high dental wear. The origins of continuous dental replacement in mammals remain unresolved. As the functional study of a trait may permit pinpointing the adaptive nature of its independent evolution, we aimed at comparing first the morpho-functional characteristics of the masticatory apparatus between the pygmy rock-wallaby and their closest relatives, and then with some published data on the silvery mole-rat. 3D geometric morphometric and biomechanical analyses were complemented by dental microwear analyses. Our results showed that the pygmy rock-wallaby clearly departs from its relatives in having a wider skull, a shorter snout, and a dentition situated more distally. These cranial modifications, previously observed in the silvery mole-rat, are probably linked with a neotenic development. Because of higher developmental constraints on marsupial skulls, such adjustment in the pygmy rock-wallaby may have improved the force generated by adductor muscles at molars for comminution of tough and abrasive plants. In contrast, the strong attrition combined with the ingestion of dust during high activity of digging and feeding might contribute to both molar damage and high wear in the silvery mole-rat. Our results stress the importance of combining morphological, developmental, and functional data to show that different behaviors related to ecology can explain the convergent occurrence of continuous dental replacement in mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of convergence is fundamental in evolutionary biology to understand the mechanisms involved in the independent origins of similar traits, as well as their putative adaptive nature (Arendt and Reznick 2008; Losos 2011; Wake et al. 2011). Mammals underwent numerous events of diversification that resulted in spectacular examples of morphological convergences. These convergent traits are generally associated with similar modes of life in response to very similar environmental conditions, as is the case for gliding, fossorial, ant-eating, or carnivorous species (McGhee 2011). The study of convergent traits in mammals is thus appropriate to investigate underlying biological mechanisms (i.e. functional, phylogenetic, and developmental constraints) and to understand the core of convergent evolutionary processes.
Teeth are intimately associated with obtaining or processing of food items, and generally play a major role in defining the ecology of mammals (Ungar 2010). Investigating an exceptional feature such as the continuous replacement of teeth is a highly promising case for examining convergent evolution. Continuous dental replacement (CDR) corresponds to the continuous addition of molars at the back of the jaw, which then migrate toward the front of the jaw to replace heavily worn teeth (Domning and Hayek 1984; Janis and Fortelius 1988; Sanson 1989; Gomes Rodrigues et al. 2011; Fig. 1a). Manatees (Trichechus sp., Sirenia, Placentalia) and the pygmy rock-wallaby (Petrogale concinna, Macropodiformes, Marsupialia) have CDR, which might allow them to withstand intense dental wear resulting from highly abrasive diets, involving mainly grasses (Domning 1982; Sanson et al. 1985). CDR has also recently been described in the silvery mole-rat (Heliophobius argenteocinereus, Bathyergidae, Placentalia), which is a subterranean rodent (Gomes Rodrigues et al. 2011). It has been hypothesized that CDR could be related to the solitary and intensive chisel-tooth digging activity of this mole-rat, because it continuously rebuilds its burrow system for foraging and dispersion (Škliba et al. 2009), which could imply high dental wear (Gomes Rodrigues et al. 2011, 2016). However, the evolutionary origin of CDR in both placentals and marsupials remains to be explained.
(a) Diagrams of mandible showing patterns of continuous dental replacement (green arrows: mesial drift; red arrows: shed tooth) in the pygmy-rock wallaby and the silvery mole-rat. (b) Phylogeny of rock-wallabies (from Potter et al. 2012), also including the silvery mole-rat, and showing the adult dental formula (dP: deciduous premolars, P: permanent premolars, M: molars, [teeth rapidly shed]) and the number of specimens studied for geometric morphometric analyses. * data from Gomes Rodrigues et al. (2016)
Although morphological dental convergences are widespread among mammals, placentals and marsupials show disparate cranial and dental patterns and ontogenies (Clark and Smith 1993; Smith 1997; Cifelli and Muizon 1998; Luo et al. 2004; Sánchez-Villagra et al. 2008). Permanent anterior teeth (incisor and canines) show a delayed eruption in marsupials while the deciduous teeth remain in a vestigial state, and only the distal-most (third) premolar is replaced. This is probably due to the prolonged lactation necessitating a period of fixation of the young to maternal nipple soon after birth (Luckett 1993; Van Nievelt and Smith 2005). In contrast, all the teeth but molars are replaced in many placentals, which are not similarly constrained during early development. Early suckling also leads to a precocious ossification of facial bones in marsupials (Clark and Smith 1993; Smith 1997; Sánchez-Villagra et al. 2008). After quantification of cranial morphospaces, Bennett and Goswami (2013) concluded that such a developmental sequence has limited the number of possible evolutionary pathways in marsupials compared with placentals. Because the development of the masticatory apparatus is constrained early during the ontogeny of marsupials, we aim to determine first how CDR can be interpreted at the functional level in the pygmy rock-wallaby, by comparing the morpho-functional characteristics of the masticatory apparatus between the pygmy rock-wallaby and its closest relatives (other rock-wallabies).
Following the proposal of Losos (2011) in order to understand the multiple occurrences of convergent traits, the study of the functional consequences of CDR is required to assess the adaptive nature of the convergent evolution of this unique developmental pattern in mammals. Consequently, we compared the morpho-functional characteristics of the masticatory apparatus of the pygmy rock-wallaby with those of the silvery mole-rat, by using 3D geometric morphometrics, biomechanical estimations, and dental microwear analyses. The characteristics of this mole-rat have been recently published and compared with its closest relatives (Gomes Rodrigues et al. 2016). The case of rock-wallabies and mole-rats is interesting because they both show a diprotodont masticatory apparatus (i.e., two lower front teeth combined with a large diastema between incisor and cheek teeth), but a very different evolutionary background including distinct developmental constraints on the masticatory apparatus. Such a comparison will be a key route to better understand the functional origins of CDR in mammals. More generally, we aimed to assess to what extent and under which conditions convergent morphological traits can occur in mammals that are distantly related.
Material and Methods
Sample Composition
We analyzed 41 mandibles and 44 crania belonging to both sexes, and representing nine species of rock-wallabies for geometric morphometrics (Fig. 1, Online resource 1) housed in the mammal collections of the Natural History Museum, London (NHM; United Kingdom), and including one specimen from the University Museum of Zoology, Cambridge (UMZC; United Kingdom) and one specimen from Museum Victoria, Melbourne (MV; Australia). This sample notably includes Petrogale concinna and its sister species, P. brachyotis, which live in sympatry. Biomechanical analyses were limited to the six species for which more than one specimen was available for study. Dental casts of 11 Petrogale concinna, nine Petrogale brachyotis, and 15 specimens of Heliophobius argenteocinereus were sourced from the Western Australian Museum, Perth (WAM; Australia) and the Royal Museum for Central Africa, Tervuren (RMCA; Belgium).
Geometric Morphometric Methods
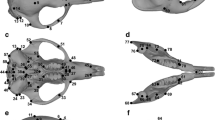
The mandibular and cranial forms were quantified with 11 and 61 anatomical landmarks, respectively (Fig. 2 and Online resource 2). Digital data of all specimens were acquired using a Microscribe 3-D digitizer. One skull of Petrogale brachyotis was scanned using Xradia Versa 520 XRM at energy of 120 kV with a cubic voxel of 46.4 μm, in order to visualize virtual deformations within the Petrogale dataset. Because the mandible of Petrogale is constituted by a dentary bone of relatively simple shape, most of the landmarks taken on the dentary were of type 2 (e.g., maxima of curvature - Fig. 2; Bookstein 1991). All configurations (sets of landmarks) were superimposed using the Procrustes method of generalized least squares superimposition (see Gomes Rodrigues et al. 2016 for more details). Shape variability of the cranium and mandible was analyzed by principal components analysis (PCA) of shape (Fig. 3). Analysis and visualization of patterns of shape variation were performed with the interactive software package MORPHOTOOLS (Specht et al. 2007; Lebrun et al. 2010). A multivariate regression of the first PCs representing 95 % of the variance (15 PCs for the mandibles and 30 PCs for the crania) on size, estimated by the logarithm of the centroid size, allowed us to take into account the potentially confounding effects of allometry on shape. Multivariate regressions were performed with PAST 2.06 (Hammer et al. 2001). To account for the potentially confounding effects of size allometry on shape of rock-wallabies, size-corrected shapes were obtained as follows: PCres corresponds to principal components of a PCA performed on shape data corrected for allometry (i.e. centroid size; Fig. 4). Additionally, univariate regressions were undertaken using the first two principal components and the logarithm of the centroid size of each specimen (Fig. 5). Younger (i.e. last molars not erupted, or premolars still present for P. concinna) and older specimens (i.e. last molars fully erupted, or premolars missing for P. concinna) of Petrogale were shown on univariate regressions.
Principal component analyses for (a) crania and (b) mandibles of Petrogale species and associated virtual deformations on the extreme sides of each axis. Symbols used: P. assimilis ( ), P. brachyotis (
), P. brachyotis ( ), P. concinna (
), P. concinna ( ), P. herberti (▲), P. lateralis (
), P. herberti (▲), P. lateralis ( ), P. penicillata (
), P. penicillata ( ), P. purpureicollis (
), P. purpureicollis ( ), P. rothschildi (
), P. rothschildi ( ), P. xanthopus (
), P. xanthopus ( )
)
Principal component analyses for (a) crania and (b) mandibles of Petrogale species with shape data corrected for allometry, and associated virtual deformations on the extreme sides of each axis. Symbols used: P. assimilis ( ), P. brachyotis (
), P. brachyotis ( ), P. concinna (
), P. concinna ( ), P. herberti (▲), P. lateralis (
), P. herberti (▲), P. lateralis ( ), P. penicillata (
), P. penicillata ( ), P. purpureicollis (
), P. purpureicollis ( ), P. rothschildi (
), P. rothschildi ( ), P. xanthopus (
), P. xanthopus ( )
)
Regression of the first and second principal components on the centroid size of (a, b) crania and (c, d) mandibles of Petrogale species, and associated virtual deformations. Symbols used: P. assimilis ( ), P. brachyotis (
), P. brachyotis ( ), P. concinna (
), P. concinna ( ), P. herberti (▲), P. lateralis (
), P. herberti (▲), P. lateralis ( ), P. penicillata (
), P. penicillata ( ), P. purpureicollis (
), P. purpureicollis ( ), P. rothschildi (
), P. rothschildi ( ), P. xanthopus (
), P. xanthopus ( ). Open symbols and the cross represent younger specimens
). Open symbols and the cross represent younger specimens
Biomechanical Analyses
The quantification of the mechanical advantage of each adductor muscle (temporalis, superficial masseter, and deep masseter) allows the measurement of force transmitted from the muscle to the bite point (cheek teeth). The mechanical advantage can be estimated as the ratio of the estimated in-lever (distance from the condyle or fulcrum to the point of muscle attachment) and the out-lever (distance from the condyle to the bite point; Hiiemae 1971). Three adductor muscles have been considered for in-levers: the temporalis, which attaches along the coronoid process (landmark 66); the superficial masseter, which attaches along the angular part of the mandible (landmark 69), and the deep masseter, which attaches in the masseteric fossa (landmark 71; Warburton 2009; Fig. 6). Only cheek teeth are considered as the bite point, and not incisors, which are less relevant here because our study focuses on cheek teeth replacement. The middle point of cheek tooth row (between landmarks 64 and 65) was measured as the bite point for the out-lever. Three estimates of biomechanical advantage (in-lever/out-lever) were thus considered for a given mandible (following Thorington and Darrow 1996; Gomes Rodrigues et al. 2016): TvsCT (temporalis/cheek teeth), SMvsCT (superficial masseter/cheek teeth), and DMvsCT (deep masseter/cheek teeth). Differences between rock-wallabies in the in-lever/out-lever ratios were tested using post-hoc multiple mean comparison tests: the Fischer’s Least Significant Difference test (LSD) and the Tukey’s Honestly Significant Difference test (HSD), the latter being less sensitive but more conservative than the former.
Mean cranial and mandibular reconstruction for the whole sample of rock-wallabies compared to P. concinna. Yellow and violet code for increases and decreases in surface area, respectively. Measures of in-levers (red lines), and out-levers (black lines) between landmarks digitized are indicated on the mandible. T: Temporalis; SM: Superficial Masseter; DM: Deep Masseter. Graphs represent the different mean in-lever/out-lever ratio with corresponding standard deviations for rock-wallabies
Dental Microwear Analysis
Dental microwear mainly results from abrasion (i.e. tooth-food contact), and its analysis permits reconstruction of the last diet items ingested by an animal prior to its death (Walker et al. 1978), involving the physical properties of the ingested matter. The protocol used here combines transmitted light stereomicroscopy and high resolution photographs (see Merceron et al. 2004). We use the method adapted to small mammals by Gomes Rodrigues et al. (2009), using translucent upper molar casts at ×100 magnification and a 0.01mm2 square at the center of dental facets to quantify dental microwear features (see Fig. 7). Three variables were used for quantification: the numbers of fine scratches (Nfs), wide scratches (Nws), and large pits (Nlp). Both Nws and Nlp have a width exceeding 5 μm. A high Nfs likely indicates an abrasive and tough component (e.g. consumption of either graminoids including small silica phytoliths and/or plants from the herbaceous stratum covered by dust); a high Nws indicates intake of coarse and tough to brittle material (e.g. fruits with hard shells, nuts) and a high Nlp likely indicates ingestion of hard material (e.g. exoskeleton of invertebrates, bones of vertebrates, dust and grit). Direct comparisons are possible between marsupials and placentals, because we only wanted to infer and compare the physical properties of material ingested by P. concinna and Heliophobius, and not the particular type of food items (Christensen 2014).
Dental characteristics of the pygmy rock-wallaby (a) and the silvery mole-rat (b). Details of occlusal surface (M: mesial, L: lingual) and enamel microstructure (pale grey corresponds to dentine and dark grey to enamel, which includes a softer outer layer and a translucent inner layer in the pygmy rock-wallaby, modified from Palamara et al., 1984 and Koenigswald, 2004) of erupting upper molars are represented with diagrams (not to scale). Photographs of dental microwear are also shown (squares indicate the facets analysed on upper molar occlusal surface). (c) Comparison of dental microwear data between P. brachyotis, P. concinna, and Heliophobius (Nfs: number of fine scratches, Nws: number of wide scratches, Nlp: number of large pits)
Results
According to both PCAs performed on the crania and mandibles (Online resource 3), P. concinna can be clearly differentiated from other Petrogale. The crania of P. concinna are clearly separated along the negative end of the first principal component (PC1, Fig. 3a), which shows a shortened snout, an increase in skull width with widened zygomatic arches, an elevation of the cranial roof, and a distal and reduced dental row. In contrast, in the positive values, most Petrogale species show a more gracile skull, enlarged snout with enlarged nasal bones and premaxillae, and a more mesial and enlarged dentition. Most of the specimens of P. concinna cluster together in the negative values of PC2 where specimens show a wider snout, but a reduced anterior part of premaxillae and a narrower posterior part of the braincase. The mandibles of P. concinna are clearly discriminated from those of other Petrogale species in the morphospace defined by the first two principal components (Fig. 3b). Negative PC1 correlates with mandibles characterized by a larger ascending ramus relative to the horizontal ramus, with a mesial dental row, whereas the tooth row is more distally located on the positive values, with mandibles showing a reduced ascending ramus compared to the horizontal ramus even if processes and condyle are slightly more expanded. PC2 displays variation in the height and width of the ascending ramus. The negative values characterize P. concinna, which show a mandible with an elevated condyle, an elongated and slender coronoid process projecting backward, and an angular process more lingual than the condyle.
A multivariate regression of the shape component on size, estimated by the logarithm of centroid size, was highly significant (cranium: F = 49.66, p = 2.38 × 10−9, df = 30; mandible: F = 6.712, p = 1.74 × 10−5, df = 15). Then, we observed less differentiation with shape data corrected for allometry (Fig. 4), especially in the mandible dataset. Specimens of P. concinna do not set apart from other Petrogale for both the cranium and mandible, which indicates that the differences in shape are related to its difference in size. However, on the negative side of PC2res for crania, P. lateralis differs from other Petrogale in having a larger braincase, a gracile snout with enlarged nasal bones, while the fronto-sphenoid part is reduced (Fig. 4a). Regressions of the first cranial and mandibular principal components on centroid size (Fig. 5; crania: r2 = 0.82; p = 2.09 × 10−17; mandible, r2 = 0.340; p = 5.71 × 10−5) show a clear allometric trends, with small specimens, including P. concinna and younger Petrogale, displaying wide crania, with a reduced snout, and a relatively shortened mandibular ramus, and a dental row distally located. However, even if regressions of the second principal component on centroid size are less obvious, although significant, for crania (r2 = 0.10; p = 0.0325), and mandibles (r2 = 0.29; p = 2.91 × 10−4), the specimens are not randomly distributed on the graph. It seems to highlight ontogenetic series of Petrogale species, in which older specimens tend to have a wider snout but a reduced anterior part of premaxillae, a narrower posterior part of the braincase, and a dental row slightly displaced mesially (Fig. 5b). It is notably expressed in P. concinna, and, to a lesser extent, in P. brachyotis, P. lateralis, and P. purpureicollis.
Estimates of biomechanical advantage for adductor muscles revealed that P. concinna present the highest values for the temporalis, the superficial masseter and the deep masseter among the reduced sample of Petrogale species (Fig. 6, Online resources 4 and 5). It significantly differs from other Petrogale species, including P. brachyotis, which show low values. Petrogale lateralis is also characterized by high values, especially for the masseters, while P. xanthopus tends to have the lowest values for all muscles but the deep masseter.
Dental microwear analyses showed that P. concinna displays a high proportion of fine scratches, likely indicating an ingestion of abrasive and tough material, and shows no significant differences with P. brachyotis. Heliophobius has a higher proportion of large pits than rock-wallabies, which is probably linked to coarse and hard elements (Fig. 7, Online resource 6).
Discussion
Heterochrony and Skull Development in the Pygmy Rock-Wallaby
The pygmy rock-wallaby has been frequently assigned to the monotypic genus Peradorcas, even in recent studies (Nowak 1999; Meredith et al. 2009), because of its unique mode of dental replacement, which is unknown in other Petrogale species. Such generic discrimination could be also substantiated by the strong cranial and mandibular morphological differentiations of P. concinna compared to its relatives. Nonetheless, we have shown that the wide but short skull of P. concinna is clearly related to its small size and is reminiscent of the overall morphology observed in young Petrogale specimens. As a result, P. concinna likely displays a neotonic morphology compared to other Petrogale (Fig. 6), as attested by the strong allometric relationships of skull shape in Petrogale, especially the cranium (Fig. 5). As a matter of fact, a young specimen of its closest relative P. brachyotis resembles the specimens of P. concinna in some aspects (see negative side of PC1 on Fig. 5a). The reduced snout of P. concinna and young Petrogale, especially in its posterior part, combined with the distal location of a shorter dentition can clearly be considered as juvenile characters because the elongation of the rostrum involves a more mesial and enlarged cheek tooth row in adults. During the prolonged growth of kangaroos and wallabies (Cockburn and Johnson 1988), there is a mesial progression of the dentition, which can be either attributed to dental shift or drift (Lentle and Hume 2010). Dental drift corresponds to the migration of teeth inside the bone, as it is continuously observed from the rear to the front of the jaw of P. concinna (Fig. 1a). It can be defined as moderate (following Gomes Rodrigues et al. 2012) in most kangaroos, which can lose their premolars completely, whereas it should be considered as slight in most Petrogale, because only tipping of premolars has been observed without dental loss (Lentle and Hume 2010). Consequently, such a mesial progression of the dentition is mainly attributed to mesial shift in rock-wallabies (Lentle et al. 2003b), which corresponds to virtual displacement of teeth due to bone apposition during growth, especially at the rear of the jaw. All these observations concur with the fact that P. concinna is a small rock-wallaby that retained a juvenile morphology, probably like the smallest rock-wallaby P. burbidgei (Kitchener and Sanson 1978), which could not be investigated here.
A Developmental Adjustment toward an Increase of the Masticatory Efficiency?
The muscular anatomy of the masticatory apparatus of rock-wallabies remains poorly studied (Parsons 1896). We showed that, despite its small size, P. concinna displays widened zygomatic arches, higher mandibular condyles, enlarged but slender coronoid processes, and more lingual angular processes (Fig. 3). All these modifications may be associated with larger areas of insertion for adductor muscles (temporalis, superficial masseter, deep masseter, and medial pterygoid). The developmental constraints on the oral region during the early development of marsupials are likely to limit morphological changes compared to placentals (Bennett and Goswami 2013). The paedomorphic nature of the skull of P. concinna might represent a suitable adjustment to improve the efficiency of the masticatory apparatus. Such an assertion is evidenced by the values obtained for the biomechanical advantages of the adductor muscles, which are the highest for P. concinna compared to other Petrogale species, especially its sister species P. brachyotis. The distal position of the tooth row in P. concinna induces a decrease of the out-lever and, as a consequence, an increase of the output force at the cheek teeth level.
The pygmy rock-wallaby has larger insertion for adductor muscles, especially for the temporalis because of a slightly enlarged coronoid processes (Fig. 6). In kangaroos and wallabies, the temporalis permits an efficient cutting of plant materials with incisors, particularly in browsers (Lentle et al. 2003a; Warburton 2009). Petrogale concinna seems to be highly prone to grazing and seasonally feeds on abrasive ferns found in floodplains during the dry season (Sanson et al. 1985). The putative enlargement of the temporalis in P. concinna linked to the development of the coronoid process could also confer to this species a more efficient cutting of highly fibrous plant material.
Morphological Convergences and Masticatory Disparities between Rock-Wallabies and Mole-Rats
Similarly, most African mole-rats show a cranial configuration characterized by a wide cranium with a short snout (Gomes Rodrigues et al. 2016), which is associated with massive adductor muscles (temporalis, superficial masseter, and deep masseter) used for digging with incisors (Cox and Faulkes 2014). They are also considered as forceful biters among mammals, especially at the level of molars (Van Daele et al. 2009). The temporalis is highly developed and greatly contributes to the jaw closing motion (Cox and Faulkes 2014), which might increase the output force at the level of both incisors and molars in these rodents (Gomes Rodrigues et al. 2016; McIntosh and Cox 2016). Interestingly, the pygmy rock-wallaby and the silvery mole-rat show similar overall morphological trends in having larger insertions for adductor muscles, especially for the temporalis.
However, the morphological convergences observed between these animals, which could be attributed to the occurrence of the CDR, do not actually reflect similar adaptations but rather an ecological differentiation. Even if the silvery mole-rat shows high values, it has lower biomechanical advantage of adductor muscles for molar bite than most of its relatives (Gomes Rodrigues et al. 2016), contrary to the pygmy rock-wallaby. Differences in the direction of the power stroke should also be integrated when considering morpho-functional aspects. Distinct orthal and transverse phases of mastication are observed in kangaroos and wallabies, contrasting with the sole efficient transverse motion noticed in most placental herbivores (Crompton et al. 2008). In kangaroos and wallabies, the insertion of the deep masseter on the mandible protrudes anteriorly in the masseteric fossa. In addition, the extended lingual angular process allows for a greater insertion of the superficial masseter and for a widening of the pterygoid fossa involving a developed medial pterygoid (Tomo et al. 2007; Crompton et al. 2008; Warburton 2009). All these characteristics are not present in most mole-rats, which have a reduced angular process potentially limiting the force generated by the superficial masseter during mastication (Gomes Rodrigues et al. 2016). In kangaroos and wallabies, the superficial masseter and the medial pterygoid greatly contribute to the orthal phase of shearing food between high transverse lophs of molars, and more importantly to the transverse phase of crushing food on interlophine links (Sanson 1980, 1989; Crompton et al. 2008; Crompton 2011; see Fig. 7). Shearing and crushing actions could be hypothesized as improved in the pygmy rock-wallaby because of its larger angular process for the insertion of the superficial masseter, as well as its wider pterygoid fossa, but this remains to be tested. The process of mastication is far less known in the African mole-rats. The main component of their mastication was assumed to be propalinal (Landry 1957). But, they more likely have a significant oblique component associated with precocious flat occlusal dental surface, due to very low cusps associated with enamel free areas present before eruption (Fig. 7).
Mosaic of Morpho-Functional Characters Associated with CDR
Alternative mechanisms preventing high dental wear and allowing a sustained function of the dentition, such as high-crowned teeth, are less common among extant marsupials (i.e., only wombats) than in placentals (e.g., “ungulates”, xenarthrans, rodents). However, P. concinna displays a tooth row more curved than other Petrogale, which more importantly limits the number of functional teeth and maintains the efficiency of the dentition throughout its lifetime (Sanson 1989). This is coupled to a reduced dental length compensated by the presence of CDR, and which facilitates dental drift. It also has a molar enamel microstructure with a soft outer layer, which is opaque, hypomineralized, and shows a high number of voids contrary to other macropodids, such as Macropus and Wallabia (other Petrogale were unfortunately not considered; Palamara et al. 1984). Such a feature might favour rapid wear at the tip of cusps, and would be appropriate to maintain precise occlusal relationships during dental drift and to limit malocclusion issues (Palamara et al. 1984). In contrast, a softer enamel would decrease the molar efficiency more rapidly, so the presence of CDR becomes an advantage (Sanson 1989), especially considering the tough and highly abrasive components of their feeding habits revealed by dental microwear analyses. However, it might not be relevant here to link the acquisition of CDR only with differences in feeding habits. This is challenged by our microwear study indicating that sympatric P. brachyotis and P. concinna might eat abrasive material or plants, such as grasses, in similar proportions, as noticed by Telfer and Bowman (2006), even if data on fern consumption are lacking in their study. Moreover, other Petrogale species, such as P. xanthopus and P. assimilis, are known to significantly feed on grasses (Arman and Prideaux 2015), but their masticatory apparatus does not show higher mechanical advantages for adductor muscles than most of the investigated Petrogale species. None of these observations take into account the potential effect of the incidental ingestion of dust deposited in the lower stratum of plant cover depending on the environment and habitat, and the fact that comminution of highly fibrous plant may involve longer chewing sequences and could lead to higher dental wear, especially due to the combined ingestion of abrasive mineral particles. The putative differences of feeding behavior (i.e. diet and mastication) and habitat (rock-habitat specialist vs. generalist) between P. concinna and P. brachyotis, could have led to niche partitioning (Telfer et al. 2008). Variations in size and dental replacement might be the consequences of such an ecological partitioning probably at the origin of vicariant speciation.
In comparison with the pygmy rock-wallaby, the masticatory apparatus of the silvery mole-rat is not especially predisposed to a more efficient use of cheek teeth compared to its relatives. In fact, the skull of Heliophobius is mainly constrained by its subterranean mode of life, and specifically adapted to chisel-tooth digging like other African mole-rats (Gomes Rodrigues et al. 2016; McIntosh and Cox 2016). This does not mean that the masticatory apparatus of Heliophobius is less efficient than that of the wallaby, because it shows relatively higher biomechanical advantages for molars than for incisors (Gomes Rodrigues et al. 2016), which however need high output force for digging. Heliophobius displays precocious flat occlusal surfaces; its high-crowned cheek teeth are likely efficient for grinding (Schmidt-Kittler 2002), but the incisors are mainly used for cutting and shearing the underground part of plants (tubers and bulbs), as well as digging. No difference in enamel microstructure was observed in comparison with other mole-rats (Koenigswald 2004), and most of them show high dental wear with apparent traces of damage, apart from dental microwear which could only be investigated in Heliophobius. One can legitimately wonder why this species developed a CDR, while it already has high-crowned teeth to withstand dental wear, which is generally associated with the ingestion of abrasive matter (Janis and Fortelius 1988; Koenigswald 2011; Madden 2015). It has been recently noted that high-crowned teeth enable improved anchorage of teeth during dental drift, which in turn can contribute to the setting up of CDR (Gomes Rodrigues and Šumbera 2015). Dental microwear analyses confirmed this assertion and revealed that strong attrition is probably combined with the ingestion of dust and grit because of high activity of digging and feeding, which leads to strong molar damages and intense wear compensated by CDR. Paradoxically, the ingestion of mineral particles associated with flat and simplified dental occlusal surface might enhance the mechanical reduction of underground part of plants, and represent an alternative to shearing crests (Madden 2015).
On the Origins of CDR and Convergent Traits in Mammals
Understanding the origins of CDR in mammals is highly challenging from evolutionary and functional points of view. It might be directly associated with the feeding behavior in the pygmy rock-wallaby, whereas it might be tightly related to the fossorial activity of the silvery mole-rat, which surely has a significant impact on dental wear. More generally, if developmental, phylogenetic and ecological constraints could have limited the acquisition of CDR in mammals, its scarce occurrence is surely a contingent phenomenon. On the one hand, different constraints do not necessarily prevent the occurrence of similar traits: for instance, CDR is combined with developmental adjustments for the skull in a marsupial, and superimposed on a digging-adapted skull in a placental. On the other hand, similar phylogenetical backgrounds do not hinder opposite evolutionary trends, as exemplified by sirenians for different types of plant comminution: manatees have a CDR, dugong has a degenerate but high-crowned dentition associated with enlarged horny pads, and the extinct Steller’s sea cow only had horny pads without teeth (Domning 1982; Lanyon and Sanson 2006). Consequently, and even if prerequisites are required for the setting of CDR (e.g., mesial drift; Gomes Rodrigues et al. 2011), only infrequent genetic processes could explain the evolution of supernumerary teeth in very distant phylogenetical taxa such as marsupials and placentals. Only then this mechanism could have been positively selected in response to environmental pressures, which vary from one group to another (i.e. food-related abrasiveness, life underground), but of which the consequences are in the end the same (i.e., high dental wear).
In conclusion, these results show that if convergent features occur in mammals, they may sometimes reflect different adaptive natures. The study of adaptation constitutes an important cornerstone of evolutionary biology, but defining an adaptation is not an easy task (Curio 1973; Gould and Lewontin 1979; Gould and Vrba 1982; Krimbas 1984; Reeve and Sherman 1993). Biological adaptation can only be built upon available variation, and evolutionary plasticity is in part limited by phylogenetic constraints and the general rules of construction of a structure. While variation is widely available in nature, the general rules of structural construction are not infinite as they depend on the preservation of the integration between different body parts, which directly reflects the dictates of past adaptations (Lessa 2000; Arthur 2001). Adaptationist hypotheses consider morphological variation as mainly reflecting adaptation in relation to organismal survival. In contrast, the structuralist viewpoints aim to understand the general rules of construction of a morphological feature (Seilacher 1970; Raup 1972; Cubo et al. 2008). In practice, these two schools of thought are not mutually exclusive. The convergent evolution of CDR in distant mammalian clades gives us an opportunity to separate the effect of ecological and developmental constraints on the morphological evolution of the skull. The difficulty lies in assessing the relative importance of constraints and selection, as well as the potential confounding effects of ecological factors that can channel morphological variation into a main direction by the acquisition of CDR. The adaptationist program is based on the observation that an adaptive balance, derived from natural selection, will lead a structure to align itself with a function. However, we showed that the analysis of this dual relationship must be set into a functional, developmental, and phylogenetic context in order to better understand the notions of convergences and adaptation as well as the respective roles played by environmental conditions and other factors susceptible to interact with selection.
References
Arendt J, Reznick, D (2008) Convergence and parallelism reconsidered: what have we learned about the genetics of adaptation? Cell 23:26–32
Arman SD, Prideaux GJ (2015) Dietary classification of extant kangaroos and their relatives (Marsupialia: Macropodoidea) Austral Ecol 40: 909–922
Arthur W (2001) Developmental drive: an important determinant of the direction of phenotypic evolution. Evol Dev 3:271–278
Bennett CV, Goswami A (2013) Statistical support for the hypothesis of developmental constraint in marsupial skull evolution. BMC Biol 11:52
Bookstein FL (1991) Morphometric Tools for Landmark Data Geometry and Biology. Cambridge University Press, Cambridge
Christensen HB (2014) Similar associations of tooth microwear and morphology indicate similar diet across marsupial and placental mammals. PloS One 9:e102789
Cifelli RL, Muizon C de (1998) Tooth eruption and replacement pattern in early marsupials. CR Acad Sci II A 326:215–220
Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215:119–149
Cockburn A, Johnson CN (1988) Patterns of growth. In: Tyndale-Biscoe CH, Janssens PA (eds) The Developing Marsupial. Springer, Berlin, pp 28–40
Cox PG, Faulkes CG (2014) Digital dissection of the masticatory muscles of the naked mole-rat, Heterocephalus glaber (Mammalia, Rodentia). Peer J 2:e448
Crompton AW (2011) Masticatory motor programs in Australian herbivorous mammals: Diprotodontia. Integr Comp Biol 51:271–281
Crompton AW, Barnet J, Lieberman DE, Owerkowicz T, Skinner J, Baudinette RV (2008) Control of jaw movements in two species of macropodines (Macropus eugenii and Macropus rufus). Comp Biochem Phys Part A 150:109–123
Cubo J, Legendre P, de Ricqlès, A, Montes L, Margerie de E, Castanet J, Desdevises Y (2008) Phylogenetic, functional, and structural components of variation in bone growth rate of amniotes. Evol Dev 10:213–223
Curio E (1973) Towards a methodology of teleonomy. Experientia 29:1045–1058
Domning DP (1982) Evolution of manatees; a speculative history. J Paleontol 56:599–619
Domning DP, Hayek, L-AC (1984) Horizontal tooth replacement in the Amazonian manatee (Trichechus inunguis). Mammalia 48:105–127
Gomes Rodrigues H, Marangoni P, Šumbera R, Tafforeau P, Wendelen W, Viriot L (2011) Continuous dental replacement in a hyper-chisel tooth digging rodent. Proc Natl Acad Sci USA 108:17355–17359
Gomes Rodrigues H, Merceron G, Viriot L (2009) Dental microwear patterns of extant and extinct Muridae (Rodentia, Mammalia): ecological implications. Naturwissenchaften 96:537–542
Gomes Rodrigues H, Solé F, Charles C, Tafforeau P, Vianey-Liaud M, Viriot L (2012) Evolutionary and biological implications of dental mesial drift in rodents: the case of the Ctenodactylidae (Rodentia, Mammalia). PloS One 7:e50197
Gomes Rodrigues H, Šumbera R (2015) Dental peculiarities in the silvery mole-rat: an original model for studying the evolutionary and biological origins of continuous dental generation in mammals. Peer J 3:e1233
Gomes Rodrigues H, Šumbera R, Hautier L (2016) Life in burrows channelled the morphological evolution of the skull in rodents: the case of African mole-rats (Bathyergidae, Rodentia). J Mammal Evol 23:175–189
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc Roy Soc B 205:581–597
Gould SJ, Vrba E (1982) Exaptation-a missing term in the science of form. Paleobiology 8:4–5
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hiiemae K (1971) The structure and function of the jaw muscles in the rat (Rattus norvegicus L) III. The mechanics of the muscles. Zool J Linn Soc 50:111–132
Janis CM, Fortelius M (1988) On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol Rev 63:197–230
Kitchener DJ, Sanson GD (1978) Petrogale burbidgei (Marsupialia, Macropodidae), a new rock wallaby from Kimberley, Western Australia. Rec W Austral Mus 6:269–285
Koenigswald W von (2004) The three basic types of schmelzmuster in fossil and extant rodent molars and their distribution among rodent clades. Palaeontographica Abt A 270:95–132
Koenigswald W von (2011) Diversity of hypsodont teeth in mammalian dentitions – construction and classification. Palaeontographica Abt A 294:63–94
Krimbas CB (1984) On adaptation, neo-Darwinian tautology, and population fitness. Evol Biol 17:1–57
Landry SC (1957) The interrelationships of the New and Old World hystricomorph rodents. Univ Calif Pub Zool 56:1–118
Lanyon JM, Sanson GD (2005) Degenerate dentition of the dugong (Dugong dugon), or why a grazer does not need teeth: morphology, occlusion and wear of mouthparts. J Zool 268:133–152
Lebrun R, Ponce de León MS, Tafforeau P, Zollikofer CPE (2010) Deep evolutionary roots of strepsirrhine primate labyrinthine morphology. J Anat 216:368–380
Lentle RG, Hume I (2010) Mesial drift and mesial shift in the molars of four species of wallaby: the influence of chewing mechanics on tooth movement in a group of species with an unusual mode of jaw action In: Coulson G, Eldridge M (eds) Macropods: The Biology of Kangaroos, Wallabies and Rat-Kangaroos. CSIRO Publishing, Collingwood, pp 127–137
Lentle RG, Hume ID, Stafford KJ, Kennedy M, Haslett S, Springett BP (2003a) Comparison of tooth morphology and tooth patterns in four species of wallabies. Aust J Zool 51:61–79
Lentle RG, Hume ID, Stafford KJ, Kennedy M, Haslett S, Springett BP (2003b) Comparisons of indices of molar progression and dental function of brush-tailed rock-wallabies (Petrogale penicillata) with tammar (Macropus eugenii) and Parma (Macropus parma) wallabies. Aust J Zool 51:259–269
Lessa EP (2000) The evolution of subterranean rodents: a synthesis In: Lacey EA, Patton JL, Cameron GN (eds) Life Underground, the Biology of Subterranean Rodents. The University of Chicago Press, Chicago, pp 389–420
Losos JB (2011) Convergence, adaptation and constraint. Evolution. 65:1827–1840
Luckett WP (1993) An ontogenetic assessment of dental homologies in therian mammals In: Szalay FS, Novacek MJ, McKenna, MC (eds) Mammal Phylogeny-Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials, Vol. 2. Springer-Verlag, New York, pp 182–204
Luo Z-X, Kielan-Jaworowska Z, Cifelli RL (2004) Evolution of dental replacement in mammals. Bull Carnegie Mus Nat Hist 36:159–175
Madden RH (2015) Hypsodonty in Mammals - Evolution, Geomorphology, and the Role of Earth Surface Processes. Cambridge University Press, Cambridge
McGhee GR (2011) Convergent Evolution-Limited Forms Most Beautiful. The MIT Press
McIntosh AF, Cox PG (2016) Functional implications of craniomandibular morphology in African mole-rats (Rodentia: Bathyergidae). Biol J Linn Soc 117: 447–462
Merceron G, Blondel C, Viriot L (2004) Tooth microwear pattern in roe deer (Capreolus capreolus L) from Chizé (western France) and relation to food composition. Small Ruminant Res 53:125–132
Meredith RW, Westerman M, Springer MS (2009) A phylogeny of Diprotodontia (Marsupialia) based on sequences for five nuclear genes. Mol Phylogenet Evol 51: 554–571
Nowak RM (1999) Walker’s Mammals of the World, Vol II, 6th ed. The Johns Hopkins University Press, Baltimore
Palamara J, Phakey PP, Rachinger WA, Sanson GD, Orams HJ (1984) On the nature of the opaque and translucent enamel regions of some Macropodinae (Macropus giganteus, Wallabia bicolor and Peradorcas concinna). Cell Tissue Res 238: 329–337
Parsons FG (1896) On the anatomy of Petrogale xanthopus, compared with that of other kangaroos. Proc Zool Soc London 1896: 683–714
Potter S, Cooper SJB, Metcalfe CJ, Taggart DA, Eldridge MDB (2012) Phylogenetic relationships of rock-wallabies, Petrogale (Marsupialia: Macropodidae) and their biogeographic history within Australia. Mol Phylogenet Evol 62:640–652
Raup DM (1972) Approaches to morphological analysis in: Schopf TJM (ed) Models in Paleobiology. Freeman, Cooper and Co, San Francisco, pp 28–44
Reeve HK, Sherman PW (1993) Adaptations and the goals of evolutionary research. Quart Rev Biol 68:1–32
Sánchez-Villagra MR, Goswami A, Weisbecker V, Mock O, Kuratani S (2008) Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evol Dev 10:519–530
Sanson, GD (1980) The morphology and occlusion of the molariform cheek teeth in some Macropodinae (Marsupialia; Macropodidae). Aust J Zool 28:341–365
Sanson GD (1989) Morphological adaptations of teeth to diets and feeding in the Macropodoidea In: Grigg G, Jarman P, Hume I (eds) Kangaroos, Wallabies and Rat-kangaroos. Surrey Beatty and Sons, Sydney, pp 151–168
Sanson GD, Nelson JE, Fell P (1985) Ecology of Peradorcas concinna in Arnhem land in the wet and dry season. Proc Ecol Soc Aust 13: 69–72
Schmidt-Kittler N (2002) Feeding specializations in rodents. Senckenberg Leth 2:141–142
Seilacher, A (1970) Arbeitskonzept zur Konstruktions-Morphologie. Lethaia 3: 393–396
Škliba J, Šumbera R, Chitaukali WN, Burda H (2009) Home-range dynamics in a solitary subterranean rodent. Ethology 115:217–226
Smith KK (1997) Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution 51:1663–1678
Specht M, Lebrun R, Zollikofer CPE (2007) Visualizing shape transformation between chimpanzee and human braincases. Visual Comp 23:743–751
Telfer WR, Bowman WNJS (2006) Diet of four rock-dwelling macropods in the Australian monsoon tropics. Austral Ecol 31:817–827
Telfer WR, Griffiths AD, Bowman WNJS (2008) The habitat requirements of four sympatric rock-dwelling macropods of the Australian monsoon tropics. Austral Ecol 33:1033–1044
Thorington RW, Darrow K (1996) Jaw muscles of Old World squirrels. J Morphol 230:145–165
Tomo S, Tomo I, Townsend GC, Hirata K (2007) Masticatory muscles of the great-gray kangaroo (Macropus giganteus). Anat Rec 290: 382–388
Ungar PS (2010) Mammal Teeth: Origin, Evolution, and Diversity. The John Hopkins University Press, Baltimore
Van Daele PAAG, Herrel A, Adriaens D (2009) Biting performance in teeth-digging African mole-rats (Fukomys, Bathyergidae, Rodentia). Physiol Biochem Zool 82:40–50
Van Nievelt AFH, Smith KK (2005) To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31:324–346
Wake DB, Wake MH, Specht CD (2011) Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science 331:1032–1035
Walker A, Hoeck H, Perez L (1978) Microwear of mammalian teeth as an indicator of diet. Science 201:908–910
Warburton, NM (2009) Comparative jaw muscle anatomy in kangaroos, wallabies, and rat-kangaroos (Marsupialia: Macropodoidea). Anat Rec 292:875–884
Acknowledgments
We are grateful to R. Portela Miguez from the NHM (United Kingdom), to R. How and C. Stevenson from the WAM (Australia), to R. Asher and M. Lowe from the UMZC (United Kingdom), and to K. Roberts from MV (Australia) for providing access to their collections of rock-wallabies. We also acknowledge W. Wendelen from the RMCA (Belgium) for allowing the study of silvery mole-rats. We thank R. Lebrun (Institut des Sciences de l’Evolution de Montpellier) who kindly gave us access to MeshTools and Morphotools, and for his help during analyses, and to G. Merceron (IPHEP, Poitiers) for technical support during dental casts. We would also wish to thanks the anonymous reviewers and the editor-in-chief for their valuable comments and suggestions. This work was supported by La Fondation des Treilles (www.les-treilles.com, grant to H.G.R.). This research also received support from the SYNTHESYS Project http://www.synthesys.info/, which is financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” Programme. L.H. acknowledges Sidney Sussex College (Cambridge, UK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1 Online resource 1
(DOCX 20 kb)
ESM 2 Online resource 2
(DOC 35 kb)
ESM 3 Online resource 3
(DOCX 13 kb)
ESM 4 Online resource 4
(DOCX 14 kb)
ESM 5 Online resource 5
(DOCX 14 kb)
ESM 6 Online resource 6
(DOC 73 kb)
Rights and permissions
About this article
Cite this article
Gomes Rodrigues, H., Hautier, L. & Evans, A.R. Convergent Traits in Mammals Associated with Divergent Behaviors: the Case of the Continuous Dental Replacement in Rock-Wallabies and African Mole-Rats. J Mammal Evol 24, 261–274 (2017). https://doi.org/10.1007/s10914-016-9348-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-016-9348-7