Abstract
The National Mastitis Council was founded in 1961 based on the desire of a forward-thinking group of individuals to bring together “all forces of organized agriculture in the United States to combat, through every practical device, the mastitis threat to the Nation’s health and food safety”. What started as a small organization focused on mastitis of dairy cattle in the United States has grown into a global organization for mastitis and milk quality. Over the last 50-plus years the concerted efforts of the membership have led to the synthesis and dissemination of a considerable body of knowledge regarding udder health, milk quality, and food safety which has improved dairy cattle health and well-being and farm productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis, an inflammation of the udder most often caused by an intramammary infection (IMI) with an invading microbial agent, is considered the most common and costly disease of dairy cattle worldwide [1]. Further, mastitis is the most common reason that dairy cattle are treated with antimicrobials [2, 3]. Because milk is an important source of human nutrition, mastitis is not only an animal health concern but a human health concern. Thus, mastitis is a disease at the interface of animal and human health fitting under the One Health paradigm. In addition, mastitis can afflict almost all mammalian species and many of the lessons learned in the study of lactation and mastitis in the bovine are relevant to other farm animals, domestic pets, and people.
Mastitis can be clinical, having visually detectable changes in the milk, udder, and sometimes the cow, or subclinical, with no overt clinical signs. The economic impacts of mastitis come from lost milk production, veterinary costs, treatment costs, increased labor, altered product quality, diagnostics, concomitant diseases, and animal replacement costs due to culling [1]. In 2013, the United States milked 9.2 million dairy cattle which produced approximately 201 billion pounds of milk (http://www.ers.usda.gov/data-products/dairy-data.aspx). Estimates on the economic impact of mastitis vary based on mastitis presentation (clinical versus subclinical), treatment, and preventative measures. For example, in their review Halasa et al. [1] reported that, on average, a case of clinical mastitis cost $367 per cow per year, whereas the cost of subclinical mastitis cost averaged $130 per cow per year. Further, they report that the average cost of mastitis prevention was $33 per cow per year [1]. While current national figures on the cost of mastitis to the U.S. dairy industry are not available, estimates from the 1990s suggest the cost is in the region of $1.5-2.0 billion per year.
In developed countries milk sold for human consumption is highly regulated to ensure safety and quality. Most countries have guidelines for saleable milk quality based on three important measures, somatic cell count (SCC; an indicator of inflammation), bacteria count (pre- and post-pasteurization), and freedom from therapeutic drug residues. While pasteurization is a common critical control point to protect human health, some regions allow sale of raw milk. Regardless, milk from clinical mastitis cases should not enter the food chain. Milk from cows with subclinical mastitis can enter comingled bulk milk that is sold for processing. However, the aforementioned regulatory standards are in place to ensure a safe and wholesome product. Because there is a significant correlation between milk SCC, milk yield from the cow, finished product yield, and shelf-life, a lot of attention has been focused on controlling mastitis on dairy farms.
The National Mastitis Council (NMC) is a not-for-profit professional organization devoted to reducing mastitis and enhancing milk quality. The NMC promotes research and provides information to the dairy industry on udder health, milking management, milk quality, and milk safety. Founded in 1961 and currently headquartered in Wisconsin, NMC has approximately 1,500 members in more than 40 countries throughout the world. While the major focus of NMC has been dairy cattle mastitis, over the years issues related to mastitis in other species such as dairy goats has been discussed, and research on mastitis and milk quality in several species other than cattle has been presented.
History of the Organization
The NMC originated from the desire of the Executive Board of the International Association of Milk and Food Sanitarians (IAMFS; now known as International Association for Food Protection), to assemble the available information on bovine mastitis and to stimulate collective action for controlling the disease. The IAMFS Executive Board, through its Committee on Farm Methods, appointed a Mastitis Action Committee in early 1960. The committee was comprised of representatives from organizations concerned with milk production, dairy processing, equipment manufacture, and food sanitation.
The Mastitis Action Committee planned and sponsored a Mastitis Action Conference in Chicago, Illinois on October 29, 1960. With 225 registrants, the conference recommended the formation of a permanent National Committee on Mastitis Action. The initial meeting to organize a National Mastitis Action Committee was held on January 20, 1961 in Chicago, Illinois. The permanent name of the organization, National Mastitis Council, was agreed upon at a session on March 17, 1961. Three more meetings were held in 1961 to plan the financing and function of the Council. A constitution and bylaws were adopted, with the purpose of the NMC being “to promote, aid or engage in educational activities and scientific research in the field of mastitis control, including the coordination and focusing of all forces of organized agriculture in the United States to combat, through every practical device, the mastitis threat to the Nation’s health and food safety.”
Much like our understanding of mastitis has evolved over the last five decades so has NMC, growing from a national organization to a global organization. NMC’s mission, however, remains essentially unchanged and that is to “Provide a forum for education and global exchange of information on milk quality, mastitis, and relevant research. Communicate that information to the dairy industry enabling it to control mastitis and improve milk quality”. Similarly, the organization’s vision is to be “The global information source for the production of quality milk”.
Membership
The NMC is a membership-based organization, independent of any university or government agency. Membership is open to anyone with an interest in udder health, milking management, and milk quality. Individuals and companies from all segments of the dairy industry are represented including dairy producers, dairy cooperatives, milk handlers, veterinarians, researchers, extension educators, industry suppliers and manufacturers, sanitarians, dairy plant field staff, regulatory officials, and students. The largest sectors of the membership include veterinarians (28 %), individuals from industry (e.g., milking machine manufacturers, pharmaceutical and animal health companies, and teat disinfectant/sanitizer companies; 26 %), university researchers and extension educators (12 %), and dairy producers (11 %). While the organization is based in the U.S., membership has expanded globally over the last 50-plus years with approximately 25 % of the membership coming from outside of the United States. In 1966, for example, 300 people registered for the 5th annual meeting and only three countries were represented. Annual meeting attendance now exceeds 400 with attendees coming from as many as 25 countries.
The comprehensive breadth of the NMC’s membership brings together professionals from all facets of the dairy industry under one umbrella making the organization distinct among professional organizations. “The NMC is a unique organization. I cannot think of any group comprised of so many segments of an industry, all linked by their common interest in a single disease” (Bob Eberhart, NMC President, 1984).
Organizational Structure
The NMC is currently governed by a fifteen-member Board of Directors and a five-member Executive Committee. An Executive Director is employed full-time to manage the organization and part-time employees are engaged to distribute the workload. The Board is elected by and from the membership. Representation on the Board is allocated to maintain a balance among the specialized groups within the membership. Board members are elected for a three-year term with the possibility of serving a second consecutive three-year term. The officers are elected by and from the Board of Directors and serve one-year terms. Many activities of the NMC are carried out by committees and task forces. These groups play a major role in NMC’s organization, providing a forum to evaluate issues and make recommendations to the Executive Committee and Board of Directors. Committee chairs and committee members are appointed for a three-year term by the Board of Directors.
One of the most important features of NMC has been the committee system. Over the years, committee work organized and carried out by NMC members has provided vital guidance to the Council and assisted in the implementation of projects that have led to new educational materials and documents.
For example, the Research Committee was developed from one of five task forces that presented reports at the first Mastitis Action Conference in 1960. Appointed in May 1961, one of the first tasks of the Research Committee was to conduct a survey of research projects with a view to coordinating research efforts. At the 1962 NMC annual meeting, the Education Committee reported that the primary need in the control of mastitis was a “well-documented compilation containing the basic non-controversial information that is known about mastitis”. Synthesis of the best available knowledge into a user friendly format for use by the dairy industry has been a cornerstone of the organization. Over the years committees have come and gone and the role of each committee has changed over time. Currently there are six core committees including Research, Teat Health, Milk Quality Monitoring, Membership & Marketing, Annual Meeting Program, and Nominating.
Educational Materials
Information exchange is a major component of the NMC mission and as such the organization has a long history of generating educational materials. These materials include fact sheets, educational pamphlets, guidance documents, peer-reviewed publications, a bimonthly newsletter, meeting proceedings, and text books. A list of printed publications (books and pamphlets) available for purchase from NMC is found in Table 1.
One of the first acts of the NMC was to develop uniform recommendations for promoting and implementing mastitis prevention and control programs. First outlined in 1962, several hundred copies of the NMC recommendations were distributed to States, local groups, and individuals, becoming the foundation for formation of State and local organization mastitis control programs. Also in 1962, a committee was constructed to draft what was known about mastitis into a booklet which would become “Current Concepts of Bovine Mastitis”, a text book that is currently being revised for release as the fifth edition.
Somatic Cell Count
A subcommittee of the Research Committee was formed in 1967 to evaluate screening tests for mastitis. The subcommittee published an improved method for direct microscopic counting of somatic cells in milk. The NMC has been involved in advising on regulatory limits for SCC for many years and these efforts are summarized below (Advisement to Government and Regulatory Agencies).
Additionally, NMC has published milk quality guidance documents centered on SCC including guidelines for normal and abnormal milk based on SCC and signs of clinical mastitis, guidelines for accurate sampling and reporting of bulk milk cell counts, and using the new SCC sire evaluations. A fact sheet specifically related to SCC is also available entitled “The Value and Use of Individual Cow Somatic Cell Count” (Table 1).
Milk Microbiology
Early on, the Research committee was also instrumental in developing standardized bacteriological methods for the diagnosis of IMI which led to the publication of a manual entitled “Microbiological Procedures for Diagnosis of Bovine Mastitis”. The fourth edition of this manual, now entitled “Microbiological Procedures for the Diagnosis of Bovine Udder Infection and Determination of Milk Quality” [4] still serves as a basic reference for mastitis researchers and those involved in mastitis diagnosis. During the 1980s, a subcommittee worked on an expanded microbiological procedures manual that became the 208-page “Laboratory and Field Handbook on Bovine Mastitis” [5]. A revised edition with the title “Laboratory Handbook on Bovine Mastitis” was published in 1999 [6]. The revised edition placed increased emphasis on appropriate use of microbiological procedures to differentiate pathogens that cause mastitis and identifying the most likely sources on the farm to allow implementation of treatment and control strategies. The laboratory handbook has become an international reference for use by researchers, veterinarians, and diagnostic laboratories, and is currently undergoing its third revision.
Milking Equipment & Teat Health
Understanding the interaction of milking equipment, udder health, and food hygiene has also been a major focus of the NMC. In 1964, the NMC first cooperated with the Milking Machine Manufacturers Council of the Farm Equipment Institute to publish a booklet entitled “Modern Way to Efficient Milking” [7]. The NMC Machine Milking Committee has provided leadership coordinating research and educational efforts aimed at understanding the milking machine’s role in mastitis and teat health. Publications have included “Procedures for Evaluating Vacuum Levels and Air Flow in Milking Systems” [8] along with an educational video on evaluating milking systems, and “Troubleshooting Cleaning Problems in Milking Systems” [9] (Table 1).
The Machine Milking Committee was instrumental in the formation of the “Teat Club International” (TCI), whose primary focus was the health and condition of the teat. This group presented a series of publications at the 2nd International Symposium on Mastitis and Milk Quality [10–13], advancing the science and practice of managing teat health. The group also produced a “Teat Condition Portfolio” on CD-ROM which gives detailed photographs for the detection, diagnosis, and scoring of various teat conditions encountered on dairy farms. This portfolio is currently being revised for release as a second edition.
Mastitis Control Programs
Work at the National Institute for Research in Dairying (NIRD) in England led by Frank Dodd and his team paved the way for what became known as the “Five-point Plan of Mastitis Control” [14, 15]. Dodd was a frequent invited speaker at NMC annual meetings during the formative years of the organization. The five-point plan encompassed strategies for preventing new IMI and eliminating existing IMI and included: 1) maintenance of a properly functioning milking machine, 2) dip teats in an effective post-milking germicide, 3) appropriate therapy of clinical mastitis, 4) treat every mammary quarter of every cow at drying off with an intramammary antibiotic preparation, and 5) cull chronically infected cattle.
The five-point plan was later expanded into the NMC 10-point plan entitled “Recommended Mastitis Control Program” (http://nmconline.org/docs/NMCchecklistNA.pdf ). Additions to the original five-point plan included establishment of goals for udder health, maintenance of a clean, dry, comfortable environment, proper milking procedures, good record-keeping, maintenance of biosecurity, regular monitoring of udder health status, and periodic review of the herd’s mastitis control program.
Fact Sheets, Protocols, Guidelines, Position Papers, & Translated Materials
Numerous fact sheets, protocols, guidelines, and position papers have been compiled over the years. Printed pamphlets on various subjects related to mastitis control and milk quality are shown in Table 1. A number of other resources including NMC Documents and Reports; NMC Protocols, Guidelines and Procedures; Fact Sheets; Resource Articles on Mastitis, Milk Quality, and Milking Management; NMC Columns developed for magazines, newsletters, and newspapers; and Translated Materials can be found on the NMC website (http://www.nmconline.org) in the resources section.
Some notable documents produced by NMC committees and available on the web page under NMC Documents and Reports include Interpreting Bacteriological Culture Results to Diagnose Bovine Intramammary Infection, NMC Position Statement on the Consumption of Raw Milk, NMC Research Committee Note: Methicillin Resistant Staphylococci and Dairy Food, and NMC Research Committee Report: Bovine Mastitis Pathogens and Trends in Resistance to Antimicrobial Drugs.
The Resources Articles on Mastitis section of the website includes approximately 200 articles on management, prevention, control, and treatment; somatic cell counts; mastitis pathogens; bacteriological culturing of mastitis pathogens; machines and milking; housing, cow comfort and welfare; teat health; teat disinfectants; and milk quality and food safety.
Newsletter
The NMC newsletter (“Udder Topics”) was first published in 1964. The bimonthly publication delivers timely news and updates to the NMC membership keeping them engaged in the activities of the organization and abreast of changes in the dairy industry pertinent to mastitis and milk quality. An e-mail-based version was launched in 2002, providing additional announcements, updates, and milk quality tips.
Social Media
As the organization has evolved, methods for communicating information have likewise evolved. Using social media platforms such as Twitter®, Facebook®, and LinkedIn®, NMC has taken on a new persona serving members of the dairy industry in a more real-time fashion providing updates on newsworthy changes in the industry and reporting significant new knowledge live from NMC meetings and other dairy industry events.
Annual and Regional Meetings
Annual Meetings
Another important element of the organization’s mission is its annual and regional meetings. The first annual meeting was held on February 15, 1962 in Chicago, Illinois with an attendance of 100 people. The program included discussions on screening tests for abnormal milk and future plans for the Council. Annual meetings were held in Chicago for most of the organizations first decade then in Louisville, Kentucky. Since 1984 the annual meeting venue has rotated around the United States.
The format of the meeting has evolved. In 1983, the Technology Transfer Session (TTS) was added to the annual meeting program. The TTS has developed into a robust component of the annual meeting program supplementing the main program by providing mastitis and milk quality information through a poster session allowing one-on-one interaction between presenters and attendees. Since 2007, TTS participants have been given the option to have their work considered for oral presentation during the Research and Development Summaries Session held during the annual meeting main program. The goal has been to provide additional opportunities for graduate students, post-doctoral fellows, research associates, and others to be involved in oral presentations at the NMC meeting.
Featured symposia have become an integral part of the annual meeting program following the success of the pre-conference symposium held at the NMC annual meeting in 1997. Some of these symposia have led to position papers such as “Human Health Risks Associated with High Somatic Cell Count Milk” [16].
Regional Meetings
The NMC regional meeting emerged from a half-day program held in Minneapolis, Minnesota in August 1966. The program was part of the NMC’s Board of Directors meeting which took place the day before the International Association of Milk, Food, and Environmental Sanitarians (IAMFES) annual meeting. Following the mid-year Board meeting in October 1967, the NMC Board recommended that the interim meetings should be continued as annual regional meetings. Until 1989 the NMC regional meeting was held in conjunction with the IAMFES annual meeting. Since 1989 the regional meeting has moved around North America most often to an area with a strong dairy industry. Canada has hosted the regional meeting three times. In 1987, the regional meeting was associated with the International Mastitis Symposium held in conjunction with the 23rd World International Veterinary Congress in Montreal, Quebec; in 1999 NMC partnered with the Ontario Large Herd Operators to host a meeting in Waterloo, Ontario; and in 2006 the regional meeting was held in Charlottetown, Prince Edward Island. In 1996, the NMC accepted an invitation to hold its regional meeting in Queretaro, Mexico furthering the organizations international outreach. The first European meeting of the NMC was held in Ghent, Belgium in August 2014 expanding the global outlook of the organization.
Short Courses
In 1990, limited enrolment 3-hour short courses were added to the annual and regional meeting programs. These courses, designed to facilitate in-depth coverage of subjects and encourage open discussion, continue to be a very popular component of both the annual and regional meetings. Short courses have included topics such as: milking equipment evaluation; how to utilize various types of data to implement a milk quality program; in-depth discussions on specific mastitis pathogens and their treatment, control and prevention; treatment of clinical mastitis; controlling mastitis on organic dairy farms; animal handling and well-being; laboratory and on-farm diagnostic procedures; and using new technologies on the farm. In 2008 and 2010 some of the short courses at the regional meeting were offered in Spanish.
Meeting Proceedings
In addition to the educational materials detailed elsewhere, thousands of papers and research summaries have been presented at the annual and regional meetings and published in the meeting proceedings since 1962. It is safe to say that there is likely no other organization in the world that has generated more information related to mastitis control and the production of high quality milk. The NMC materials have been referenced worldwide and have contributed to the increased quality of the global milk supply.
Advisement to Government and Regulatory Agencies
The NMC has a long history of providing information and feedback to government and regulatory agencies in the United States on issues related to milk quality and mastitis control.
Somatic Cell Count Limits in Bulk Tank Milk
The regulatory aspects of abnormal milk and somatic cell count standards have been addressed by the NMC since the 1960s. The NMC has worked closely with the National Conference on Interstate Milk Shipments (NCIMS) advising on National milk quality standards for the United States. In the early 1960s, NMC worked with the Abnormal Milk Committee of NCIMS to develop the Abnormal Milk Program, which was ultimately incorporated in the Grade A Pasteurized Milk Ordinance setting the first U.S. regulatory limit for SCC at 1,500,000 cells/ml. Also, in 1963, the United States Public Health Service adopted NMC’s recommendations for mastitis screening procedures and developed a pamphlet that was distributed throughout many states. In his presidential report in 1965, R.W. Metzger stated “the latest draft of the Public Health Service code devotes considerable attention to abnormal milk and urges the development of mastitis control programs based on National Mastitis Council’s published material”.
Throughout the 1970s and early 1980s NCIMS evaluated several proposals to lower the U.S. regulatory limit for SCC, but it was not until 1986 that a drop from 1,500,000 to 1,000,000 cells/ml was implemented. During this period the NMC Board of Directors did not take official action in supporting a decrease in the U.S. SCC regulatory limit. However, in 1991, NMC submitted a proposal to NCIMS recommending a reduction in the regulatory limit of SCC in bulk tank milk to 500,000 cells/ml. An amended version of the proposal was adopted in setting the SCC limit at 750,000 cells/ml and became effective in 1993. Between 1999 and 2011, NMC submitted five separate proposals to NCIMS recommending the U.S. adopt a SCC limit of 400,000 cells/ml matching the limit in the European Union. Although NCIMS has not approved a change in the SCC limit, in 2011 a proposal to lower the SCC limit to 400,000 cells/ml failed by only a single vote.
Teat Dips
Based on evidence that teat-dipping was an important control measure for preventing intramammary infection and thus mastitis, many teat dipping products were introduced to the market in the late 1960s and early 1970s. The efficacy of products was highly variable, and there was confusion regarding teat dip use, proper classification for teat dips, labeling requirements, and regulation. In response, the NMC Research Committee established a subcommittee on teat dips in 1972, which later became the Teat Dip Committee and subsequently the Teat Health Committee. The original subcommittee was charged with working in cooperation with the U.S. Food and Drug Administration (FDA) to ensure that products sold for teat dipping were safe and effective. In 1974, the NMC Teat Dip Committee submitted a comprehensive review of the literature on teat dips to the FDA Bureau of Veterinary Medicine at the FDA’s request. The FDA also requested information on protocols for determining effectiveness and safety of teat dip products. A series of recommended protocols for teat disinfectant manufacturers to use for determining product efficacy were later developed by NMC and included protocols A) germicidal effectiveness in the lab (excised teats), B) efficacy in challenge trials, and C) efficacy in field trials.
Current NMC recommended protocols are based on revisions of these earlier protocols. In 1990, Hogan et al., published protocols for evaluating efficacy of post-milking teat dips describing three methods including 1) efficacy following experimental exposure of teats to mastitis pathogens, 2) efficacy based on reduction of naturally-occurring IMI, and 3) comparing an experimental post-milking teat dip with a product of known efficacy based on incidence of naturally-occurring IMI [17]. These revised protocols were published in response to a 1989 vote of the NMC membership to amend recommended protocols for teat dip efficacy testing to include technological updates, enhanced scientific merit, and to further ensure standardization of testing procedures [17]. As part of this revision the excised teat model (Protocol A) was eliminated from the “NMC-recommended” methodologies because it was recognized that the ability to reduce bacterial numbers on excised teats in vitro was easier to demonstrate and only measured germicidal activity not efficacy of preventing IMI in a lactating cow. In 2004, Nickerson et al., published revised guidelines which included a fourth protocol for determining the efficacy of a post-milking barrier teat dip based on reduction of new IMI [18]. The recommended protocol for determining efficacy of a pre-milking teat dip based reduction of naturally-occurring new IMI was published in the annual meeting proceedings in 1991 [19].
The NMC has also published guidelines for evaluating teat condition, guidelines for proper storage and handling of teat disinfectants, guidelines for on-farm transfer of teat disinfectants, and guidelines for developing teat sanitizer concentrates. These protocols and guidance documents can be found on the NMC website (http://nmconline.org/protocols.html). Most recently, Schukken et al. published standardized protocols for determining the efficacy of a post-milking teat disinfectant following experimental exposure of teats to mastitis pathogens that builds on previously published NMC protocols to allow for trials that can be done under Good Clinical Practice guidelines [20].
A comprehensive review on post-milking teat disinfection was published by Pankey et al. in the early 1980s [21]. In 1988, an attempt was made to develop a “teat dip fact sheet”, but was never published. At the 1994 annual meeting a request was made that the Research Committee develop a bibliography of teat disinfectants as a means of providing factual information on teat disinfectant efficacy that would be available to members of the dairy community and other interested parties. A subcommittee was appointed and developed a “Summary of Peer-Reviewed Publications on Efficacy of Pre-milking and Post-milking Teat Disinfectants Published Since 1980”. The criteria for inclusion of manuscripts in the bibliography were: 1) only information from peer-reviewed scientific journals published since 1980 was included (The year 1980 was chosen as a comprehensive review was published in 1984 [21]), 2) only information from peer-reviewed scientific journals was used (Peer-reviewed scientific journal was defined as a journal with an editor and an editorial board that reviewed the scientific merit of a manuscript), 3) the study had to follow protocols essentially as described by the NMC, 4) any reference to non-significant results was not included except for natural exposure studies that used a positive control, and 5) products with neither trade name nor manufacturer information mentioned in the publication were not included.
This document also known as the teat dip bibliography was updated annually and published in the annual meeting proceedings from 1996 until 2014 when it was decided that, because of the ability to readily perform an online search of the peer-reviewed literature, continuing to update and publish the document was not necessary. The historical bibliography (last updated in 2009) is available on the NMC website (http://nmconline.org/documents.html) along with instructions on how to perform an online literature search with suggested key words.
Intramammary Infusion Products
In 1970, the NMC Executive Committee appointed a Mastitis Treatment Committee which was charged with counseling the FDA on policy matters regarding mastitis therapy. In 1974, the Infusion Products Committee was appointed to review current FDA guidelines on mastitis infusion products and suggest revisions, if needed. Lactating and dry cow mastitis product guidelines were prepared by the committee and submitted to the FDA the following year. In 1981, the FDA published draft guidelines for the evaluation of antimicrobial drugs for intramammary infusion, outlining the regulatory requirements and procedures for conducting evaluations for drug products being considered for approval. NMC submitted written comments in 1982. In June 1985, guidelines for anti-infective bovine mastitis product development were issued by the FDA. The NMC along with the Animal Health Institute (AHI), American Association of Bovine Practitioners (AABP), and the American Veterinary Medical Association (AVMA) worked with FDA in the early 1990s, following the agency’s request for comments on the 1993 draft guidelines for developing and manufacturing anti-infective bovine mastitis products. The draft guidelines were presented at the 1993 NMC annual meeting. The final guidelines “Target animal safety and drug effectiveness studies for antimicrobial bovine mastitis products (lactating and non-lactating cow products)” were published by the FDA in 1996. The NMC also worked with AHI, National Milk Producers Federation (NMPF), and the FDA Center for Veterinary Medicine to develop guidelines for the uniform labeling of antimicrobial drugs for intramammary infusions which were published by the FDA in 1983.
For many years, a subcommittee of the Residue Avoidance Committee was assembled annually at the NMC annual meeting to advise on the content of the Milk and Dairy Beef Residue Prevention Protocol Dairy Quality Assurance Manual, a component of the Milk and Dairy Beef Quality Assurance Program published by Agri-Education, Inc. Specifically, the NMC representatives on the Technical Review Committee advised on content of the manual with particular attention being paid to the approved therapeutic agents for use lactating and non-lactating dairy cattle and approved methods of detection in raw milk. National Milk Producers Federation now publishes this booklet and a group of NMC members is currently serving as content advisors to NMPF.
Recombinant Bovine Somatotropin
Recombinant bovine somatotropin (rBST) was approved by the FDA for use in dairy cattle in the United States in 1993. Marketed in the United States under the name Posilac®, it is labeled “To increase production of marketable milk in healthy lactating dairy cows”. In March 1993, the FDA Veterinary Medicine Advisory Committee held a meeting to address concerns regarding rBST use in dairy cattle. Bob Harmon, University of Kentucky, testified on behalf of the NMC, summarizing factors influencing mastitis incidence in dairy cattle. In 1988, NMC had submitted a protocol for the evaluation of mastitis in efficacy studies of bovine somatotropin and production drugs in dairy cattle. The protocol was developed to assist FDA during their review of rBST and any impacts it may have on the incidence of mastitis. While rBST is still marketed for use in dairy cattle, public perception about exogenous hormones in milk has driven many milk processors to not accept milk from rBST treated cattle despite lack of evidence of adverse effects on human health [22].
Milk Quality Monitoring
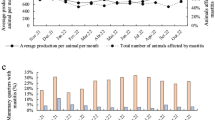
Since 1997, the NMC Milk Quality Monitoring Committee has collaborated with the United States Department of Agriculture (USDA) Animal Plant Health Inspection Service’s Center for Epidemiology and Animal Health as well as USDA’s Agricultural Marketing Service to monitor U.S. milk quality using bulk tank SCC (BTSCC) data. Data is evaluated from four of the Nation’s ten Federal Milk Marketing Orders (Upper Midwest, Central, Mideast, and Southwest orders) which accounts for approximately one-half of the milk marketed in the U.S. During the period of this collaboration (1997-Present) BTSCC has decreased about 35 % from 295,000 cells/ml to 194,000 cells/ml. Although the overall BTSCC has decreased, relative elevations in BTSCC are still observed during the summer months when higher temperatures and humidity increase stress on cows and provide conditions more favorable for bacterial growth in the environments of dairy cows (Fig. 1).
Milk-weighted U.S. Bulk Tank Somatic Cell Count (BTSCC) trends 1996–2014. Although the overall BTSCC has decreased, relative elevations in BTSCC are still observed during the summer months when higher temperatures and humidity increase stress on cows and provide conditions more favorable for bacterial growth in the environments of dairy cows
The Milk Quality Monitoring Committee has also coordinated presentation of reports on BTSCC from different countries around the world at the NMC annual meeting. As part of this process, the committee has developed uniform reporting guidelines and a report template to improve consistency in reporting between different countries. Reports from many countries show similar trends to Fig. 1.
Over the 50-plus years of NMC’s existence, the regulatory thresholds for acceptable levels of SCC in bulk tank milk have become more stringent with progressively lower SCC thresholds being implemented in many regions. The trend towards improved milk quality over time is likely influenced by multiple factors including changing demands of processors and regulatory bodies to meet domestic and export market requirements. As the demand for high quality milk with a low SCC continues to increase, the knowledge synthesized and disseminated by the NMC on mastitis control will have continued importance and relevance in helping dairy farmers meet industry standards for milk quality.
Collaboration at Home and Abroad
Partnerships with Other Organizations
In order to expand upon the multifaceted nature of the organization, NMC has sought partnerships with other organizations throughout its 50-plus year history. Partnerships with the AABP led to joint meetings in 1970, 1982, 1990, 2001, and 2011. The 1990, 2001, and 2011 joint meetings were international symposia on mastitis and milk quality held in Indianapolis, Indiana, Vancouver, British Columbia, Canada, and St. Louis, Missouri, respectively. These latter three co-sponsored symposia brought together international leaders to address current and future issues in mastitis research, mastitis control, and the production of high quality milk.
The NMC partnered with the AVMA in 1976, 1980, 1981, and 1982. Seminars were sponsored by NMC during the Western Veterinary Conference in 1985 and the Eastern States Veterinary Conference in 1987. Other collaborations have included holding the NMC annual meeting in conjunction with the International Dairy Housing Conference (hosted by American Society of Agricultural and Biological Engineers) in 1994, 1998, and 2003 and NMC regional meetings held jointly with the Empire State Mastitis Council (now Empire State Milk Quality Council) in 1993, 1997, and 2002; with the Professional Dairy Producers of Wisconsin in 2001; and with the Mid-Atlantic Consortium in 2009.
International Collaboration
Collaborating with individuals and groups outside of the U.S. has been an important component of NMC’s mission. As early as 1967, speakers from outside the U.S. were invited to present at the annual meeting with the first international speaker coming from Canada followed in 1968 with a speaker from Sweden. The tradition of inviting distinguished internationally recognized mastitis researchers to speak at the annual meeting lasted for many years with speakers during the organization’s formative years coming from New Zealand, England, Denmark, Ireland, Australia, and Israel. Today, speakers from all over the world are featured on the annual meeting program.
The NMC has also fostered relationships with international organizations who share similar goals and objectives. During the 1970s at the urging of the Research Committee, NMC initiated a formal cooperation with the International Dairy Federation (IDF). In 1976, NMC accepted an invitation by the IDF A2 Group of Mastitis Experts to appoint an observer from the U.S. to attend their meetings. NMC representation continues to this day, and has provided valuable consultation between researchers and strengthened ties within the international scientific community.
In 1978, the NMC annual meeting program featured an International Symposium on Machine Milking. Over 500 people from 15 countries attended the symposium, which summed up the current knowledge on machine milking. Forty-six papers presented by speakers from 11 countries were published in the 477 page proceedings. In 1997, the NMC and the IDF A2 Group of Mastitis Experts co-sponsored a special pre-conference symposium at the NMC annual meeting where over 25 % of the registrants came from 15 countries outside the United States. Also, as detailed above, NMC has co-sponsored three international symposia on mastitis and milk quality with AABP.
In 2014, the NMC regional meeting was held in conjunction with the M-teamUGent and the Mastitis Research Workers meeting in Ghent, Belgium. Not only was this the first NMC meeting outside of North America, but the meeting had a record high enrolment of over 650 attendees from approximately 50 countries.
Overall, these joint efforts have expanded the global outreach of the NMC and encompassed the shared missions of multiple organization by communicating knowledge and ideas regarding mastitis and milk quality.
Research
While the NMC does not specifically conduct research, the organization has always been a forum for presentation and sharing of research and synthesis of research findings into recommended best practices. The distillation of the research presented through NMC and other sources has been and continues to be the basis of educational materials made available and promoted by NMC. As discussed above the proceedings of the annual and regional meetings as well as papers from the Technology Transfer Session are published on an annual basis to keep the membership informed of the latest research related to mastitis and milk quality. While a large body of knowledge had been assembled, it was questioned in the 1980s whether a research foundation could be formed to financially support research efforts on mastitis and milk quality.
National Mastitis Research Foundation
In 1983, the NMC Past Presidents Committee and Long Range Planning Committee recommended that NMC study the possibility of establishing a research foundation representing all segments of interest in the industry. After several years of planning, the National Mastitis Research Foundation (NMRF) was formally established awarding its first grant in 2000 during the 39th annual meeting of the NMC. The NMRF is funded by donations from NMC members and supporters and through fund-raising activities held at the NMC annual meetings. Additional grants were funded in 2004 and 2006. In 2007, the focus of the foundation switched from funding research grants to funding the NMC Scholars Program. This program provides travel scholarships for students who have a strong interest in udder health and quality milk production to attend and participate in NMC meetings and activities. The overarching goal of the program is to support the development of future milk quality researchers and specialists.
Looking to the Future
The NMC was founded in 1961 to “to promote, aid or engage in educational activities and scientific research in the field of mastitis control, including the coordination and focusing of all forces of organized agriculture in the United States to combat, through every practical device, the mastitis threat to the Nation’s health and food safety”. What started as a National organization focused on combatting a single disease in the United States, has grown into a global effort to coordinate and synthesize available resources to help the dairy industry control mastitis and improve milk quality through research, exchange of information, and education.
The net effect has been a reduction in the incidence of mastitis and improvement in milk quality. Hence, the vision of NMC’s founders has largely been realized. That said, the world is an ever changing place. With the globalization of the dairy industry and evolution of the way cows are managed and interact with their environment, new challenges with regard to mastitis and milk quality will always exist. Technological advances in the research tools used to understand the host-pathogen-environmental interaction are revealing new information about mastitis pathogen ecology and host susceptibility which will potentially impact how we control, treat, and prevent mastitis in the future.
Public perceptions about animal well-being, therapeutic drug-use, and antimicrobial resistance are ever present and will potentially impact how we treat and prevent mastitis in the future. The continued success of the NMC will depend, as always, on the engagement of its membership to synthesize the next generation of research, design best practices for the dairy industry, and deliver a scientifically sound and reasoned message about mastitis and milk quality to not only its membership, but consumers of dairy products.
Abbreviations
- AABP:
-
American Association of Bovine Practitioners
- AHI:
-
Animal Health Institute
- AVMA:
-
American Veterinary Medical Association
- BTSCC:
-
Bulk Tank Somatic Cell Count
- FDA:
-
Food and Drug Administration
- IAMFES:
-
International Association of Milk, Food, and Environmental Sanitarians
- IDF:
-
International Dairy Federation
- IMI:
-
Intramammary Infection
- NCIMS:
-
National Conference on Interstate Milk Shipments
- NIRD:
-
National Institute for Research in Dairying
- NMC:
-
National Mastitis Council
- NMPF:
-
National Milk Producers Federation
- NMRF:
-
National Mastitis Research Foundation
- rBST:
-
Recombinant Bovine Somatotropin
- SCC:
-
Somatic Cell Count
- TCI:
-
Teat Club International
- TTS:
-
Technology Transfer Session
- USDA:
-
United States Department of Agriculture
References
Halasa T, Hujips K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: A review. Vet Q. 2007;29:18–31.
Pol M, Ruegg PL. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin. J Dairy Sci. 2007;90:249–61.
USDA. Dairy 2007, Part III: Reference of dairy cattle health and management practices in the United States, 2007. Fort Collins, Colorado: USDA-APHIS-VS, CEAH. #N482.0908; 136.
Microbiological procedures for use in the diagnosis of bovine udder infection and determination of milk quality. 4th ed. Verona, Wisconsin: National Mastitis Council, Inc.; 2004.
Barnes-Pallesen FD, Blackmer P, Britten A, Bushnell RB, Van Damme DM, Welcome F. Laboratory and field handbook on bovine mastitis. Arlington: National Mastitis Council, Inc.; 1987.
Hogan JS, Gonzalez RN, Harmon RJ, Nickerson SC, Oliver SP, Pankey JW, et al. Laboratory handbook on bovine mastitis. Madison: National Mastitis Council, Inc.; 1999.
The modern way to efficient milking. Chicago, Illinois: Milking Machine Manufacturers Council of the Farm Equipment Institute; 1964.
Procedures for evaluating vacuum levels and airflow in milking systems. Verona, Wisconsin: National Mastitis Council, Inc.; 2012.
Troubleshooting cleaning problems in milking systems. Madison, Wisconsin: National Mastitis Council, Inc.; 2004.
Mein GA, Neijenhuis F, Morgan WF, Reinemann DJ, Hillerton JE, Baines JR, et al. Evaluation of bovine teat condition in commercial dairy herds: 1. Non-infectious factors. Vancouver: Proceedings of the 2nd International Symposium on Mastitis and Milk Quality; 2001. p. 347–51.
Hillerton JE, Morgan WF, Farnsworth R, Neijenhuis F, Baines RJ, Mein GA, et al. Evaluation of bovine teat condition in commercial dairy herds: 2. Infectious factors and infections. Vancouver: Proceedings of the 2nd International Symposium on Mastitis and Milk Quality; 2001. p. 352–6.
Reinemann DJ, Rasmussen MD, LeMire S, Neijenhuis F, Mein GA, Hillerton JE, et al. Evaluation of bovine teat condition in commercial dairy herds: 3. Getting the numbers right. Vancouver: Proceedings of the 2nd International Symposium on Mastitis and Milk Quality; 2001. p. 357–61.
Neijenhuis F, Mein GA, Britt JS, Reinemann DJ, Hillerton JE, Farnsworth R, et al. Evaluation of bovine teat condition in commercial dairy herds: 4. Relationship between teat-end callosity or hyperkeratosis and mastitis. Vancouver: Proceedings of the 2nd International Symposium on Mastitis and Milk Quality; 2001. p. 362–6.
Dodd FH, Westgarth DR, Neave FK, Kingwill RG. Mastitis – the strategy of control. J Dairy Sci. 1969;52:689–95.
Neave FK, Dodd FH, Kingwell RG, Westgarth DR. Control of mastitis in the dairy herd by hygiene and management. J Dairy Sci. 1969;52:696–707.
Human Health Risks Associated with High Somatic Cell Count Milk. Madison, Wisconsin: National Mastitis Council, Inc.; 2005. http://nmconline.org/docs/SCChealthrisks.pdf
Hogan JS, Galton DM, Harmon RJ, Nickerson SC, Oliver SP, Pankey JW. Protocols for evaluating efficacy of postmilking teat dips. J Dairy Sci. 1990;73:2580–5.
Nickerson SC, Saxon A, Fox LK, Hemling T, Hogan JS, Morelli J, Oliver SP, Owens WE, Pawlak M, Petersson L. Recommended protocols for evaluating efficacy of postmilking teat germicides. Proceedings of the 43rd Annual Meeting of the National Mastitis Council. 2004:379–99.
Hogan JS, Eberhart RJ, Galton DM, Harmon RJ, Nickerson SC, Oliver SP, Pankey JW. Protocol for determining efficacy of pre-milking teat dips. Proceedings of the 30th Annual Meeting of the National Mastitis Council. Reno, Nevada. 1991:157–9
Schukken YH, Rauch BJ, Morelli J. Defining standardized protocols for determining the efficacy of a postmilking teat disinfectant following experimental exposure of teats to mastitis pathogens. J Dairy Sci. 2013;99:2694–704.
Pankey JW, Eberhart RJ, Cuming AL, Daggett RD, Farnsworth RJ, McDuff CK. Update on post-milking teat antisepsis. J Dairy Sci. 1984;67:1336–53.
Collier RJ, Bauman DE. Update on human health concerns of recombinant bovine somatotropin use in dairy cows. J Anim Sci. 2014;92:1800–7.
Acknowledgments
Much of the information presented in this manuscript was previously published in a booklet distributed by NMC to its membership in 2012 commemorating 50 years of the National Mastitis Council 1961–2011, and in documents available at http://www.nmconline.org.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Middleton, J.R., Saeman, A., Fox, L.K. et al. The National Mastitis Council: A Global Organization for Mastitis Control and Milk Quality, 50 Years and Beyond. J Mammary Gland Biol Neoplasia 19, 241–251 (2014). https://doi.org/10.1007/s10911-014-9328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-014-9328-6