Abstract
Ants use chemical, visual, magnetic and/or solar cues during foraging activity. Orientation is important, since foragers need to return to the nest. In this study, we analyze the maze performance of the Dinoponera quadriceps using chemical and visual cues to study spatial orientation. We used a white maze with seventeen chambers and we allowed the ants to explore for ten minutes in each session. Six treatments performed by manipulating presence or absence of chemical and visual cues. Two treatments occurred in the presence and absence of odor, but without visual cues. In four treatments, we introduced visual cues, in two upper visual cues and in two frontal visual cues, both with and without odor. Our results showed that both chemical and visual cues improve maze performance during ant movement to the food source and to the nest. We suggest bimodal navigation in D. quadriceps. The association of multiple cues (chemical and visual) improves workers navigation performance, which probably enhances foraging rate and individual fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All moving organisms spend part of their time orienting themselves while interacting with their environment (Jander 1975). Important resources, such as food, are distributed in space and time. Thus, in order to optimize their behavior, it is important to determine the most viable route to access resources. Spatial orientation is the ability to avoid or return to specific locations in the home range. It may involve spatial memory (Benhamou et al. 1990), but the choice of routes does not necessarily reflect spatial knowledge (Collett et al. 2007).
Ants present a variety of orientation strategies. During displacement from one location to another, ants may follow their own chemical trails or those left by conspecifics, heterogeneities in the substrate, or use a memory configuration of terrestrial or aerial landmarks around the nest (Hölldobler and Wilson 1990). For instance, Cataglyphis fortis learns how to associate nest entrance with environmental odor (Steck et al. 2009) and Melophorus bagoti discriminates among visual stimuli in its inbound trip back to the nest (Schwarz and Cheng 2011). Other species use the sun and the earth’s magnetic field to guide them. Cataglyphis noda relies on magnetic cues to reference nest location (Buehlmann et al. 2012). The leaf-cutting ant (Atta columbica) follows magnetic and solar orientation in the absence of chemical and visual cues (Banks and Srygley 2003). The use of multiple cues simultaneously is beneficial because it increases the accuracy of navigation information (Buehlmann et al. 2020).
Neotropical ants include poneromorphs who blend simple social organization with the high diversity of morphological, behavioral and ecological traits (Schmidt and Shattuck 2014). The Neotropical genus Dinoponera is one of the largest known ants (Kempf 1971). A study realized by Fourcassié and collaborators (Fourcassié et al. 1999) demonstrated that in the natural environment of poneromorph ants Dinoponera gigantea that chemical cues are not obligatory, but it depends on each, indeed, chemical cues are not indispensable in familiar routes.
Although most ant species use chemical cues as their main orientation (Hölldobler and Wilson 1990), visual cues may be more stable over time (Harrison et al. 1988). Experiments conducted in small arenas show that specific paths are learned through orientation cues (Nicholson et al. 1999). The cues may be proprioceptive in nature, as ants can trace their own movement and direction (De Agro et al. 2020). Thus, the use of an experimental maze is an appropriate methodology in orientation studies (Jaffé et al. 1990; Macquart et al. 2008; Cammaerts and Lambert 2009). The aim of this study is to analyze the maze performance of the ant Dinoponera quadriceps using chemical and visual cues to study spatial orientation. This species occurs in the Brazilian Caatinga and Atlantic Forest (Kempf 1971). It forages alone and shows no evidence of recruitment (Araújo and Rodrigues 2006; Azevedo et al. 2014). After leaving the nest to search for food, workers move slowly in a zigzag pattern and return directly to the nest once they find food (Araújo and Rodrigues 2006; Azevedo et al. 2014), thereby showing directional fidelity (Azevedo et al. 2014, 2021).
It is important to underscore that most studies on spatial orientation are conducted with desert and/or recruiting ants; however, little is known about orientation performance of Neotropical ants that do not recruit new individuals during the foraging activity. Based on these above features, we hypothesize that D. quadriceps use visual and chemical cues to locate both their nest and food site. Our prediction is that the presence of a visual cue, in addition to chemical cues, improves the workers movement efficiency.
Material and Methods
Animal Collection and Housing
We collected five colonies of Dinoponera quadriceps (Hymenoptera, Formicidae) at the Central Campus of the Universidade Federal do Rio Grande do Norte (UFRN). The Instituto Chico Mendes de Biodiversidade (ICMBio) under license numbers 10602–1 and 12547–1 authorized the ant collection.
The study was conducted at the Behavioral Biology Laboratory of the Universidade Federal do Rio Grande do Norte (UFRN), Natal, Brazil. Each colony was kept in a dark box (50 × 50x20 cm) divided into chambers to be used as a nest. The dark box was inside a foraging arena acrylic (100 × 50 cm) filled with vermiculite as substrate. The laboratory was maintained at a temperature of 25 ± 2 °C, humidity of 67 ± 3% and 12:12 h light–dark cycle. In the foraging arena, food was available three days a week and water ad libitum. The ant diet consisted of Tenebrio molitor (adults and larvae) and fruit. The ants were marked before the study using a colored label with an alphanumeric code affixed to the thorax (Corbara et al. 1986). Moreover, the alphanumeric code identified each individual and the color label its colony. We used the ad libitum method to observe in the five colonies for one week to select the individuals, and we used those that left the nest to engage in external activity in the experiment.
Test Apparatus
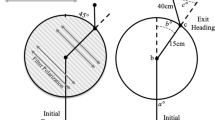
The apparatus consisted of a white wooden maze (80 × 80x15 cm) with seventeen compartments (Fig. 1). The surface and the walls of the maze were covered with Formica. We connected the inlet chamber to a start box that represents the nest (black box; 20 × 10 cm) (Fig. 1). During the testing, the workers can return to the start box at any time during testing. A Sony HDR-AS10-ExmorR camcorder was placed 1 m above in the center of the maze to record the tests. The camera was on a metal frame attached to the edges of the maze. The maze was completely covered with white cloth put on the metal frame that supported the camera, to prevent individuals from seeing outside, in this way we avoid any possible outside information that eventually could disturb the experiment.
Schematic diagram of the maze used in the experiments showing the chamber number used to describe the ant movement sequence. The chambers 1, 3, 6, 7, 10, 11, 16 and 17 have 14 × 12 cm; chambers 2, 5, 9 and 15 have 12 × 12 cm; chambers 4, 8, 12 and 14 have 22 × 12 cm; and chamber 13 has 22 × 22 cm. The black circle in chamber 10 indicates the box where food was available. Inlet chamber 0 (gray) corresponds to the start box connected to the maze. The rectangular marks indicate the presence of colored cards (5 × 3 cm), blue and yellow, corresponding to upper visual placed on the upper part of the chamber walls (b), and frontal visual marks placed on the lower part of chamber walls (c). The location of the colored cards on maze wall is indicated in the image
Data Collection
The maze did not undergo any modification throughout the experimental treatments. We manipulated presence and absence of odor and visual cues in each treatment (Table 1). Figure 1 shows the manipulation: O (only odor) and Ø (without odor) corresponds to Fig. 1a; blue and yellow cards (Cammaerts and Lambert 2009) were placed on the wall of the chambers at the beginning of the treatments, and kept during each treatment. The upper visual card was on top of the wall, while frontal visual card was on the wall in front as follows indicated in the Fig. 1b and c. OVup (odor plus upper visual) and ØVup (upper visual without odor) correspond to Fig. 1b, and OVfr (odor plus frontal visual) and ØVfr (frontal visual without odor) to Fig. 1c. The choice of the places of the visual cues indicates the shortest paths from start box to food chamber.
We tested each ant for three sessions, in each treatment. Each session lasted 10 min, which was enough time to perform exploratory behavior, and at the end of the session, we removed the individual, regardless of its position in the maze. We represented in the flowchart of Fig. 2 the development of the experimental testing. Before starting treatments with odor, we used five ants that did not participate in the tests to explore the maze, and possibly leave odor marks in the maze, since in the natural environment there are several scents. In the treatments without odor, we cleaned the maze with 70% alcohol. There was no food deprivation between treatment sessions. The ants were not trained before the experiments. We released the ants in the start box of the maze without a predefined sequence, which means, each individual is randomly released in the maze at the beginning of the sessions (based on Cammaerts and Lambert 2009).
Flowchart representing the three basic parts of the experiment: the session, the treatment and the sequence of six treatments. Each event last for ten minutes, the individuals can follow a set of possibilities: go to the food chamber, wander in the maze or even do not enter in the maze. Each treatment spans one week and consists of three sessions that took place at 48 h intervals for a total of 33 ants
During the tests, we recorded the chemical marking, a behavior that indicates worker odor perception in natural environment: the individual rubs its abdomen on the substrate with a quick zigzag movement or a continuous linear motion. Chemical marking mostly takes place near nest entrance and in foraging areas (Azevedo 2009).
We used the all-occurrences sampling technique to register ant behavior (Martin and Bateson 1994). We recorded the sequences of the ant path in the maze and all the events in which the workers reached the food chamber. The food chamber always has ten available food items (Tenebrio molitor larvae). If the ant collected any piece of food from the chamber, the item was replaced. The experiments took place from January to March 2013. We consider that the possible sounds and / or odors produced by Tenebrio's larvae, did not generate bias in the results, since they were present in all experimental sessions.
Statistical Procedures
We applied the Monte Carlo technique, or resampling methodology, to compute the confidence intervals for the following measures: chemical marking behavior, the number of times the ants reached the food chamber and sequences to the food and to the nest. A total of 1000 random samples were performed for each treatment. The p-value associated with each pair of treatments was calculated by comparing the confidence intervals. We used the Bonferroni-Holm correction to correct the p-value in multiple comparisons (Rice 1989). Mauchly’s Test showed not sphericity of data (p ≤ 0.05) violating an assumption for performing a repeated measure analysis.
Frequency of the following paths were analyzed: sequences toward the food: 0 → 1 → 2 → 4 → 5 → 8 → 9 → 10, 0 → 1 → 2 → 4 → 13 → 12 → 9 → 10, 0 → 1 → 2 → 14 → 15 → 12 → 9 → 10; and sequences to return to the start box: 10 → 9 → 12 → 15 → 14 → 2 → 1 → 0, 10 → 9 → 8 → 5 → 4 → 2 → 1 → 0, 10 → 9 → 12 → 13 → 4 → 2 → 1 → 0. These sequences represent the shortest (most efficient) paths between the start box and the food chamber without passing through any other chamber. We use these shortest paths to attach visual cues in 3rd, 4th, 5th and 6th treatments.
We estimate the Markov transition matrix corresponding to the ant movement in the maze (Basawa and Prakasa Rao 1980). In addition, we estimate the probability corresponding to the shortest sequences. In this analysis, we do not perform any resampling procedure. All statistical analyses were performed using R software (R version 3.1.1, the R Foundation for Statistical Computing, 2014).
Results
The total experiment time was 99 h, and 33 ants were tested in each treatment. The analysis of the videos revealed that few times the tested ant did not enter the maze, but it remained in the start box. We also observed that when the ant collected the food it returned to the start box and did not go back to the maze. However, most of the time the individuals leave the start box and come back more than once to explore the maze. Moreover, the individuals reached the food chamber several times without collecting any food item. Even though the maze is not connected directly to the nest, all ants in reached the chamber of food at least once. This shows that workers were not disoriented in the maze.
The ants laid more scent marks in treatments when the maze was cleaned with alcohol (Ø: N = 213, ØVup: N = 243 and ØVfr: N = 201) than without cleaning (O: N = 117, OVup: N = 171 and OVfr: N = 140). However, the comparison of the number of chemical markings among treatments showed significant difference only between O and ØVup treatments (Table 2) (Fig. 3a).
Frequency of chemical marking (a) and frequency of arrivals at food chamber (b) for the six treatments. Treatments O, OVup and OVfr occurred in the presence of odor, and upper and frontal visual cues; treatments Ø, ØVup and ØVfr occurred in the absence of odor, and presence of upper and frontal visual cues. The confidence intervals were created by data randomization
More ants reached the food when both odor and visual cues were present (O: N = 109, OVup: N = 134 and OVfr: N = 184; Ø: N = 85, ØVup: N = 68 and ØVfr: N = 83). Analysis of the number of times ants reached the food chamber showed differences between treatments (Table 2). The number of arrivals at the food when odor was the only cue did not differ significantly from the number of arrivals when there was no odor. However, there were significantly more arrivals when visual cues were available (Fig. 3b).
In general, specific sequences to the food chamber were more frequent when the maze was not cleaned with alcohol (O: N = 22, OVup: N = 30 and OVfr: N = 39) (Fig. 4a) than when it was odorless (Ø: N = 14, ØVup: N = 11 and ØVfr: N = 12) (Fig. 4a). The addition of visual cues improved the frequency further (Table 2) (Fig. 4a).
Frequency of optimal sequence to reach the food (a) and to return to the start box (b). Treatments O, OVup and OVfr occurred in the presence of odor, and upper and frontal visual cues; treatments Ø, ØVup and ØVfr occurred in the absence of odor, and presence of upper and frontal visual cues. The confident intervals were created by data randomization
The shortest paths to the start box frequency is also larger in treatments with odor (O: N = 42, OVup: N = 33 and OVfr: N = 56) (Fig. 4b) than in treatments without odor (Ø: N = 9, ØVup: N = 14 and ØVfr: N = 10) (Fig. 4b). The multiple comparisons showed a significant effect of the visual cues in the frequency of shortest paths to the start box (Table 2).
The probability estimated based on the Markov chain transition matrix, that describes the ant movement in the maze, showed that the chance of ants performing the shortest paths is greater in the cases: O and OVfr (Fig. 5). It means that the probability of making an efficient route increase in the presence of more than one type of guidance cue. In addition, if the odor cue is not present, the visual cue helps the ant in its movement. This result corroborates our findings about the frequency of optimal sequences to the food chamber and to the start box.
Start box to food shortest path probability computed with Markov chain transition matrix. Treatments O, OVup and OVfr occurred in the presence of odor; treatments Ø, ØVup and ØVfr in the absence of odor; treatments OVup and ØVup presented upper visual cues and treatments OVfr and ØVfr frontal visual cues
Discussion
Our results showed that D. quadriceps improves the movement performances when the individuals associated visual and odor cues, that is, the ants performed bimodal orientation. The decision of individuals to continue moving toward either the food or the nest depends primarily on chemical cues, and performance improves with the addition of visual cues.
Considering the chemical marking behavior, the ants rubbed their abdomen further into the substrate when no odor was present. This suggests that individuals primarily need chemical cues and that the odor of other ants could be an important factor for movement in the natural environment. Chemical marking was also observed in this species in a natural environment, where a few D. quadriceps workers perform this marking (Araújo and Rodrigues 2006). It suggested that the deposition of chemical material by D. quadriceps (Araújo and Rodrigues (2006), and by D. gigantea (Fourcassié et al. 1999) is used to establish individual paths. Another study with D. quadriceps proposed that chemical marking may be related to territorial delimitation (Medeiros and Araújo 2014). In the present study, we found that chemical marking seems to be indispensable and that it is individually performed with lingering effect.
Observations in both the natural environment and the maze show that odor perception is important for decision making during foraging. In general, ants have a strong sense of smell and we can assume that they perceive surrounding odors other than pheromones (Ehmer 1999). Indeed, the environment provides effective olfactory landmarks (Steck et al. 2009). Dinoponera quadriceps workers do not put on a continuous odor trail. Some individuals perform more chemical marking than others do. This marking occurs sporadically and seems to serve to inform the ant about its individual location. However, the deposited odor could possibly also help other individuals who carry out activities in the same area. In the natural environment, some workers leave the nest and perform much chemical marking (personal observation), which occurs mostly in areas of overlapping foraging activity (Azevedo 2009).
In general, studies on orientation evaluate the cues used by ants to return to the nest. A forager learns to locate landmarks along the route and path choice depends on ant familiarity with the area (McLeman et al. 2002). In our study, we show that D. quadriceps reached the food site more frequently when both frontal and upper visual cues were present. In the natural environment, D. quadriceps collect food at 5.4% of the trips (Azevedo et al. 2014), as in colonies in the laboratory. Frontally displayed objects can help insects distinguish near objects, and guide them (Collett 1996). Dinoponera quadriceps workers look forward more often when they explore the environment, although sometimes they rotate about their body axis, with their head toward the canopy (personal observation). This behavior described for wood ants as saccade-like turns, it is part of the visual control of the direction during the zigzag movement (Lent et al. 2013). The use of frontal landmarks to guide the direction to food was described for Formica rufa (Nicholson et al. 1999). By contrast, Myrmica ruginodis workers only use landmarks that are above them (Cammaerts et al. 2012).
Sometimes, visual cues can overcome chemical cues (Collett et al. 2014). The routes displayed by D. quadriceps workers in the maze showed that they are better oriented if visual and chemical signals are available. If only one of them is present in the maze, the performance is reduced and/or less efficient. Thus, we suggest that D. quadriceps uses a combination of orientation cues, and together they increases orientation efficiency during displacement. The reinforcement of one cue to another indicates that chemical and visual information are adapted to work together (Collett et al. 2014). Leaf-cutting ants (Atta cephalotes, A. laevigata and Acromyrmex octospinosus) are guided by olfactory and visual cues (Vilela et al. 1987). Pheromone trails and learned visual information guide Lasius niger foragers to previously found food sites (Evison et al. 2008). Myrmica sabuleti workers respond more to odor than to visual cues (Cammaerts and Rachidi 2009).
The natural environment of D. quadriceps provides abundant visual landmarks and it can incorporate new visual information’s, such as new barriers represented by branches, trunks along the way. Experiments conducted in the field showed that the artificial barriers placed on the D. quadriceps workers' route are surrounded in a few attempts and the familiar route reestablished in two or three trips with the maintenance of the same direction (Azevedo et al. 2021). Ants memorize those that are close to the nest or on fixed routes (Collett et al. 2007). Foragers following the correct path can change their direction slightly to check information acquired from the landmark (Cammaerts and Lambert 2009; Lent et al. 2013).
Bees (Apis mellifera) combine familiar landmarks and odometry to ensure a more efficient foraging (Vladusich et al. 2005). The results from path sequence and the probability of shortest path sequences suggest that ants learn cues and move following patterns during environmental exploration. Moreover, D. quadriceps workers exhibit directional fidelity (Azevedo et al. 2014). If an individual repeatedly travels along the same path, it acquires visual memories associated with this route (Collett 2014). The use of visual cues, represented by the colored cards in our experiment, probably has both an innate and a learned component. The innate component would be the predisposition to seek visual stimuli present in their natural environment. While the learned component would be the learning and memorization of these stimuli in the individual familiar foraging route.
In this study, we demonstrated that D. quadriceps workers use an association of olfactory and visual cues. When individuals leave the nest in search of food, navigation improves as they memorize visual landmarks, especially frontal ones. When ants return to the nest, chemical and visual cues together seem to be more efficient in orientation. Bimodal cues are learned individually, but later work as a single unit (Buehlmann et al. 2020). Ants memorize overall visual context instead of specific landmarks in the environment (De Agro et al. 2020). The odor trail is a way to stabilize the ant`s route, and they may learn landmarks by detecting odors (Collett and Collett 2002). The learning process makes it possible to reduce navigation randomness and increase ant performance (Deneubourg et al. 1987). Bees can learn to orient in a maze without visual cues, but their performance will be lower than in a maze with visual cues. After learning to move around in a maze with colored marks, they find their way even after marks are removed, however with less accuracy (Zhang et al. 1996).
Our results suggest that the unpredictability of D. quadriceps’s natural environment does not seem to interfere in worker movement; they maintain their fidelity route (Azevedo et al. 2014, 2021). This is likely because there are more cues available along the fidelity routes, and the workers use only a subset of all visual landmarks. The use of a subset of landmarks explains the flexibility in D. gigantea orientation (Fourcassié et al. 1999). Gigantiops destructor learn how to recognize and identify landmarks at the final point of foraging routes, thereby locating nest and food sites (Macquart et al. 2006). Neoponera apicalis memorize one or more paths and their surroundings (Fresneau 1985). In turn, visually guided paths enable F. rufa to maintain their trajectory without the main visual landmark (Lent et al. 2009).
Thus, we can affirm that D. quadriceps exhibit an orientation mechanism that associate chemical and visual cues, to both reach food and return to the nest. The ability to learn a route and make it familiar indicates that the establishment of route fidelity may be related to memory consolidation based on orientation cues. Maintain fidelity to route is associated with ant regularity in movement, which allows recognition and use of cues, even in a maze, as demonstrated by Zhang et al. (2000) from experiments carried out with bees. The combination of chemical and visual cues to orientation improves performance of workers with displacement more efficient, probably enhancing their foraging rate and individual fitness.
References
Araújo A, Rodrigues Z (2006) Foraging behavior of the queenless ant Dinoponera quadriceps Santschi (Hymenoptera: Formicidae). Neotrop Entomol 35:159–164
Azevedo DLO (2009) O papel das rotas e da obtenção de informações sobre a eficiência no forrageio de Dinoponera quadriceps em ambiente natural. Dissertation, Universidade Federal do Rio Grande do Norte
Azevedo DLO, Medeiros JC, Araújo A (2014) Adjustments in the time, distance and direction of foraging in Dinoponera quadriceps workers. J Insect Behav 27:177–191
Azevedo DLO, Medeiros JC, Araújo A (2021) Flexibility in the integration of environmental information by Dinoponera quadriceps Kempf during foraging. Rev Bras Entomol 65:e20210084
Banks AN, Srygley RB (2003) Orientation by magnetic field in leaf-cutter ants, Atta colombica (Hymenoptera: Formicidae). Ethology 109:835–846
Basawa IV, Prakasa Rao BLS (1980) Statistical inference for stochastic processes. Academic Press, London
Benhamou S, Sauvé J-P, Bovet P (1990) Spatial memory in large scale movements: efficiency and limitation of the egocentric coding process. J Theor Biol 145:1–12
Buehlmann C, Hansson BS, Knaden M (2012) Desert ants learn vibration and magnetic landmarks. PLoS One 7(3):1–4
Buehlmann C, Mangan M, Graham P (2020) Multimodal interactions in insect navigation. Anim Cogn 23:1129–1141
Cammaerts M-C, Lambert A (2009) Maze negotiation by a myrmicine ant (Hymenoptera: Formicidae). Myrmecol News 12:41–49
Cammaerts M-C, Rachidi Z (2009) Olfactive conditioning and use of visual and odourous cues for movement in the ant Myrmica sabuleti (Hymenoptera: Formicidae). Myrmecol News 12:117–127
Cammaerts M-C, Rachidi Z, Beke S, Essaadi Y (2012) Use of olfactory and visual cues for orientation by the ant Myrmica ruginodis (Hymenoptera: Formicidae). Myrmecol News 16:45–55
Collett TS (1996) Insect navigation en route to the goal: multiple strategies for the use of landmarks. J Exp Biol 199:227–235
Collett M (2014) A desert ant’s memory of recent visual experience and the control of route guidance. P Roy Soc B-Biol Sci 281:20140634
Collett TS, Collett M (2002) Memory use in insect visual navigation. Nature 3:542–552
Collett TS, Graham P, Harris RA (2007) Novel landmark – guided routes in ants. J Exp Biol 210:2025–2032
Collett TS, Lent DD, Graham P (2014) Scene perception and the visual control of travel direction in navigating wood ants. Phil Trans R Soc B 369:20130035
Corbara B, Fresneau D, Lachaud J-P, Leclerc Y, Goodall G (1986) An automated photographic technique for behavioural investigations of social insects. Behav Process 13:237–249
De Agro M, Oberhauser FB, Loconsole M, Galli G, Dal Cin F, Moretto E, Regolin L (2020) Multi-modal cue integration in the black garden ant. Anim Cogn 23:1119–1127
Deneubourg J-L, Goss S, Pasteels JM, Fresneau D, Lachaud J-P (1987) Self-organization mechanisms in ant societies (II): learning in foraging and division of labor. Experientia Suppl 54:177–196
Ehmer B (1999) Orientation in the ant Paraponeraclavata. J Insect Behav 12:711–722
Evison SEF, Petchey OL, Beckerman AP, Ratnieks FLW (2008) Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behav Ecol Sociobiol 63:261–267
Fourcassié V, Henriques A, Fontella C (1999) Route fidelity and spatial orientation in the ant Dinoponera gigantea (Hymenoptera, Formicidae) in a primary forest: a preliminary study. Sociobiology 34:505–524
Fresneau D (1985) Individual foraging and path fidelity in a ponerine ant. Insect Soc 32:109–116
Harrison JF, Fewell JH, Stiller TM, Breed MD (1988) Effects of experience on use of orientation cues in the giant tropical ant. Anim Behav 37:869–871
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Jaffé K, Ramos C, Lagalla C, Parra L (1990) Orientation cues used by ants. Insect Soc 37:101–115
Jander R (1975) Ecological aspects of spatial orientation. Annu Rev Ecol Syst 6:171–188
Kempf WW (1971) A preliminary review of the Ponerinae ant genus Dinoponera Roger (Hymenoptera: Formicidae). Studia Entomologica 14:369–394
Lent DD, Graham P, Collett TS (2009) A motor component to the memories of habitual foraging routes in wood ants? Curr Biol 19:115–121
Lent DD, Graham P, Collett TS (2013) Phase-dependent visual control of the zigzag paths of navigation wood ants. Curr Biol 23:2393–2399
Macquart D, Garnier L, Combe M, Beugnon G (2006) Ant navigation en route to the goal: signature routes facilitate way-finding of Gigantiops destructor. J Comp Physiol A 192:221–234
Macquart D, Latil G, Beugnon G (2008) Sensorimotor sequence learning in the ant Gigantiops destructor. Anim Behav 75:1693–1701
Martin P, Bateson P (1994) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
McLeman MA, Pratt SC, Franks NR (2002) Navigation using visual landmarks by the ant Leptothorax albipennis. Insect Soc 49:203–208
Medeiros J, Araújo A (2014) Workers’ extra-nest behavioral changes during colony fission in Dinoponera quadriceps (Santschi). Neotrop Entomol 43:115–121
Nicholson DJ, Judd SPD, Cartwright BA, Collett TS (1999) Learning walks and landmarks guidance in wood ants (Formica rufa). J Exp Biol 202:1831–1838
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Schwarz S, Cheng K (2011) Visual discrimination, sequential learning and memory retrieval in the Australian desert ant Melophorus bagoti. Anim Cogn 14:861–870
Steck K, Hansson BS, Knaden M (2009) Smells like home: desert ants Cataglyphis fortis, use olfactory landmarks to pinpoint the nest. Front Zool 6(5):1–8
Vilela EF, Jaffé K, Howse PE (1987) Orientation in leaf-cutting ants (Formicidae: Attini). Anim Behav 35:1443–1453
Vladusich T, Hemmi JM, Srinivasan MV, Zeil J (2005) Interactions of visual odometry and landmark guidance during food search in honeybees. J Exp Biol 208:4123–4135
Zhang SW, Bartsch K, Srinivasan MV (1996) Maze learning by honeybees. Neurobiol Learn Mem 66:267–282
Zhang SW, Mizutani A, Srinivasan MV (2000) Maze navigation by honeybees: learning path regularity. Learn Memory 7:363–374
Acknowledgements
We thank the Universidade Federal do Rio Grande do Norte for logistical support and the Instituto Chico Mendes de Biodiversidade (ICMBio) for collection licenses. We are also grateful to the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) (Grant 302012 / 2006-0; 401738 / 2007-8 and 307907/2019-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Apoio à Pesquisa do Estado da Bahia (FAPESB) (PRONEX FAPESB-CNPq PNX 0011-2009) for funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation were performed by Dina L.O. Azevedo and Arrilton Araujo, data collection was performed by Dina L.O. and Pablo F.G.A. Santos, and statistical analysis were performed by André G.C. Pereira, Gilberto Corso and Dina L.O. Azevedo. The first draft of the manuscript was written by Dina L.O. Azevedo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Aspects
Study authorized by Instituto Chico Mendes de Conservação da Biodiversidade, Ministry of Environment, Brazil (authorization nº 10602–1 and 12547). As it is an invertebrate, in accordance with Brazilian legislation (Law No. 11.794/2008), there was no need to submit it to the Ethics Committee on the Use of Animals. We also take into account the recommendations made in the Guidelines for the Use of Animals (Animal Behavior Society).
Conflict of Interest
The authors have no conflicts of interest in relation to the subject of this manuscript, regarding specific financial interests, relationships or affiliations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 2625 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azevedo, D.L.O., Santos, P.F.G.A., Pereira, A.G.C. et al. Effect of Chemical and Visual Cues in the Maze Performance of the Ant Dinoponera quadriceps. J Insect Behav 35, 103–113 (2022). https://doi.org/10.1007/s10905-022-09803-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-022-09803-6