Abstract
The small cockroach Attaphila paucisetosa, a myrmecophilic species, was recently reported in nests of the leafcutter ant Atta cephalotes in Southwestern Colombia. We carried out both behavioral bioassays and field observations to learn more about this cockroach-myrmecophilous association. When we excavated the nests of A. cephalotes did not found cockroaches out of the anthills. We collected all lifecycle stages of At. paucisetosa (ootheca, nymphs, male and female adults) inside of fungus chambers of the leafcutter ants’ nests. In lab, we observed that At. paucisetosa recognized artificial trails of A. cephalotes as well as worker ants. Our results show that At. paucisetosa is recognized by A. cephalotes workers as an intruder, triggering aggressive behavior, but the aggressiveness reduced in the presence of the ants’ fungus. Also, the cockroach may use foraging trails and its attachment abilities to ride on foraging ant, facilitating the entrance to the stablished colonies, but the cockroach must take risks (from being mutilated or killed) since it is recognized as an intruder, so it takes advantage of its small size to try to find refuge in the fungus crevices, taking recognition profile hydrocarbons from the ants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large variety of arthropods live in association with ants (Hölldobler and Wilson 1990; Allan and Elgar 2001). This association, called myrmecophily, occurs because ant nests represent well-protected and stable environments that are rich in various resources, mainly stored food and waste materials (Maurizi et al. 2012). The myrmecophilic arthropods display several adaptations, including morphological and chemical mimicry, behavioral mimicry, feeding behaviors, and corporal modifications that allow them to avoid ant attacks and be accepted by ants (di Giulio et al. 2011; Maurizi et al. 2012).
A good example of the of this adaptation is represented by species of the genus Attaphila Wheeler 1900 (Blattodea: Blaberoidea) which are associated with the monophyletic group of the genera Atta, Acromyrmex and Amoimyrmex (Bohn et al. 2021). Attaphila species are distributed from Louisiana and Texas (USA) to Uruguay and Argentina (Bolivar 1905; Moser 1964; Rodríguez et al. 2013; Nehring et al. 2015). Until recently, Attaphila was composed of six recognized myrmecophilous species (Beccaloni 2014). Recently, three additional species, among them At. paucisetosa (Fig. 1), were described (Bohn et al. 2021).
All known Attaphila cockroaches live in colonies of leaf-cutting and fungus-gardening ants, including the recently proposed Amoimyrmex (Phillips et al. 2017; Cristiano et al. 2020; Bohn et al. 2021). Overall, these roaches are small (2.6–3.0 mm length), which is a very common characteristic of social insect guests (Kistner 1982; Hölldobler and Wilson 1990; Parker 2016). Also, this myrmecophilous organism has short legs and antennae. Females are apterous while males are brachypterous (Wheeler 1900; Nehring et al. 2015; Bohn et al. 2021). Attaphila roaches display cryptic habits and have never been collected independently of the ants. There are three known dispersal strategies well described by Phillips et al. (2021): First, vertical transmission or riding female alates during mating flights. Second, direct horizontal transmission or independent movement of the roaches between nests. Third, horizontal transmission which roaches ride on female alates and abandon them during dispersal to entry in matures colonies, such behavior also was observed by Zubarán and Di Iorio (2020) with Acromyrmex lundi and its host At. bergi.
In contrast, At. schuppi individuals follow Ac. niger foraging trails by joining ant workers (Bolivar 1905). Moser (1964) also observed that At. fungicola individuals perceived artificial foraging trails of A. texana and Trachymyrmex septentrionalis. The recently described At. paucisetosa, formerly misidentified as At. fungicola, was recorded in nests of Atta cephalotes in Colombia (Rodríguez et al. 2013). In this study, we investigated if Attaphila paucisetosa is recognized as an intruder by Atta cephalotes and what strategy it uses to avoid attacks inside the nest. For this reason, we propose as objectives: To study the strategy of entry and establishment of At. paucisetosa in a nest of A. cephalotes and to analyze the role of the fungus as a possible place of refuge for these individuals.
Materials and Methods
Species and Sample Collection
During January to May 2015 a total of 70 ant colonies of Atta cephalotes nests were entirely excavated in two separated places in Cali, Colombia: Los Andes, a rural area (3°24′58.78″ N; 76°35′30.69″ W); and campus of the Universidad del Valle (3°22′23.07″ N; 76°31′50.59″ W). The collected material, which included queens, workers, mutualistic fungus and At. paucisetosa cockroaches, was transported in plastic bowls to the entomological lab of the Universidad del Valle. Ant colonies were maintained under a photoperiod 12:12 at room temperature and fed on Mangifera indica leaves and oat flakes. Experiments were carried out over the five days following collection.
Tracking Experiments
Artificial trails were prepared following the technique of Moser and Blum (1963). Separately, the gasters of a minor (head length: 0.4 -2.0 mm) and a major (head length: 4.4–8.8 mm) worker were macerated in 1 mL hexane. We considered the fully pigmented workers to avoid using young workers. An extract was prepared with ants gaster from each collection site. The extract was used to draw 18 cm diameter circles on acid free paper (attempting to avoid any chemical cue that affect the experiment). In the center of each circle, an ant and a cockroach were deposited separately. An event was considered positive when the ant or the cockroach perceived and walked more than half of the circle in a time of five minutes. This experiment was done with 20 ants and 20 cockroaches. The ants were from same colony, and we used four colonies; two replicated for each colony. The cockroaches were from a single colony. Serial dilutions were used to estimate the minimum concentration that roaches could perceive. As control tests, artificial trails were made only of hexane.
Aggression Test
The methods proposed by Larsen et al. (2014), with slight modifications, were used to measure the aggressiveness of the ants toward the cockroaches. Using cockroaches from a same colony, we performed aggression tests which consisted of an encounter between three discriminator ants and one target organism (this was a cockroach or an ant, depending on the test). The arena was a fluon-coated plastic container (40 mm diameter, 60 mm height) the bottom of which was covered with filter paper exposed for 24 h inside a discriminator colony (Guerrieri et al. 2009). Discriminator ants were placed in the arena five minutes before the beginning of each test. Each test was recorded with an observer using a DinoLite AM4113T camera for five minutes or until the test organism was killed. The behavior of the discriminator ant toward the target organism was classified as follows: (i) no contact, (ii) antennation, (iii) mandible opening, and (iv) biting. Each of these behavioral responses was scored from 0 to 2, where 0 was antennation (lowest), 1: mandible opening, and 2: biting (aggressive response). The aggression index (AI) for each encounter was calculated following the d’Ettorre et al. (2000) formula:
where, \({AI}_{i}\) is the aggression level and \({t}_{i}\) is duration of event. T is total interaction time, defined as the sum of times (in seconds) which a discriminator ant was in physical contact with the target organism. Ants and cockroaches were used only once and not returned to the colonies, and only cockroaches from Los Andes site were used (Additional file 1).
Ant-Cockroach Encounters
To test if ants recognize At. paucisetosa as an intruder, a test with six replicates was carried out allowing the encounter between three-discriminator ants (cockroach nestmate or non-nestmate) vs one cockroach, with six replicates of each test. The possible combinations were: At. paucisetosa vs three major non-nestmate, At. paucisetosa vs three medium non-nestmate (head length: 2.1–4.3 mm), and At. paucisetosa vs minor non-nestmate ants (Additional file 1). An identical set of combinations was used as control but employing nestmate ants instead. In all behavioral essays we used once the organisms (ants and cockroaches).
Ant-Cockroach-Mutualistic Fungus Encounters
To determine whether the aggressiveness of A. cephalotes against At. paucisetosa is modified by the presence of the mutualistic fungus, encounters between three-discriminator ants and one cockroach were carried out as just described but adding a piece of fungus with a volume of 0.5 g was added in the arena five minutes before each test. For this test, we considered nestmate fungus as the fungus from colony where the cockroach was found, and non-nestmate fungus as the fungus from another colony where cockroaches were not found. Six replicates of each of the possible combinations were carried out: At. paucisetosa with a non-nestmate fungus vs. non-nestmate workers; At. paucisetosa with non-nestmate fungus vs. nestmates workers; At. paucisetosa with nestmate fungus vs. non-nestmate workers. As a control event, At. paucisetosa with nestmate fungus vs nestmate workers was performed (Fig. 2, Additional file 1).
Statistical Analyses
Data were analyzed using a generalized linear mixed model lme4 (Bates et al. 2011) and an aggression index (AI) as the dependent variable. As explanatory factors, worker castes (major, medium, minor), presence of fungus in the arena, origin of colony and fungus (Cali or Los Andes), and target organism (ant or cockroach) were considered. Tracking experiments were analyzed using the Zero Inflated Poisson regression model (Jackman 2008), considering worker size, origin of collected ants, and dilution of extract as random variables. For all data, the R statistical package was used (v. 3.2.1, R Core Team 2020).
Results
A total of 210 Attaphila paucisetosa cockroaches were collected from 30 ant nests of ages ranging from one to four years, in Los Andes (rural area); 42.8% of the nests had at least one roach. No cockroaches were founded in the other place (Universidad del Valle). One colony, with 100 cockroaches, was 12 years old (Table 1). All At. paucisetosa individuals, including nymphs and adults, were inside the fungus chamber; 46.67% of the cockroaches were nymphs (Table 1). The sex ratio (female: male) was 3:1, and of all roaches collected, 73.3% were females. A female with an ootheca attached to her abdomen was found in the largest excavated nest (Fig. 3). Many of the cockroaches had mutilated limbs: 29.2% had lost at least one leg, and 71.9% had a mutilated antenna.
Tracking Artificial Trails of Atta cephalotes
Both ants and cockroaches perceived artificial trails, but ants had more positive events (Z = 23,605; p < 0.005). In the case of At. paucisetosa, all individuals detected artificial trails until 10–3 dilutions (Table 2). At the first phase of the tracking, the cockroach started to move slowly on the trail, then it stayed still and finally began to move faster. We observed that most positive events were those tracks made with gaster extract from the major worker (t37 = 5.30; p < 0.05). On the other hand, if we consider the origin of the gaster extract, the number of positive events increased if cockroaches and the extract came from the same colony (Z = 23.605; p < 0.05). Neither ants nor cockroaches perceived control (hexane) trails.
Aggression tests
Ant-Cockroach Interaction
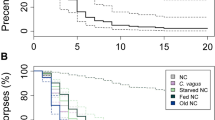
Aggressiveness levels of A. cephalotes toward At. paucisetosa differed depending on nest origin and worker caste (Fig. 4). The minor workers displayed the highest level of aggression against non-nestmate cockroaches (p < 0.001). Medium workers occasionally reacted aggressively while major workers did not attack. No cockroaches were attacked by their ant nestmates (p < 0.001 compared with non-nestmates). We observed cockroaches avoid touching the most of time the ants and shows no interaction between the ants and the cockroach, however it uses the fungus to hide from non-nestmate ants.
Aggression indices of three different Atta cephalotes castes (major, medium, minor) were greater toward Attaphila paucisetosa cockroaches when they were exposed to non-nest mate (left) than to nest mate (right) target workers. Box plots depict the median and interquartile range. Significance indicators above the boxes are derived from a generalized linear mixed model (*** p < 0.001). Circles denote atypical or extreme data. It is noteworthy that each encounter combination was replicated six times
Ant-cockroach-symbiont fungus interaction
Ant reactions toward cockroaches changed when mutualistic fungus was added to the arena (Fig. 5). Nestmates and non-nestmate worker ants reacted more aggressively against the cockroaches when a piece of non-nestmate fungus was placed in the arena (p < 0.01); both biting and mandible opening behavior increased. Again, the minor workers were the most aggressive caste (p < 0.05). In one event a non-nestmate major worker killed a cockroach, the cockroach was moving towards the arena so fast that it ran into a major worker’s mandible with a fatal result, this may have been an artifact of captivity.
Aggression indices of three different Atta cephalotes castes (major, medium, minor) towards Attaphila paucisetosa with a piece of mutualistic fungus in the arena. a) Worker castes and non-nestmates of cockroaches, coming from Cali nests, the piece of fungus belonging to Los Andes nests (left side) and to Cali nests (right side). b) Worker castes and nestmates of cockroaches coming from Andes nests are both confronted with a piece of fungus from a Cali nest (left side) and an Andes nest (right side). The significance indicators above the boxes are derived from a generalized linear mixed model (** P < 0.01; ***P < 0.001). Circles denote atypical or extreme data. Each combination depicted had six replicates
When nestmate ants were used as discriminators, they did not react aggressively when a piece of nestmate fungus was placed in the arena. However, when the origin of fungus changed, we observed more aggressive behavior toward the cockroach (p < 0.01). As in other cases, minor workers were the most aggressive (p < 0.01).
Discussion
This study presents new insights and corroborates previous information about the biology and life history of Attaphila sp. cockroaches. The At. paucisetosa cockroaches were mainly collected moving through the fungus chambers, and 90% of the specimens were obtained from mature nests (estimated to be more than four years old). The large number of cockroaches found in mature nests, as well as the different developmental stages of At. paucisetosa, including an ootheca attached to the body of a female; suggesting their entire life cycle occurs inside the nests. Our field observations are supported by Phillips et al. (2021) which Attaphila roaches are not compatible with incipient or recent nests given their extremely fragility.
According to the sex present in the colonies, Waller and Moser (1990) during an excavation of A. texana nests in Austin (Texas, US) reported a lack of males of At. fungicola males; but Phillips et al. (2017) proposed that a limited sampling or a parthenogenesis strategy (at least for North America) could explain this absence of males. Nevertheless, both sexes of At. paucisetosa were found in the present study.
But how does the cockroach interact with ants to integrate into the colony? In our study we found that At. paucisetosa cockroaches recognize ant trails in lab. During the field phase we did not find any cockroach attached to an alate queen like At. fungicola in or out of the colony. However, it is noteworthy that our study was in few places, possibly would be the exception but not the rule. Bohn et al (2021) mention two ways to disperse to reach another colony: join to the ant dispersal activities (known as vertical transmission, e.g., mate flights) or move by its own, independent of the ants (or horizontal transmission). As we mention before, in this study we did not find any At. paucisetosa cockroach in newly founded colonies, indicating possibly vertical transmission is rare or not very effective, since according with Phillips et al. (2017) observations At. fungicola females did not survive in newly founded nest, corroborating our field observations.
Then the option of horizontal transmission would acquire ever more relevance, since as mentioned Bohn et al. (2021) one prerequisite is Attaphila cockroaches can follow the pheromone traces, something observed under lab conditions that At. paucisetosa followed artificial trails of A. cephalotes. But it is not only study with the same behavior. Moser (1964) found At. fungicola in lab conditions not only follow pheromone trails of its host Atta texana, but also Trachimyrmex ants. On another hand, At. schuppi has been found on foraging trails in field (Bolivar 1905), however this strategy is not a plausible and unique way to disperse because foraging trails are unlikely connected between nests.
Phillips (2021) suggests that Attaphila cockroaches do not use just one way, but rather a combination of ways of dispersion from observations in field which showed At. fungicola uses foraging trails after it abandoned A. texana foundress. Once found the foraging trail, the cockroaches use presumably its strong attachment abilities via well-developed pretarsal arolia and it is riding on foraging ant workers or foraged leaf carried by ants (Brossut 1976; Bohn et al. 2021; Phillips 2021).
This combined strategy possibly function as safety way to reach and enter a new colony as Bohn et al. (2021) suggest cockroaches take advantage of being on the back of the worker to avoid the hosts' recognition system, that is probably via chemical odor (Nehring et al. 2016; Adams et al. 2020), and in lab we could corroborate At. paucisetosa individuals tries riding on foraging worker, without success, in change, the ant reacted aggressively. While one individual was killed in this study, occasional killings do occur in many others ant-myrmecophile systems (e.g., Rettenmeyer 1963; Akre and Torgerson 1969; Witte et al. 2009; von Beeren et al. 2011; Parmentier et al. 2016).
The aggressiveness in ants is determined by the size of the opponent and the task inside the nest. We know that At. paucisetosa is recognized as intruder by Atta workers, however the major caste did not react aggressively as expected, and this is possibly because as mentioned Montoya-Correa et al. (2007) the major workers are the first line of defense if a nest is disturbed, and they modulate their aggression according to the opponent size to maintain energy (Nowbahari et al. 1999). Therefore, the major caste might perceive cockroach as a threat that minor castes could face easily. Another possible reason lies in the task for each caste to accomplish within the colony. Leaf cutting ants have a very stratified society where minor workers accomplish maintenance tasks. One of their functions is prevent external contaminants that affect symbiotic fungus. An effective way to keep off potential infecting organisms is by attacking aggressively regardless of whether it is a nestmate or not (Larsen et al. 2014). Hence, we consider that myrmecophile’s life represent a risk in or out the nest.
The next step is: How could At. paucisetosa establish inside the colony? It can be explained in terms of our results that showed when the fungus comes into equation. The ants perceived the cockroach did not belong to their colony, but when we put the fungus piece the ants decrease or did not attack, but why? As Jaffé (1983) mentioned, ants do not react aggressively if intruders do not move. So, when the cockroach was in the arena, the ants reacted aggressively, but immediately the cockroach reach the fungus and hiding in its crevices, reducing the ants’ aggression (Nehring et al. 2015). Then, At. paucisetosa could use the fungus as a shelter, but while trying to reach it, the roach must avoid the contact with ant workers. For this, its small size could help to avoid the attacks (Kistner 1979; Buschinger 2009; Parker 2016), but this physical trait is not completely effective since in many cases by accident or by aggressive response could suffer mutilation (Wheeler 1900). However, body size has not been studied as a predictor of host attacks and it is not clear whether this trait is related to host tolerance (Parker 2016; Parmentier et al. 2016, 2017). Once inside the fungus garden, At. paucisetosa like other Attaphila species probably they start acquiring chemical substances from their host ants (while mounted) or fungus garden via constant touching (Akino 2008; Nehring et al. 2016; Mendoça et al. 2019; Bohn et al. 2021).
Nevertheless, despite our findings, we are aware the limitations of our research. The cryptic peculiarities of the studied species, determining with certainty the interaction that occurs within an underground system brings logistic inconveniences such as a possible alteration of the behavior of cockroaches and ants while the nest is extracted and transported to the laboratory, which would be difficult to know their behaviors are natural or a consequence of the stress due to researcher handling in lab. In addition, another logistical limitation is the reduced number of ant workers used, since this number interacting with the cockroach may be much higher than that proposed in our aggression tests. Hence, we believe that this factor must be considered in the future tests. Although this study is a step closer to elucidating the biology of this cockroach, it is important to be clear that communication between ants occurs mainly through the chemical pathway (d’Etorre et al. 2017; Adams et al. 2020). Nehring et al. (2016) observed A. paucisetosa bear same cuticular hydrocarbon profile than its nestmate workers, which confirms such species uses chemical mechanisms to live inside the nests. Our results show the key role.
of symbiont fungus as a recognition substances source. We assume then that At. paucisetosa possibly exploits the A. cephalotes colonies using chemical mechanisms to enter and integrate. Hence, it would be advisable in future studies use chemical approaches to better explain the ant-cockroach observed behaviors.
In conclusion, the present study highlights the possible mechanism of entry and interaction between the At. paucisetosa in an A. cephalotes colony. Evidently, the cockroach recognizes the artificial trails, possibly it uses this ability along its capacity of attachment to ride on ants’ backs, but this interaction is not friendly, due to is recognized as a foe, but, when it achieves its goal, the ant may be useful as a less aggressive entry mechanism. Once inside, the establishment may bring certain risk complexities such as mutilation or death, and the ant symbiont fungus stars playing a fundamental role for the survival of the myrmecophilic roach inside the colony.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Akre RD, Torgerson RL (1969) Behavior of Vatesus beetles associated with army ants (Coleoptera: Staphylinidae). Pan-Pac Entomol 45:269–281
Adams RMM, Wells RL, Yanoviak SP, Frost CJ, Fox EGP (2020) Interspecific eavesdropping on ant chemical communication. Front Ecol Evol 8:24
Akino T (2008) Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol News 11:173–181
Allan R, Elgar M (2001) Exploitation of the green tree ant, Oecophylla smaragdina, by the salticid spider Cosmophasis bitaeniata. Aust J Zool 49:129–137
Bates D, Machler M, Bolker BM (2011) lme4: Linear mixed-effects models using s4 classes. http://cran.R-project.org/package=lme4.Rpackageversion0.999375-42.
Beccaloni, GW (2014). Cockroach Species File Online. Version 5.0/5.0. World Wide Web electronic publication. http://Cockroach.SpeciesFile.org. Accessed 3 September 2020.
Bolivar I (1905) Les blattes myrmécophiles. Mitt Schwei Entomol Ges 3:134–141
Bohn H, Nehring V, Rodríguez J, Klauss-Dieter K (2021) Revision of the genus Attaphila (Blattodea: Blaberoidea) myrmecophiles living in the mushroom gardens of leaf-cutting ants. Arthropods Syst Phylogeny 79:205–280
Brossut R (1976) Étude morphologique de la blatte myrmécophile Attaphila fungicola Wheeler. Insectes Soc 23:167–174
Buschinger A (2009) Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol News 12:219–35
Cristiano MP, Cardoso DC, Sandoval-Gómez VE (2020) Amoimyrmex gen. nov. and relevant taxonomic changes attributed to Cristiano, M. P.; Cardoso, D. C.; Sandoval-Gómez, V. E. Pp. 647–674 in: Cristiano MP, Cardoso DC, Sandoval-Gómez VE, Simões-Gomes F (2020) Amoimyrmex Cristiano, Cardoso and Sandoval, gen. nov. (Hymenoptera: Formicidae): a new genus of leaf-cutting ants revealed by multilocus molecular phylogenetic and morphological analyses. Austral Entomol. 59: 643–676
d’Ettorre P, Errard C, Ibarra F, Francke W, Hefetz A (2000) Sneak in or repel your enemy: Dufour’s gland repellent as a strategy for successful usurpation in the slave-maker Polyergus rufescens. Chemoecology 10:135–142
d’Etorre P, Deisig N, Sandoz JC (2017) Decoding ants’ olfactory system sheds light on the evolution of social communication. Proc Nat Acad Sci 114:8911–8913
di Giulio A, Maurizi E, Hlaváč P, Moore W (2011) The long-awaited first instar larva of Paussus favieri (Coleoptera: Carabidae: Paussini). Eur J Entomol 108:127–138
Guerrieri FJ, Nehring V, Jorgensen CG, Nielsen J, Galizia CG, d’Ettorre P (2009) Ants recognize foes and not friends. Proc R Soc Lond [b] 276:2461–2468
Hölldobler B, Wilson EO (1990) The ants. Belknap press of Harvard University Press, Cambridge, MA
Jackman S (2008) Classes and methods for R developed in the political science. Computational Laboratory, Stanford University. Department of Political Science, Stanford University, Stanford, California. R package version 0.95
Jaffé K (1983) Chemical communications systems in the ant Atta cephalotes. In: Johnson K, Jaisson P (eds) Social Insects in the Tropics. Presses de l’Université Paris-Nord, France, pp 165–180
Kistner DH (1979) Social and evolutionary significance of social insect symbionts. In: Hermann HR (ed) Social Insects. Academic Press, New York, pp 339–413
Kistner DH (1982) The social insects’ bestiary. In: Hermann HR, editor. Social Insects. New York: Academic Press; 1982. p. 1–244
Larsen J, Fouks B, Bos N, d’Ettorre P, Nehring V (2014) Variation in nestmate recognition ability among polymorphic leaf-cutting ant workers. J Ins Physiol 70:59–66
Maurizi E, Fattorini S, di Giulio A (2012) Behavior of Paussus favieri (Coleoptera, Carabidae, Paussini), a myrmecophilous beetle associated with Pheidole pallidula (Hymenoptera, Formicidae). Psyche 1–9
Mendoça CAF, Pesquero MA, Carvalho RSD, Arruda FV (2019) Myrmecophily and myrmecophagy of Attacobius lavape (Araneae: Corinnidae) on Solenopsis saevissima (Hymenoptera: Myrmicinae). Sociobiology 66:545–550
Montoya-Correa M., Montoya-Lerma J, Armbrecht I, Ropero MCG (2007) ¿Cómo responde la hormiga cortadora de hojas Atta cephalotes (Hymenoptera: Myrmicinae) a la remoción mecánica de sus nidos? – Boletín del Museo de Entomología de la Universidad del Valle 8: 1–8.
Moser JC (1964) Inquiline roach responds to trail-marking substance of leaf-cutting ants. Science 143:1048–1049
Moser JC, Blum MS (1963) Trail marking substance of the Texas leaf-cutting ant: Source and potency. Science 140:1228
Nehring V, Dani FR, Calamai L, Turillazzi S, Bohn H, Klass KD, d’Ettorre P (2016) Chemical disguise of myrmecophilous cockroaches and its implications for understanding nestmate recognition mechanisms in leaf-cutting ants. BMC Ecol 16:1–11
Nehring V, Dani FR, Turillazzi S, Boomsma JJ, d’Ettorre P (2015) Integration strategies of a leaf-cutting ant social parasite. Anim Behav 108:55–65
Nowbahari E, Fénéron R, Malherbe MC (1999) Effect of body size on aggression in the ant, Cataglyphis niger (Hymenoptera; Formicidae). Aggressive Behav 25:369–379
Parker J (2016) Myrmecophily in beetles (Coleoptera): Evolutionary patterns and biological mechanisms. Myrmecol News 22:65–108
Parmentier T, Dekoninck W, Wenseleers T (2016) Survival of persecuted myrmecophiles in laboratory nests of different ant species can explain patterns of host use in the field (Hymenoptera: Formicidae). Myrmecol News 23:71–79
Parmentier T, Vanderheyden A, Dekoninck W, Wenseleers T (2017) Body size in the ant-associated isopod Platyarthrus hoffmannseggii is host-dependent. Biol J Linn Soc Lond 121:305–311
Phillips ZI (2021) Emigrating together but not establishing together: A cockroach rides ants and leaves. Am Nat 197
Phillips ZI, Reding L, Farrior CE (2021) The early life of a leaf-cutter ant colony constrains symbiont vertical transmission and favors horizontal transmission. Ecol Evol 11:11718–11729
Phillips ZI, Zhang MM, Mueller UG (2017) Dispersal of Attaphila fungicola, a mutualistic cockroach of leaf-cutter ants. Insectes Soc 64:277–284
Rettenmeyer CW (1963) The behavior of Thysanura found with army ants. Ann Entomol Soc Am 56:170–174
R Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing
Rodríguez J, Montoya-Lerma J, Calle Z (2013) Primer registro de Attaphila fungicola (Blattaria: Polyphagidae) en nidos de Atta cephalotes (Hymenoptera: Myrmicinae) en Colombia. Bol Cient Mus His Nat 17:219–225
von Beeren C, Schulz S, Hashim R, Witte V (2011) Acquisition of chemical recognition cues facilitates integration into ant societies. BMC Ecol 11:30
Waller DA, Moser JC (1990) Invertebrate enemies and nest associates of the leaf-cutting ant Atta texana (Buckley) (Formicidae, Attini). In: Vander Meer RK, Jaffé K, Cedena A (eds) Applicated Myrmecology. Westview Press, A world Perspective, pp 256–273
Wheeler WM (1900) A new myrmecophile from the mushroom gardens of the Texan leaf-cutting ant. Am Nat 34:851–862
Witte V, Foitzik S, Hashim R, Maschwitz U, Schulz S (2009) Fine tuning of social integration by two myrmecophiles of the Ponerine army ant, Leptogenys distinguenda. J Chem Ecol 35:355–367
Zubarán GE, Di Iorio OR (2020) The genus Attaphila Wheeler, 1900 (Blattaria: Polyphagidae) in Argentina. Historia Natural 10:219–228
Acknowledgements
We thank Elsy Alvear and Sayra Mina for their support in collecting and maintaining ant colonies, and Cristian Roman Palacios for his comments and technical support in statistical analysis. In addition, the authors thank GEAHNA (Grupo de Investigaciones de Ecología de Agrosistemas y Hábitats Naturales) for providing laboratory facilities, and Dr. Horst Bohn, Germany for identifying of the roach species. Special thanks to Professor William Eberhard for his valuable comments.
Funding
This study did not receive any founding resources.
Author information
Authors and Affiliations
Contributions
BOJ and JR conceived the idea and designed the study. BOJ and JR collected the nest samples. BOJ performed the experiments, analyzed the recordings, took the pictures, and provided the first draft. BOJ, JR, and JML wrote and edited the manuscript.
Corresponding author
Ethics declarations
Compliance with Ethical Standards
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species. The study was carried out under the collection permit granted by the environmental licensing authority (ANLA) to the Universidad del Valle (Resolution 1070 issued on August 28, 2015).
Financial Interests
The authors have no financial or proprietary interests in any material discussed in this article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ospina-Jara, B., Rodríguez, J. & Montoya-Lerma, J. Intruders in the Nest: Interaction of Attaphila paucisetosa (Blattodea: Blaberoidea) with Atta cephalotes Workers (Hymenoptera: Formicidae). J Insect Behav 35, 1–10 (2022). https://doi.org/10.1007/s10905-022-09794-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-022-09794-4