Abstract
In the multimodal communication of Schizocosa ocreata wolf spiders, males respond to chemical signals from females with visual and substrate-borne vibratory signals for courtship. We examined the effect of wet vs. dry leaves on transmission of male courtship signals, responses of male spiders to female chemical cues, responses of courting males to bird calls indicating predator presence, and mating success. Laser Doppler vibrometry showed that spider stridulation and percussive signals maintain higher amplitude over distance on dry leaves than on wet leaves. Male response to chemical cues (courtship latency and rate) declined after leaves with female silk became wet. In response to predatory bird calls (Blue Jays) transmitted through leaf surfaces, courting male spiders on dry leaves responded with anti-predator “freeze” behaviors more often and with longer duration than those on wet leaves, and with longer latency to return to courtship on wet leaves. Laser Doppler vibrometry confirmed that bird calls on dry leaves had significantly greater average amplitude and different spectral profiles than those on wet leaves. Males courted females on wet and dry leaves with equal frequency, but subsequent mating success was significantly greater on dry leaf litter. Interestingly, visual signals increased on wet leaves, suggesting compensatory behavior in response to moisture. Given a predicted change in precipitation in parts of North America because of global climate change, these results suggest potential for impact on behavior of invertebrates at the microhabitat level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environment can play an important role in animal signal transmission as well as detection, but its effects can vary with animal size and environmental conditions. While the physical structure of forests, fields and urban environments have been shown to influence signals of birds, anurans and mammals (Hedwig et al. 2018; Halfwerk et al. 2019; Charlton et al. 2019; Velásquez et al. 2018; Marín-Gómez et al. 2020), the leaf litter microenvironment of deciduous forests has also been shown to impact communication of small invertebrates (Hill 2008; Hill and Wessel 2016; Yack 2016; Hill et al. 2019; Cividini and Montesanto 2020; Stritih-Peljhan and Virant-Doberlet 2021). For example, recent research revealed that specific aspects of the forest floor leaf litter environment (leaf structure, soil surface, moisture) may play a role in transmission and detection of vibratory/seismic courtship signaling of spiders (Elias et al. 2004; Hebets et al. 2008; Elias et al 2010; Gordon and Uetz 2011; Uetz et al. 2013; Elias and Mason 2014; Rosenthal et al. 2019; Sun et al. 2021). Litter environments can also affect chemical communication of many animal species (amphibians: Byrne and Keogh 2007; reptiles: Howey and Snyder 2020; insects: Salmon et al. 2019; spiders: Persons et al. 2001; Wilder et al. 2005).
Detection of other vibratory/acoustic information, e.g., airborne predator cues, may also be affected by leaf litter characteristics. Previous research has observed changes in wolf spider courtship behavior (e.g., “freeze” or sudden cessation of courtship) in response to airborne predatory bird calls, which spiders perceive as substratum-conducted vibrations transduced through a leaf surface (Lohrey et al. 2009; Gordon and Uetz 2012). Importantly, the mating season of many North American wolf spider species occurs in spring, when there are frequent rains, and when insectivorous birds are selectively provisioning nestlings with spiders (Grundel and Dahlsten 1991; Arnold et al. 2007). Consequently, increased seasonal rainfall owing to climate change (Meehl et al. 2005; Papalexiou and Montanari 2019) may impact the leaf litter microhabitat disproportionately by changing the transmission properties of the leaf substrates used for communication by spiders and other invertebrates.
The overall goal of this research was to assess the impact of environmental variation, specifically wet vs. dry leaves, on courtship and mating of the brush-legged wolf spider Schizocosa ocreata. We examined four aspects of the influence of wet vs. dry leaves in four separate experimental studies: (I) transmission of vibratory signals from male courtship; (II) detection of female chemical cues; (III) detection of airborne acoustic predator stimuli (bird calls) and subsequent anti-predator responses of wolf spiders; and (IV) effects of leaf moisture on mating success.
General Methods
Juvenile S. ocreata wolf spiders were collected from the Cincinnati Nature Center (39˚ 7′31.15″N; 84˚ 15′4.29″W) during Spring and Fall of 2018 and Spring 2019 and raised to maturity in the lab. Spiders were isolated in small round opaque plastic deli containers (9 cm diam x 5 cm ht), kept under controlled lab conditions (13/11 h L:D; stable temperature and RH%), and raised to maturity. Spiders had access to water ad libitum and were fed twice weekly with two appropriately sized crickets. Molts of each spider were tracked, and their penultimate molt and maturity dates were recorded, so that males and females could be matched for time since maturity.
In all the experimental studies below, we compared dry vs. wet leaves. We collected recently fallen leaves of Sycamore (Platanus occidentalis) from field study sites at the Cincinnati Nature Center and placed them between pages of a telephone book to dry and flatten them. Once fully dried and flattened, half of leaves were chosen at random for the “wet” treatment and soaked in water for one hour before use in experimental studies to standardize moisture level. Based on our observation and handling of leaves, we felt this treatment was sufficient to match the condition of leaves after a soaking rain in nature.
Animal Welfare/Ethical Note
We adhered to the ASAB/ABS Guidelines for the Use of Animals in Research (https://doi.org/https://doi.org/10.1016/j.anbehav.2019.11.002). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Effect of Leaf Moisture on Transmission of Vibration through Wet and Dry Leaves
Playback signals We used playback of pre-recorded spider vibration signals via piezoelectric elements to test the effects of moisture on signal transmission through leaves. We used a Polytec PDV-100 laser Doppler vibrometer (LDV) to record and measure playback stimuli (from pre-recorded vibratory signals of courting male S. ocreata). Twenty-five males were selected at random from the lab population and recorded in advance to match the amplitude of the single vibratory courtship stimulus to the population average. The recording of vibratory signals used in playback was one from previous studies representing the mean value for body size and vibratory signal parameters (Roberts et al. 2006).
Playback apparatus and calibration Vibratory playback was done with piezoelectric elements (APC International) attached to selected specimens of comparably sized leaves of sycamore (Platanus occidentalis). We collected all P. occidentalis samples from leaf litter in the same location and at the same time. We deliberately selected 20 individual leaves (10 each treatment) that were complete (no damage or herbivory) and at least 10 cm in length from the base.
The vibration signal recordings were played from an Apple iPod (4th edition) connected to an amplifier (Pyle Mini 2 × 40W Stereo Power Amplifier). The output of the amplifier was connected to the piezoelectric element via speaker wire. The piezoelectric element was affixed to the leaf with tape and then signals were calibrated. Vibratory recordings and playback were calibrated to match that of the average percussive amplitude in our sample population of S. ocreata using the Polytec PDV-100 laser Doppler vibrometer (125 mm/s/V sensitivity, 100 mm/s max, 96 mm standoff distance). The laser Doppler vibrometer (LDV) was connected to an external sound card (Roland QuadCapture) and calibrated with a 1 kHz tone (50% FS). We analyzed the vibratory signals from the sample of 25 courting males with SpectraPLUS-SC acoustic software (24 kHz sampling rate, 2048 FFT, Hanning window) to calculate the root mean square (RMS) amplitude of signals. Once this value was obtained (mean RMS: 4.565 mm/s), the previously described arena was used to play the vibratory courtship signal through a leaf, and the LDV was used to calibrate the percussive amplitude. The amplifier was then adjusted until it matched the population mean as measured by the LDV. We measured signal amplitude at the source and at 1 cm increments up to 10 cm away from the playback source on both 10 wet and 10 dry leaves for a total of 220 sample measurements (n = 110 each treatment, N = 220 overall).
Effect of Leaf Moisture on Chemical Cues
Male Schizocosa wolf spiders respond with courtship behavior when exposed to chemotactile cues from female silk (Tietjen and Rovner 1982; Roberts and Uetz 2004a, b, 2005). In this study, we tested whether female silk-borne chemotactile cues were impacted or deactivated by leaf moisture, as has been suggested by others (Tietjen and Rovner 1982; Wilder et al. 2005), by measuring responses of males to female cues on wet or dry leaves. We also tested whether the order of silk deposition (e.g., before or after rainfall) affected detection by males.
We chose mature, unmated female spiders and assigned them randomly to one of five treatment groups (N = 15 each group) to deposit silk cues. We chose mature, unmated male spiders and assigned them randomly to all treatment and control groups (N = 15 each group). Females and males used matured around the same date. We had three treatment groups and two control groups: dry leaf w/female silk, wet leaf w/female silk, as well as dry and wet leaves without chemical cues from female silk (controls) plus an additional treatment to test the importance of the timing of silk deposition relative to wet conditions (e.g., from rainfall), with dry-then-wet leaves—i.e., initially dry when females deposited cues on them, then soaked after silk deposition. The trial arenas were clear, cylindrical shaped containers, 7 cm in diameter and 7.5 cm in width, placed on top of a flattened sycamore leaf (wet or dry; dry-then-wet). Five trials (three treatments and two controls) were prepared during the same set, with spiders assigned at random to sets and trials. Each set of trials was prepared for 3 h, when females were allowed to lay down silk. In between sets of trials, leaves and containers were cleaned with ethanol.
To determine how leaf moisture affects male courtship behavior, male spiders’ visual signals in response to the presence or absence of cues in each treatment/control group were observed. Males were allowed to move about freely on leaves inside of arenas while behaviors were recorded with a Sony digital camcorder for 10 min. Videos were scored for the number of three visual signals: leg waves, body bounces, and leg tapping in the 10-min trial (see Stratton and Uetz 1986; Meyer and Uetz 2019 for description).
Leaf Moisture and Responses to Airborne Predator Cues
In order to understand how the environment can affect information indicating danger, we explored whether the moisture content of leaves affected detection of bird calls by courting male spiders. We addressed this by examining anti-predator responses of male S. ocreata courting on wet vs. dry leaves exposed to playback of a Blue Jay (Cyanocitta cristata) call.
Because previous studies determined that spiders perceive airborne bird calls as vibration in leaf substrates (Lohrey et al. 2009; Gordon and Uetz 2012), we measured how leaf moisture affects transmission of airborne predator stimuli through wet vs. dry leaves. Substratum-borne vibrations from airborne bird calls were recorded in the experimental arena on wet and dry leaf surfaces using a laser Doppler vibrometer (as described for experiments above). The laser recordings were made from below the arena through a 1 cm hole in the base; the laser was aimed at a 2 mm mylar “dot” attached to the underside surface of the leaf. To avoid pseudoreplication, we made four different replicate recordings (four separate leaves) of wet and dry leaves with each of four bird call exemplars.
Male and female S. ocreata (N = 60 each) were randomly selected from the sample population of spiders in the lab. A circular (17.5 cm diam.) plastic arena was used for trials with a speaker placed 15 cm above it. Sycamore leaves were collected from field sites in Ohio. Half the leaves were soaked in water for a minimum of 1 h (as described above) before trials to serve as wet leaf litter, while the other half remained dry. Before each trial, females laid silk on leaf litter for at least 1 h. The leaf litter was then transferred into a circular arena, with a male placed inside.
Trials began with the onset of male courtship, which was recorded with a camcorder. After one minute of courtship, acoustic cues (a 10 s recording of a Blue Jay call, Peterson Field Guide, A Field Guide to Bird Songs; ISBN- 10: 0618225943) were played from a speaker mounted 15 cm above the arena (at a level similar to field conditions (82 dB), as in Lohrey et al 2009), and spider responses were observed. Male behavior recordings were analyzed later for the occurrence of anti-predator “freeze” responses to calls (cessation of courtship/immobility), as well as number of taps, waves, bounces, and latency to resume courtship before and after exposure to the bird call. Recordings of bird calls were analyzed with SpectraPlus® software (as described above) to determine average RMS (velocity in mm/sec) amplitude on wet vs. dry leaves, then amplitude was analyzed in a 2 × 4 factorial design. Spectral properties of the bird calls were analyzed with the Raven software package, which generated a sound spectrogram (a.k.a. “sonogram”) and allowed us to compare the same exemplar recordings under wet and dry conditions.
Effect of Leaf Moisture on Mating Success
We randomly paired selected male and female S. ocreata (N = 71 pairs) in plastic arenas on wet and dry leaf litter substrates to assess the effect of leaf moisture in live mating encounters. The circular mating arenas were 22 cm in diameter constructed from plastic flowerpots; each contained either wet or dry litter placed upon a 2 cm soil base. Prior to the trial, unmated females were placed in the arenas overnight to lay silk to initiate male courtship. The females were removed, allowed to hydrate, and then placed back into the arenas. Unmated males were placed on the opposite side of the arena and allowed to court for one hour. We used 71 pairs of spiders selected randomly from the lab population in each treatment (36 dry, 35 wet); spiders were used only once. The trials were videotaped from above for one hour or stopped if/when copulation or cannibalism occurred.
Statistical Analyses
Statistical analyses were performed using JMP® (JMP Statistical Discovery LLC, Cary, NC). Different parts of this study generated both categorical (e.g., mate/not, occurrence of behaviors) and continuous variables (e.g., rates and amplitudes) and were analyzed in several ways. Categorical data were analyzed with chi-squared tests. Continuous variable data were first tested for fit to a normal distribution and were subsequently analyzed with Generalized Linear Model (GLM) analyses using a normal or Poisson distribution as the basis, or two sample tests, or their non-parametric equivalents. Latency to court or to mate in wet-dry treatments was analyzed using a Log-rank survival test of cumulative proportion of responses over time.
Results – I: Vibration Transmission
Data on RMS amplitude (mm/s) of stridulation signals fit a normal distribution (Shapiro-Wilk W = 0.992; P = 0.317) while amplitude of percussion did not (Shapiro-Wilk W = 0.824; P < 0.0001). Consequently, we used a GLM with a normal distribution basis for stridulation, while for percussion we used a GLM with a Poisson distribution as its basis. Both analyses showed that signal amplitude varied significantly with distance, treatment (dry vs. wet leaves) and the distance x treatment interaction (Table 1).
As expected, attenuation of male vibratory signals increased with distance on both wet and dry leaves, but the louder spider signals (percussion) were still measurable at up to 10 cm away on both wet and dry leaves. Treatment (dry vs. wet leaves) significantly affected playback signal amplitude for both stridulation and percussion signals across the active space of the leaf (Fig. 1a, b).
Stridulation amplitude was greater on dry leaves up to 5 cm from the source but had much overlap between treatments beyond that distance (Fig. 1a). Percussive elements of the spider signal attenuated significantly as distance from the source increased on both wet and dry leaves, but moisture level resulted in significantly greater attenuation (Fig. 1b). Spectral analyses showed leaf moisture filters the frequency of recorded signals, as dry leaves transmit male spider courtship signals at greater amplitudes across almost all frequencies than wet leaves (Fig. 2), and again this effect is more pronounced for percussive elements.
Results – II: Chemotactile Cues
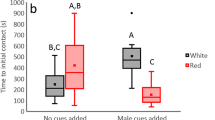
Results show significant differences in courtship latency and number of visual signals across treatment and control groups. Data on courtship response latency show significant differences among treatments (Log-rank analysis: X2 = 16.554; P = 0.0024); male spiders display visual signals faster to chemical cues on dry leaves than all other experimental treatments (Fig. 3).
Rates of individual behaviors (waves, bounces, taps, sums of displays and mean displays/min) did not fit a normal distribution (Shapiro-Wilk W values: 0.489—0.679; P < 0.0001) and were subjected to GLM analysis with a Poisson distribution as its base. GLM analysis showed that the rates of individual visual signals (displays/minute) as well as sum and mean of total displays varied significantly across treatments (Table 2; Fig. 4).
Rates of male behaviors on dry and wet leaves with silk were greater than both the control groups and dry-then-wet leaves (Newman-Keuls test, p < 0.05). Male spiders exhibited a greater total number of signals on dry leaves w/silk vs. wet leaves w/silk, although post hoc tests show overlap (Fig. 4). This shows that even when a female spider deposits silk chemical cues on a wet surface, male spiders can detect them. Results also show far fewer male courtship behaviors on leaves that received female silk chemical cues prior to being inundated with moisture (dry-then-wet leaves). This indicates cues may become less potent when leaves become wet after silk is deposited, suggesting a role for rainfall and/or morning dew in deactivating chemical communication (Tietjen and Rovner 1982; Wilder et al. 2005).
Results – III: Predator Cues
Prior to the bird call, a log-rank survival analysis of cumulative proportion of responses over time (Fig. 5a) showed no differences in latency to begin courtship (Log-rank X12 = 0.126; P = 0.724). However, upon hearing the bird call, spiders on dry leaves responded to bird calls more often and with longer duration than those on wet leaves. Freeze behavior was not independent of leaf moisture treatment (X12 = 9.609; P = 0.019); spiders were more likely to freeze upon hearing bird calls when on dry leaves (71.43%) than wet leaves (29.63%). We also used a GLM analysis with an underlying binomial distribution basis to compare effects of leaf treatment (wet, dry) and bird call exemplar on frequency of occurrence of freeze behavior (Table 3). While we found only marginal significance for the whole model (X52 = 10.569; P = 0.060), leaf treatment showed significance (X12 = 10.049; P < 0.0015), while bird call exemplar (X22 = 0.572; P = 0.751) and the bird call x treatment interaction (X22 = 0.106; P = 0.948) did not.
Duration of “freeze” behavior did not fit a normal distribution (Shapiro-Wilk W = 0.638; P < 0.0001) and was subjected to non-parametric analysis and GLM. Freeze duration differed significantly between treatments in a two-sample test Wilcoxon test (X12 = 12.801; P = 0.0003) (Fig. 6). GLM analysis with an underlying Poisson distribution (Table 4) showed significance for the whole model, leaf treatment and bird call exemplar, but not the bird call x treatment interaction. Log-rank survival analysis of latency to resume courtship post-bird call, analyzed as cumulative proportion of responses over time (Fig. 5b) showed significant differences between wet-dry treatments (Log-rank X2 = 14.487; P = 0.0001).
GLM Analysis of vibration amplitude data recorded with the LDV on both wet and dry leaves did not show a significant effect for the whole model (Table 5), bird exemplar or treatment x exemplar interaction, but did show significance arising from leaf moisture treatment. The significant difference in vibration level with leaf moisture (Fig. 7) likely resulted from differences in the power spectra of sound/vibration on dry vs. wet leaves. Spectral analyses of bird calls conducted as vibration on wet vs. dry leaves varied across frequencies. Bird calls on dry leaves (Fig. 8a) had higher amplitude in a range between 1500 Hz—7000 Hz, but bird calls on wet leaves (Fig. 8b) had reduced amplitude in frequencies below 2000 Hz and above 4000 Hz.
Results – IV: Mating Success
Mating success was not independent of treatment (X 2 = 4.999; P = 0.0254) and was lower in wet leaf treatments (37.5%) than dry leaves (62.5%), despite no significant difference in occurrence of male courtship (nearly 100% on both wet and dry leaves: X 2 = 0.009; P = 0.925) or latency to court (Log-rank test: X 2 = 0.0059; P = 0.9389). However, we found that males increased their use of visual signals when courting on wet environments. Rates of male “wave and arch” displays did not fit a normal distribution (Shapiro-Wilk Test, W = 0.7339; P < 0.0001) and were subsequently analyzed with a non-parametric Wilcoxon test. Results show that successfully mated males displayed “wave and arch” signals (Fig. 9) significantly more often on wet leaves than dry leaves (Wilcoxon X1 2 = 7.563; P = 0.0062), as was seen in Gordon and Uetz (2011) when spiders were on substrates that did not transmit vibratory signals (stone, soil, wood). This result was also apparent in the chemical cues study (Section II), where in contrast to other male signals (which are greater on dry leaves than wet leaves), leg waves were performed at a higher rate on wet leaves (see Table 1).
Discussion
Overall, our results suggest that along with other previously studied environmental characteristics of leaf litter such as structure and substrate type (Hebets et al. 2008; Elias et al. 2010; Gordon and Uetz 2011; Uetz et al. 2013; Rosenthal et al. 2019; Sun et al. 2021), moisture conditions can influence communication in wolf spiders as well. Similar results have been seen in other animal taxa, demonstrating the importance of micro-environment in vibrational communication (Hill 2009; Yack 2016; Stritih-Peljhan and Virant-Doberlet 2021). The efficacy of spider vibratory signaling, as well as detection of chemical cues in female silk and substrate vibration from airborne cues indicating potential predators, are all affected by increased moisture. This is potentially important during periods of precipitation throughout the mating season, when increased substrate moisture might impact signal transmission and overall mating success of males, as well as their vulnerability to predation (Arnold et al. 2007). In addition to impacting these potential fitness-related aspects of communication, leaf litter moisture also has a significant negative impact on actual mating success. It is notable that while spiders increase the number of specific visual displays (wave and arch) when on wet leaves, this does not appear to increase mating success.
Our results support previous research on the role of environmental influence on transmission and reception of spider signals (Lohrey et al 2009; Gordon and Uetz 2011) and add a new finding—that differences in leaf moisture affect the response of spiders to stimuli indicating predators nearby. Detection of predators is essential for animal survival, and this study demonstrates that spiders, like other animals, can detect airborne predator sounds though leaf substrates (Castellanos and Barbosa 2006; Hill 2009; Sitvarin et al. 2016; Lohrey et al. 2009; Gordon and Uetz 2012; Virant-Doberlet et al 2019). However, results also suggest leaf moisture affects spider detection of specific bird calls, and is further supported by vibration recordings, which illustrate the dampening of sound on wet leaves in comparison to dry leaves. Given a predicted change in precipitation in parts of North America as a consequence of global climate change (Meehl et al 2005; Kharin et al. 2013; Papalexiou and Montanari 2019), these results take on new relevance. Further studies need be conducted to see if spiders from wetter or drier climates are more responsive to sounds and better able to cope with leaf moisture, which could provide additional insight to what traits might be selected for under conditions of greater precipitation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from Mendeley Data, V1, https://doi.org/10.17632/xzyxcccp5h.1.
References
Arnold KE, Ramsay SL, Donaldson C, Adam A (2007) Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring. Proc Roy Soc b: Biol Sci 274:2563–2569
Byrne PG, Keogh JS (2007) Terrestrial toadlets use chemosignals to recognize conspecifics, locate mates and strategically adjust calling behaviour. Anim Behav 74:1155–1162
Castellanos I, Barbosa P (2006) Evaluation of predation risk by a caterpillar using substrate-borne vibrations. Anim Behav 72:461–469
Charlton BD, Owen MA, Swaisgood RR (2019) Coevolution of vocal signal characteristics and hearing sensitivity in forest mammals. Nature Comm 10:1–7
Cividini S, Montesanto G (2020) Biotremology in arthropods. Learn Behav 48:281–300. https://doi.org/10.3758/s13420-020-00428-3
Elias DO, Mason AC (2014) The role of wave and substrate heterogeneity in vibratory communication: Practical issues in studying the effect of vibratory environments in communication. In: Cocroft R, Gogala M, Hill P, Wessel A (eds) Studying vibrational communication. Animal signals and communication, vol 3. Springer, Heidelberg. https://doi.org/10.1007/978-3-662-43607-3_12
Elias DO, Mason AC, Hoy RR (2004) The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: Salticidae). J Exp Biol 207:4105–4110
Elias DO, Mason AC, Hebets EA (2010) A signal-substrate match in the substrate-borne component of a multimodal courtship display. Curr Zool 56:370–378
Gordon SD, Uetz GW (2011) Multimodal communication of wolf spiders on different substrates: evidence for behavioural plasticity. Anim Behav 81:367–375
Gordon SD, Uetz GW (2012) Environmental interference: impact of acoustic noise on seismic communication and mating success. Behav Ecol 23:707–714
Grundel R, Dahlsten DL (1991) The feeding ecology of mountain chickadees (Parus gambeli)—patterns of arthropod prey delivery to nestling birds. Can J Zool 69:1793–1804
Halfwerk W, Blaas M, Kramer L, Hijner N, Trillo PA, Bernal XE, Page RA, Goutte S, Ryan MJ, Ellers J (2019) Adaptive changes in sexual signalling in response to urbanization. Nature Ecol Evol 3:374–380
Hebets EA, Elias DO, Mason AC, Miller GL, Stratton GE (2008) Substrate dependent signaling success in the wolf spider Schizocosa retrorsa. Anim Behav 75:605–615
Hedwig D, DeBellis M, Wrege PH (2018) Not so far: attenuation of low-frequency vocalizations in a rainforest environment suggests limited acoustic mediation of social interaction in African forest elephants. Behav Ecol Sociobiol 72:1–11
Hill PS (2008) Vibrational communication in animals. Harvard University Press
Hill PS (2009) How do animals use substrate-borne vibrations as an information source? Naturwissenschaften 96:1355–1371
Hill PS, Wessel A (2016) Biotremology. Curr Biol 26:R187–R191
Hill PS, Lakes-Harlan R, Mazzoni V, Narins PM, Virant-Doberlet M, Wessel A (eds) (2019) Biotremology: Studying vibrational behavior (No. 6). Springer, Heidelberg
Howey CA, Snyder EM (2020) Substrate type affects scent-trailing behavior of newborn timber rattlesnakes (Crotalus horridus). Copeia 108:772–777
Kharin VV, Zwiers FW, Zhang X, Wehner M (2013) Changes in temperature and precipitation extremes in the CMIP5 ensemble. Clim Change 119:345–357. https://doi.org/10.1007/s10584-013-0705-8
Lohrey AK, Clark DL, Gordon SD, Uetz GW (2009) Antipredator responses of wolf spiders (Araneae: Lycosidae) to sensory cues representing an avian predator. Anim Behav 77:813–821
Marín-Gómez OH, Dáttilo W, Sosa-López JR, Santiago-Alarcon D, MacGregor-Fors I (2020) Where has the city choir gone? Loss of the temporal structure of bird dawn choruses in urban areas. Landsc Urban Plan 194:103665
Meehl GA, Arblaster JM, Tebaldi C (2005) Understanding future patterns of increased precipitation intensity in climate model simulations. Geophys Res Lett 32:18
Meyer TB, Uetz GW (2019) Complex male mate choice in the brush-legged wolf spider. Behav Ecol 30:27–38. https://doi.org/10.1093/beheco/ary172
Papalexiou SM, Montanari A (2019) Global and regional increase of precipitation extremes under global warming. Water Resour Res 55:4901–4914
Persons MH, Walker SE, Rypstra AL, Marshall SD (2001) Wolf spider predator avoidance tactics and survival in the presence of diet-associated predator cues (Araneae: Lycosidae). Anim Behav 61:43–51
Roberts JA, Uetz GW (2004a) Chemical signaling in a wolf spider: A test of ethospecies discrimination. J Chem Ecol 30:1271–1284
Roberts JA, Uetz GW (2004b) Species-specificity of chemical signals: Silk source affects discrimination in a wolf spider (Araneae: Lycosidae). J Insect Behav 17:477–491
Roberts JA, Uetz GW (2005) Information content of female chemical signals in the wolf spider, Schizocosa ocreata: male discrimination of reproductive state and receptivity. Anim Behav 70:217–223
Roberts JA, Taylor PW, Uetz GW (2006) Consequences of complex signaling: predator detection of multimodal cues. Behav Ecol 18:236–240
Rosenthal MF, Hebets EA, Kessler B, McGinley R, Elias DO (2019) The effects of microhabitat specialization on mating communication in a wolf spider. Behav Ecol 30:1398–1405
Salmon S, Rebuffat S, Prado S, Sablier M, d’Haese C, Sun JS, Ponge JF (2019) Chemical communication in springtails: a review of facts and perspectives. Biol Fertil Soils 55:425–438
Sitvarin MI, Gordon SD, Uetz GW, Rypstra AL (2016) The wolf spider Pardosa milvina detects predator threat level using only vibratory cues. Behaviour 153:159–173. https://doi.org/10.1163/1568539X-00003332
Stratton GE, Uetz GW (1986) The inheritance of courtship behavior and its role as a reproductive isolating mechanism in two species of Schizocosa wolf spiders (Araneae:Lycosidae). Evolution 40:129–141
Stritih-Peljhan N, Virant-Doberlet M (2021) Vibrational signalling, an underappreciated mode in cricket communication. Sci Natur 108:1–12
Sun Y, Brandt E, Elias DO, Rosenthal M, Kamath A (2021) Jumping spiders (Habronattus clypeatus) exhibit substrate preferences that partially maximize vibration transmission efficiency. J Insect Behav 34:151–161
Tietjen WJ, Rovner JS (1982) Chemical communication in lycosids and other spiders. In: Witt PN, Rovner JS (eds) Spider communication. Mechanisms and ecological significance. Princeton, Princeton University Press, pp 249–279
Uetz GW, Roberts JA, Clark DL, Gibson JS, Gordon SD (2013) Multimodal signals increase active space of communication by wolf spiders in a complex litter environment. Behav Ecol Sociobiol 67:1471–1482
Velásquez NA, Moreno-Gómez FN, Brunetti E, Penna M (2018) The acoustic adaptation hypothesis in a widely distributed South American frog: Southernmost signals propagate better. Sci Rep 8:1–12
Virant-Doberlet M, Kuhelj A, Polajnar J, Šturm R (2019) Predator-prey interactions and eavesdropping in vibrational communication networks. Front Ecol Evol 7:203
Wilder SM, DeVito J, Persons MH, Rypstra AL (2005) The effects of moisture and heat on the efficacy of chemical cues used in predator detection by the wolf spider Pardosa milvina (Araneae, Lycosidae). J Arachnol 33:857–861
Yack J (2016) Vibrational signaling. In: Pollack G, Mason A, Popper A, Fay R (eds) Insect hearing. Springer handbook of auditory research, vol 55. Springer, Cham. https://doi.org/10.1007/978-3-319-28890-1_5
Acknowledgements
This research was supported by National Science Foundation grants IOS‐1026995 and DBI-1262863 (to G.W.U) and the UC Department of Biological Sciences. We also thank the other members of the Uetz lab for helping collect and care for the spiders. Finally, thank you to the Cincinnati Nature Center for allowing the collection of spiders from their Rowe Woods property. We acknowledge with respect that our study areas are on forest land originally occupied by the Myaamia (Miami) and Shawandasse (Shawnee) nations prior to European invasion.
Funding
This research was supported by National Science Foundation grants IOS‐1026995 and DBI-1262863 and the University of Cincinnati Department of Biological Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, and execution. Material preparation, data collection and analysis were performed by [George Uetz, Alex Sweger, Emmanuel Bagirov, Madeline Lallo, Christina Horton, Olivia Bauer-Nilsen, Riddhi Upadhyaya, Abbey Miles, and Rachel Gilbert]. The first draft of the manuscript was written by [George Uetz] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant competing interests to declare.
Financial Interests
The authors have no relevant financial or non-financial interests to disclose.
Animal Care
There are no institutional guidelines or regulations at the University of Cincinnati, the State of Ohio or U.S. government pertaining to invertebrates (spiders). Our research was conducted with attention to the ASAB/ABS Guidelines for the Use of Animals in Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uetz, G.W., Sweger, A.L., Bagirov, E. et al. Effects of Leaf Moisture on Transmission and Detection of Communication by a Wolf Spider. J Insect Behav 36, 318–331 (2023). https://doi.org/10.1007/s10905-023-09843-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-023-09843-6