Abstract
Metarhizium anisopliae and Beauveria bassiana are commonly used entomopathogenic fungi, but their non-target effects over generalist predatory insects, which can contribute to pest control, are not well known. We studied the capacity of the social wasp Polistes myersi to detect the pathogens in either a powdered form or in Galleria mellonella larvae infected with either of the two pathogens offered as prey. The effects of these treatments were compared considering wasp behaviors such as prey preference, frequency, duration and transitions of both hunting and grooming behaviors. Additionally, the effects of each entomopathogenic fungus on the wasp’s mortality were measured. Wasps seem not to detect the pathogens in powdered form but preferred healthy over infected larvae. Seventeen behavioral units for hunting and 34 for grooming were recognized. There were no differences in grooming frequency but there were significant differences on grooming duration, hunting behaviors and the patterns of transitions. Exposure of wasp colonies to either B. bassiana or M. anisopliae had no detectable impact on the mortality of adults, but mortality of larvae increased. For the first time, this study documented behavioral changes that indicated the capacity of social wasps to detect pathogens before physical contact and the display of hygienic strategies once contact occurs. The study also suggested a potential non-target effect of these entomopathogenic fungi on a generalist predator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern agriculture has faced pest problems with different strategies. The application of pesticides has been the most common strategy. However, despite initial successes, there are multiple examples of pests that have shown resistance (Van Leeuwen et al. 2010; Casida and Durkin 2013). Thus, alternative approaches have been developed seeking greater efficacy, specificity, and lower environmental impacts. Entomopathogenic organisms have been extensively used seeking these goals; however, effects on non-target organisms have also been detected. For example, Otterstatter and Thomson (2017), reported spillover infections and population reductions in native bumble bees from infected commercial bee populations with Crithidia bombi (Euglenozoa). Prior (1997), Espinosa-Ortiz et al. (2011), and de Conceição et al. (2014) recorded increased mortality of pollinators from areas where pathogens were used. Similarly, Danfa and Van Der Valk (1999) and Hokkanen et al. (2003) detected increased mortality on herbivores and parasitoids.
Metarhizium anisopliae and Beauveria bassiana have been extensively used as control agents (Cruz et al. 2017; Meikle and Nansen 2007) and are important fungi used in biological control (Lacey et al. 2015; Joop and Vilcinskas 2016). These have been targeted towards a wide array of species, including orthopterans, coleopterans, lepidopterans, and mites (Mesquita and Lacey 2001). However, the results of these applications are variable and depend on the strain, the species, the dose, and the timing of application (Prior 1997). For example, Harris et al. (2000) have observed strong differences in the efficacy of pathogenic species and strains for the control of Vespula vulgaris. Thus, studies to analyze the effect of entomopathogens on generalist predators, such as social wasps, need to have increased knowledge on the consequences of entomopathogens use on these predators.
There are many publications on the effective responses of insects towards pathogens, such as the hygienic behaviors of honey bees or the use of metapleural gland products in ant grooming (Roy et al. 2006). In social wasps a large array of defensive strategies against pathogens have been detected ranging from behavioral responses (Sumana and Starks 2004), beneficial microbial associations (Madden et al. 2013), secretion of antimicrobial substances (Baracchi et al. 2012), phagolysosomes, and humoral responses (Manfredini et al. 2008; Sadd and Barribeau 2013). However, the study of olfactory capacities has focused on beneficial microorganisms, food baits, and sexual selection of the wasps (Mackenzie et al. 2008; Davis et al. 2013; Landolt et al. 2014; Elmquist and Landolt 2018; Babcock et al. 2019), while behavioral responses against entomopathogens have been characterized by classic selection tests with very broad descriptions of the animal’s responses (Meyling and Pell 2006; Mburu et al. 2009). Detailed analyses of the behavioral response of an insect towards challenges of infection are relatively scarce.
Many species of the eusocial wasp genus Polistes have been studied as generalist predators in agroecosystems identifying them as important factors in pest control (Andrade and Prezoto 2001; Elisei et al. 2011). In Colombia Polistes myersi (8.5–15 mm long) is a common species in crops, farmlands, and in generally perturbed habitats. The nests of this species can have up to 100 cells. This species is distributed from Panama to south Venezuela. It is a widespread species in Colombia, found up to 2000 m above sea level (Sarmiento 1997). Thus, it is a good model to understand the effects of entomopathogens on a generalist predator.
The goal of this research was to assess the effect of prey larvae infected with either B. bassiana or M. anisopliae on the generalist predator wasp P. myersi and to compare their behavioral defensive response to these pathogens. The study could shed light on the non-target effects of these fungi on beneficial elements of the agroecosystem.
Materials and Methods
Wasp Colony Maintenance
A total of 20 colonies of Polistes myersi were collected from the field (Colombia: Cundinamarca, Fusagasugá, 1309 m, 4°18`996”N, 74°26`475”W) looking for nests that were highly distanced from each other along an area of 3 × 1 km; colonies were collected at three field trips along two years. Each nest was established in transparent PVC cages (25 × 16 × 16 cm) and stored in a rearing chamber under controlled conditions that resemble the climate of their environment (28 °C, 44% RH, 12/12 h photoperiod). Collected colonies ranged in size from two adults and 10 cells to 15 adults and 100 cells. All colonies had either late larvae or pupae. Strips of different paper qualities were provided for nest construction. Colonies were provided with water and a mixture of water, honey and pollen at libitum. Later instar larvae of the greater wax moth Galleria mellonella (Pyralidae) were offered every other day. Galleria larvae are commonly reared moths that naturally attack honeycombs, and the species is also a common insect model for diverse studies (Mylonakis et al. 2005; Mukherjee et al. 2010). A single larva was provided to most of the colonies, but large colonies received two larvae. No additional larvae were given if these were not hunted by the wasps. PVC cages were thoroughly cleaned every other day. Colonies were used for the studies if they regularly hunted and fed on the food items provided for approximately a month after being established in the rearing chamber. All the colonies increased their nest size and their larvae reached adulthood. In three cases new colonies were begun within the same cage. These observations suggest that they were maintained under appropriate captivity conditions.
Larvae Infection Procedure
Galleria mellonella larvae were infected through an immersion in a water-based solution with either of the two pathogens. The immersion lasted two seconds. In order to replicate field conditions, the pathogen solution was prepared at the concentration recommended by the producer (Biológicos y Ecológicos de Colombia Company, Colombian Institute of Agriculture certificate number 2593 of 1999) as follows: for B. bassiana at 1 × 108 spores/g (Strain BEC01) added to a liter of water, and for M. anisopliae at 1 × 109 spores/g (Strain BEC12) added to a liter of water. This means that we add a gram of product to a liter of water. According to the producer, germination rate is 85% for both B. bassiana and M. anisopliae.
The infection rate for each pathogen was characterized by exposing a separate batch of larvae (n = 10 each) to the pathogens following the above described protocol. The infection rate was of 80% for B. bassiana, and 100% for M. anisopliae. Infected larvae always showed the same symptoms: slower movement, clear delay in their response to tactile stimulus, and their body became less turgent and irregularly darker. In both cases, after 36 h of infection larvae showed the first symptoms but were still active, a key characteristic to induce hunting behavior by the wasps, thus, this lapse of 36 h was established as the appropriate timing to offer the larvae to the wasps. Late instar larvae of Galleria mellonella were used as prey in all tests.
Olfaction Tests
Given the preliminary results, tests to proof whether foragers showed any behavioral differences towards the odor of larvae infected with the pathogen were performed. Protocols by Mackenzie et al. (2008), Landolt et al. (2014), and Elmquist and Landolt (2018) were considered. Colonies used for these tests were different from those used for the hunting tests. Foragers were starved 24 h before olfactory tests and were extracted from the colony and placed individually in small containers for 30 min within the white styrofoam cage used for the video recording described above. In this way stress due to manipulation was reduced. The glass Y-tube (4 cm diameter, 19 cm stem length, 16 cm branch length) used for the olfaction tests was placed horizontally inside the styrofoam cage described before for the behavioral repertoire analyses.
Constant airflow was provided into each branch of the Y-tube with an aquarium pump. Air passed through each treatment container to provide the corresponding odor. To avoid infection of wasp foragers by the pathogens, standard filter paper (Whatman®) was placed between the exit of the treatment container and the Y-tube branch. No nest or wasp showed signs of infection after the olfaction tests. A total of six treatments were applied in order to compare between pathogen and control as follows. Three treatments used the pathogens in the powder presentation accompanying the commercial pesticide, and a control of rice flour, since this is used as an excipient for the pesticide commercial presentation. Three treatments used infected larvae as described previously (see 2.2.) and healthy larvae were used as controls. The pairs compared by treatment were as follow: 1. M. anisopliae vs. rice flour; 2. B. bassiana vs. rice flour; 3. M. anisopliae vs. B. bassiana; 4. Larvae infected with M. anisopliae vs. healthy larvae (control); 5. Larvae infected with B. bassiana vs. healthy larvae; 6. Larvae infected with M. anisopliae vs. larvae infected with B. bassiana.
Each treatment was tested with 30 forager wasps taken randomly from any of the 16 colonies available. Tested wasps were individually marked with color paints (either Revell® color 312 or red acrylic polish) and then were returned to their nests. Thirty-three percent of wasps were used a second time but tests were separated by at least three days. The effect of these individuals tested twice was explicitly analyzed as explained below. Given preliminary results, treatments four and five were tested 30 times more.
Following Mackenzie et al. (2008), a series of five wasps were tested successively with a single treatment and then the location of both odor sources was switched to eliminate left or right potential bias. After performing ten tests all the equipment was cleaned with Micro 90 Solution 2% (International Products Corp., Burlington, NJ) and then rinsed with deionized water, then again with acetone and then finally with hexane.
Individual foragers were placed into the stem of a Y-tube and observed for 2 min. If the wasp moved upwind until its entire body was inside any of the branches it was scored as a positive result. Data were analyzed with standard chi-squared tests available in R. The effect of using foragers more than once on the selection outcome was assessed using mixed model analysis where a forager was a random effect and the treatment was a fixed effect. This analysis was performed using the packages car, MASS, and lme4 of R (Fox and Weisberg 2019; Venables and Ripley 2002; Bates et al. 2015; respectively).
Hunting Tests
A total of 15 hunting tests were conducted for each entomopathogen as follows: an infected larva and a healthy larva were placed simultaneously on the floor of the PVC cages separated from each other by a 15 mm tall × 25 cm long transparent acetate wall to avoid cross contamination. Tests were videotaped after placing the PVC box with the colony into a white styrofoam cage (36 cm height, 77 cm width, 70 cm depth) at 26 °C and 3276 lm. This cage facilitated a similar temperature to that of the rearing chamber and a neutral visual background during the tests. Colonies were randomly selected for each treatment. An equal number of colonies was used for every test (healthy- B. bassiana and healthy - M. anisopliae) of the same size as given by the number of adults. Hunting tests were used to resemble natural conditions as wasps in the field may face an array of prey varying in health. In this sense, a wasp may be in an area where entomopathogens have been applied but never select infected prey, avoiding pathogen contact. Single treatment tests, with either infected or healthy larva, would show the wasp’s immune resistance but will not reveal whether they have an array of behaviors to prevent disease.
Infected larvae were handled with separate tweezers. Tests were video recorded until the wasp chewed the selected prey turning it into a ball and then left the hunting place. Half of the 20 colonies studied were tested up to two times. Colonies were included in a second test if the wasps did not attack a larva or if only healthy larvae were attacked the first time. Following the studies of Landolt et al. (2014) and Elmquist and Landolt (2018) on Polistes behavior, tests on the same colony were separated by three days to reduce the effect of previous experiences. The effect of these repetitions on the selection outcome was assessed using mixed model analysis where the colony was a random effect and the treatment was a fixed effect. This analysis was performed using the statistics packages described previously for the olfaction tests.
Characterization of Wasp Behavioral Repertoire
Hunting and grooming behaviors were identified and typified by looking for clear changes in the activity and the movement of specific body parts. Repertoire completeness for grooming and hunting behaviors were analyzed using the Chao1 estimator run under the package EstimateS version 9.1.0. (Colwell 2013). Duration and frequency of each behavior, and the sequences of behaviors were recorded and compared, discriminating whether it occurred with healthy or infected larvae. Comparisons were also performed with the baseline of either the hunting or grooming behaviors.
Behavioral sequences per treatment were analyzed as follow: the frequency of a transition from one behavior to another was recorded and that value was divided by the total of observed transitions. In this way, the frequency of a specific transition regarding all records was assessed. The inclusion of behaviors and sequences in the graphs followed two criteria: behaviors with higher frequencies and most common transitions between behaviors. Thus, a graph included those behaviors that summed about 80% of the total frequency observed and other behaviors that were less frequent but part of often repeated transitions.
As normality and homogeneity of variances requirements were not met from our four data sets for hunting frequency, hunting duration, grooming frequency, and grooming duration, the Wilks’ Lambda type nonparametric multivariate test available in the npmv package of R (Liu et al. 2011; Ellis et al. 2017) was performed. The method also allowed us to identify changes in probability of occurrence between treatments for every single behavior (Ellis et al. 2017).
The baseline of the hunting behaviors was established after 13 hunting events as follows: two healthy larvae of Galleria mellonella, were placed on the floor of the PVC cages separated by 3 cm from each other and videos of the adult wasp behavior were recorded under the conditions described previously. Videos lasted until the wasp chewed the prey, turning it into a ball and left the hunting area. When larvae were offered, usually a single wasp from the colony attacked one larva; only in three cases a second wasp simultaneously flew to the hunting area and in all three cases each wasp attacked a different prey.
A baseline of wasp grooming behavior was established using 68 units of video recording as follows: five-minute video recordings were focused on individuals displaying grooming. Following classical ethological techniques (Lehner 1979; Wagner-Ziemka et al. 2008) grooming behaviors were classified into different types by observing within a sequence what body regions were cleaned and what body parts were used to clean those regions.
Adult and Larvae Wasp Mortality
Adult and larvae wasp mortality was recorded up to 24 days after exposure to the infected prey larvae discriminating by treatment (healthy larvae, larvae infected with M. anisopliae, or larvae infected with B. bassiana). Colonies were labeled for each treatment depending on the prey selected by the wasps the first time: If a single wasp of a colony attacked an infected larva the first time, the colony was used in a second test offering the same pathogen and a healthy larva; thus the mortality results were attributable to a single pathogen in this case. Data were then compared with standard chi-squared tests available in R. As colonies were producing new adults constantly, there was difficult to establish the size of a starting cohort and it requires to mark every single individual increasing the level of stress in the colonies potentially affecting more the behaviors recorded.
Results
Olfactory Selection Tests
The mixed model analysis showed that the use of a colony more than once has no effect on the results (variance = 0.0214, SD = 0.14); and that, overall, there were no differences between treatments (ANOVA, chi-squared = 3.6608, df = 1, P = 0.05). Consequently, the chi-squared analysis of the selection tests with the pathogens presented as powder showed no differences, while tests with larvae suggested a trend towards healthy prey. This trend was significant only in those cases where larvae were infected with M. anisopliae (Table 1) and no difference was observed when both pathogens were present in the infected larvae.
Hunting Selection Tests
Of the 20 colonies tested, six did not attack larvae. Of the three that attacked a healthy larva the first time, one attacked an infected larva the second time, one attacked a healthy larva the second time, and one did not attack a larva the second time. One colony that did not attack larvae the first time, attacked a healthy larva the second time. Consequently, the mixed model analysis indicated that repetitions have no effect on the outcome of the tests (variance = 0.0416, SD = 0.2149). Of the 15 hunting tests with B. bassiana two infected larvae and five healthy larvae were selected. In the tests with M. anisopliae six infected larvae and one healthy larva were selected. However, overall there were no differences between treatments (ANOVA, chi-squared = 0.6656, df = 1, P = 0.41).
Behavioral Responses to Pathogens
Hunting Behaviors
A total of seventeen hunting behaviors were recorded (Table 2), the chao1 index estimated 20 behaviors implying that our sampling covered 85% of the repertoire. A total of 34 grooming behaviors were recorded (Table 3); the chao1 index estimated 34.5 behaviors implying that our sampling covered 98.6% of the repertoire.
The frequency of hunting behaviors for healthy prey (control) showed the following patterns: prey dragging (HE, 19.6%), food ball making (HF, 16.9%), and kneading (HD, 14.2%) accounted for the 50.7% of the total recorded (Fig. 2a). Only antennal waving (HB, 24%), observing the prey (HA, 22.7%) and attacking (HC) were required to reach 61.3% of the total behaviors in wasps exposed to larvae infected with B. bassiana (Fig. 2b). For M. anisopliae, antennal waving (HB, 19%), kneading (HD, 16.5%), observing the prey (HA, 10.1%), and antennal cleaning (HI, 8.9%) summed up 54.4% of the total frequency (Fig. 2c, Supplementary Fig. S1). These differences were statistically significant (Wilks´ lambda = 2.711, df = 20,40, P = 0.004). The relative effects of the analysis indicate that HD, HDG, HE, and HF exhibited higher frequencies in wasps exposed to healthy larvae while other behaviors did not. Kneading (HD) and prey dragging (HE) decreased with larvae infected with B. bassiana (Supplementary Fig. S1). These data described changes related to direct interaction with the prey and not to previous or posterior behaviors.
The duration of separate hunting behaviors showed the following patterns: food ball making (HF, 41.3%) and prey handling II (HDG, 26.8%) made up to 68.1% in wasps exposed to healthy larvae (Fig. 2a). In wasps exposed to B.bassiana 64.8% was reached with food ball making (HF, 29.1%), communal food ball making (HFH, 18.8%) and kneading (HD, 20.1%) (Fig. 2b). Finally, for wasps exposed to M. anisopliae kneading (HD, 43.7%) and food ball making (HF, 13.8%) comprised 57.5% of the time spent (Fig. 2c, Supplementary Fig. S2), which was a similar pattern to that of B. bassiana exposed wasps. Differences between treatments were statistically significant (Wilks´ lambda = 3.003, fd = 20, 40, P = 0.001). The relative effects showed that prey handling II (HDG) and prey dragging (HE) decreased with larvae infected with B. bassiana (Supplementary Fig. S2).
Transitions of hunting behaviors also showed strong differences between the three treatments (Fig. 2). Wasps exposed to healthy larvae displayed 17 behavioral types and a total of 46 transitions representing 16% of the potential events. Wasps exposed to larvae infected with B. bassiana displayed 11 behavioral types and a total of 25 transitions representing 20.7% of the potential events to occur. Wasps exposed to larvae infected with M. anisopliae displayed 12 behavioral types and a total of 37 transitions representing 26% of the potential events to occur. Overall, wasps exposed to healthy larvae displayed the following sequence: observing the prey (HA) followed by antennal waving (HB) (7.0%), then two behaviors showing the same transition probability, attacking (HC) (4.3%) or direct kneading (HD) (4.3%). The latter was followed by food ball making (HF) (8.7%) and this was followed by simultaneous food ball making while performing strong metasoma pumping (HDG) (7.0%) or prey dragging (HE) (5.2%) (Fig. 2a). Wasps exposed to larvae infected with B. bassiana started from observing the prey (HA) to either antennal waving (HB) (13.3%) or antennal cleaning (HI) (1.7%). Antennal waving (HB) was followed by attacking (HC) (8.3%) which could be followed by kneading (HD) (8.3%), prey dragging (HE) (3.3%) or food ball making (HF) (3.3%) (Fig. 2b). Three behavioral loops were recorded: observing the prey (HA) (11.7%); antennal waving (HB) (5.0%); and attacking (HC) (1.7%). The last one was due to a series of pauses that the wasps performed while attacking. Wasps exposed to larvae infected with M. anisopliae, started from observing the prey (HA) followed by either antenna waving (HB) (7.58%) or attacking (HC) (3.03%). Those that proceeded through antennal waving continued with attacking (HC) (3.03%) followed by kneading (HD) (6.06%), then prey dragging (HE) (4.55%), Prey handling II (HDG) (3.03) or food ball making (HF) (3.03) and finally, metasoma movements (HG) (3.03%), antennal cleaning (HI) (1.52%), and abandonment (HX) (7.58%) (Fig. 2c).
Grooming Behaviors
Five grooming behaviors evenly contributed to 48.6% of the frequency of all patterns observed in colonies of wasps that attacked healthy larvae (Fig. 3a). These behaviors were grooming of front legs with the mouth (N, 12.3%), hind legs with the metatibial spurs (M, 9.4%), antennae with front legs (A, 9.4%), genae and front legs with the front legs and mouth (WN, 9.4%), and front legs and one of the midleg with the front legs (VV, 8.1%). In contrast, only three behaviors were required to reach over 50% of the total frequency of activities in colonies of wasps which attacked larvae infected with either B. bassiana (54.3%, Fig. 3b, Supplementary Fig. S3) or M. anisopliae (55.2%, Fig. 3c, Supplementary Fig. S3). These overall differences between treatments were not statistically significant (Wilks’lambda = 1.786, df = 18, 30, P = 0.078). However, the most frequent behaviors differed between treatments. In colonies exposed to B. bassiana grooming of hind legs with the metatibial spurs (M, 18.8%), anterior wings with the anterior legs (L, 18.5%), and grooming of front legs with front legs and mouth (AN, 17%) were the most frequent, while in colonies exposed to M. anisopliae grooming of hind legs with the metatibial spurs (M, 25.1%), grooming of anterior wings with the anterior legs (L, 17%), and grooming of metasoma with hind legs (J, 13.1%) were the most frequent (Fig. 3, Supplementary Fig. S3). The relative effects of the nonparametric Wilks´ lambda analysis concurred with most of this description indicating that A, N, VV, and WN exhibited higher frequencies when exposed to healthy larvae while other behaviors do not. However, grooming of the hind legs with the metatibial spurs (M) was not suggested by this test as a high frequency behavior. Interestingly these behaviors involved cleaning the anterior parts of the body (Fig. 3, Supplementary Fig. S3).
Differences between treatments were detected for the duration of grooming behaviors (Wilks´ lambda = 2.308, fd = 18, 30, P = 0.02). Wasps that selected healthy larvae spent up to 54.4% of the time with the following five behaviors (Fig. 3a, Supplementary Fig. S4): anterior wings with the anterior legs (L, 13.2%); hind legs with the metatibial spurs (M, 11.8%); gena and front legs with the front legs and mouth (WN, 9.9%); grooming of front legs with front legs and mouth (AN, 9.8%); and hind legs and one of the mid legs with metatibial spurs (Q, 9.8%). In wasps exposed to larvae infected with B. bassiana spent 66.3% of the time in the following four behaviors (Fig. 3b, Supplementary Fig. S4): grooming of anterior wings with the anterior legs (L, 23.2%); grooming of front legs with front legs and mouth (AN, 18.3%); hind legs with the metatibial spurs (M, 13.1%); and grooming of hind legs and one of the mid legs with metatibial spurs (Q, 11.7%). In wasps exposed to larvae infected with M. anisopliae 74.5% of grooming time was invested in the following five behaviors (Fig. 3c, Fig. S4): cleaning of anterior wings with the anterior legs (L, 21.4%); hind legs with the metatibial spurs (M, 19.5%); grooming of front legs with front legs and mouth (AN, 15.1%); hind legs and one of the mid legs with metatibial spurs (Q, 11.2%); and grooming of antennae with front legs (A, 7.4%) (Supplementary Fig. S4). These changes concurred with the variation in the relative effects of three behaviors as given by the Wilks´ lambda analysis, although these behaviors were not among those of higher duration for healthy larvae. They showed higher duration for A, N, and VV in wasps exposed to healthy larvae (Supplementary Table S5). Only cleaning the metasoma with hind legs (J) increased in B. bassiana treatment, and cleaning front legs with the mouth (N) was strongly reduced in M. anisopliae treatment (Supplementary Table S5).
The transition for grooming behaviors of wasps exposed to healthy larvae showed very important differences compared to those of wasps exposed to either M. anisopliae or B. bassiana (Fig. 3). Wasps exposed to healthy larvae displayed 31 behavioral types and a total of 374 transitions representing 39% of the 961 potential events. However, only 47 of the observed frequencies showed values above 0.5% representing 13% of observed events (Fig. 3a). Wasps exposed to larvae infected with B. bassiana displayed 29 behavioral types and a total of 191 transitions representing 23% of the 841 potential events to occur. Of these observed 39 showed frequencies above 0.5% and represented 20% of observed events (Fig. 3b). Cleaning of the tip of the front wings with the hind legs (L) transited to grooming of the hind legs with the metatibial spurs (M) or the other way around, and these behaviors reached 10.42% of the events (Fig. 3b). Repeated sequences of behaviors, named behavioral loops, were recorded in four of the five more frequent behaviors. Wasps exposed to larvae infected with M. anisopliae displayed 25 behavioral types and a total of 172 transitions representing 28% of the 625 potential events to occur. However, only 39 of these observed showed frequencies above 0.5% and they represented 23% of observed events. Loops were also observed with grooming of the hind legs with the metatibial spurs (M) as one of the most common behaviors (Fig. 3c).
Survival of Individuals
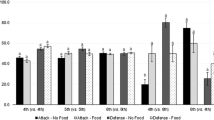
After 24 days of observation of colonies exposed to larvae either healthy or infected with pathogens, the number of dead adults did not differ between treatments (chi-squared = 1.931, df = 2, P = 0.381), while, there were differences in the number of dead wasp larvae (chi-squared = 10.211, df = 2, P = 0.006) (Fig. 1).
Flowcharts of the hunting behaviors observed in Polistes myersi colonies exposed to healthy (control) (a), and infected larvae of Galleria mellonella with B. bassiana (b) and M. anisopliae (c). Behaviors are indicated by the codes provided in Table 2. The size of the boxes indicates the approximate frequency of each behavior expressed as a percentage. The duration of each behavior is expressed by the filling of the boxes. The thickness of the arrows shows frequency of transitions. The exact percentile distribution of the transitions is indicated by the lines, those above and to the right go with the arrowhead, those below and to the left show the transition value against the arrowhead. Transitions below 0.5% are not shown
Flowcharts of the grooming behaviors observed in Polistes myersi colonies exposed to healthy (a) and infected larvae of Galleria mellonella with B. bassiana (b) and M. anisopliae (c). Behaviors are indicated by the codes provided in Table 3. The size of the boxes indicates the approximate frequency of each behavior expressed as a percentage. The duration of each behavior is expressed by the filling of the boxes. The thickness of the arrows shows the higher frequency of the transitions. The exact percentile distribution of a transition is indicated by the lines, those above or to the right go with the arrowhead, those below or to the left show the transition value against the arrowhead. Transitions below 0.5% are not shown. Dashed squares in (a) enclose behaviors classified by the region of the body cleaned
Discussion
Olfactory Selection Test
Overall, foragers of Polistes myersi seemed not to detect the pathogen in its powdered form; however, larvae infected with the pathogens, especially with M. anisopliae, were not preferred over healthy larvae. These differences suggested that, even before physical contact, the forager might detect the pathogen’s metabolic activity or the physiological changes in the infected larvae. Davis et al. (2013) report several types of interactions between microorganisms and insects where compounds produced by the microorganism’s act as semiochemicals for the insects. Vespula pensylvanica and Vespula germanica are attracted to the fungus Aureobasidium pullulans as some of the chemicals it produces may simulate a food source for wasps. They also report that parasitoid wasps preying on bark beetles (Ips pini) use the odor of their symbiotic fungi to locate the prey. Sirex noctilio (Hymenoptera: Siricidae) avoid localities with the odor of the symbiotic fungi of I. pini as they compete for the same resources. Babcock et al. (2019) also report the capacity of V. pensylvanica to detect yeast-produced semiochemicals to locate food sources. Mburu et al. (2009) report that the termite Macrotermes michaelseni (Termitidae) discriminates and avoids the most virulent strains of both B. bassiana and M. anisopliae found in the soil where they forage.
Hunting Selection Tests and Behavioral Changes
The presence of the entomopathogen in prey larvae had significant effects on hunting behavior for Polistes myersi. The number of attacked larvae was reduced by half when they were infected with any of the entomopathogens. The time spent in hunting behaviors also showed important differences; wasps spent 1% of the time displaying surveying behaviors such as observing the prey (HA) and antennal waving (HB). Wasps exposed to larvae infected with either B. bassiana or M. anisopliae increased those exploratory behaviors up to 13.4% and 2.2% respectively. In addition, kneading (HD) reached 7.5% in control tests, while it reached 16.9% when the wasps were attacking B. bassiana infected larvae and 43.7% when exposed to M. anisopliae infected larvae. These changes revealed a reluctance of the wasps towards the use of infected prey and the results agree with studies on other predators such as Anthocoris nemorum (Heteroptera, Anthocoridae) which reject prey infected with pathogens (Meyling and Pell 2006). Likewise, Rännbäck et al. (2015) found that the parasitoid Trybliographa rapae lays more eggs in healthy larvae of Delia radicum than in infected larvae.
Despite the lack of statistical differences for grooming frequencies between the three treatments, data suggest that wasps altered their grooming behavior. When they are in contact with healthy larvae, a richer and more evenly distributed array of behaviors and their transitions are observed. In contrast, wasps exposed to infected larvae concentrated their grooming activities on a few behaviors, especially cleaning the hind legs with metatibial spurs (M), cleaning the apex of wings with the hind legs (L) and cleaning of the antennae and front legs with front legs and mouth (AN).
These differences might imply a response of the wasps for protecting themselves from the pathogen through exocrine gland products or behavioral alterations because of the effects of the pathogen on the nervous system. There is a large body of evidence showing that hymenopterans reduce pathogenic effects through grooming, such as the hygienic behaviors in Apis mellifera that deter the spreading of disease (Arathi et al. 2000; Masterman et al. 2001; Cheruiyot et al. 2018). In ants, antimicrobial compounds are produced with their metapleural glands. For example, in Solenopsis invicta spray alkaloids protect the brood and nestmates against fungi and other pathogens (Oi and Pereira 1993). Poulsen et al. (2003) describe how leaf cutting ants deter microorganisms through focused grooming, spreading compounds present in their metapleural gland. To our knowledge this is the first report where detailed records of behavioral changes have been analyzed when a predator faces an immunological challenge.
Wasp Mortality
Exposure of wasp colonies to B. bassiana and M. anisopliae, prepared at the commercially recommended concentrations, did not impact the mortality of adults but did affect larvae. Even though the cause of death of adults and larvae was not identified, the trends observed concur with the expectations. In addition, these results agree with Espinosa-Ortiz et al. (2011) who report that larvae of Apis mellifera are more susceptible than adult bees. Similarly, Lord (2001) finds that adults of Cephalonomia tarsalis (Hymenoptera: Bethylidae) experience lower mortality (68.6%) when exposed to beetle hosts infected with B. bassiana, while none of the wasp larvae complete development in such hosts. However, other experiments report different results. For example, Harris et al. (2000), studying V. vulgaris, show no differences in mortality between larvae and adults.
The generality of these mortality results must be handled with caution as both the pathogen strain and the application method have profound effects on the results. Espinosa-Ortiz et al. (2011), for example, report mortalities between 12 and 100% for adult honey bees depending on the strain of B. bassiana applied. Likewise, Harris et al. (2000) report changes in larvae survival and adult longevity of V. vulgaris depending on the strain used of B. bassiana or Aspergillus flavus. Poidatz et al. (2018) compare different infection methods on adults of Vespa velutina and report that direct immersion is more efficient than other approaches, including consumption of cooked tuna infected with spores of either B. bassiana or Metarhizium robertsii. Harris et al. (2000) observe that adult longevity of V. vulgaris is reduced if infection occurs through dipping, compared to other infection strategies. These studies on vespids were designed to test methods of controlling predator species that have become a nuisance to human populations or ecosystem invaders, while our goal, like Espinosa-Ortiz et al. (2011), was to understand the effects of accidental infection by entomopathogens of a species that naturally exhibits positive benefits to crops. Thus, our protocol was closer to the field conditions experienced by these predators and may have provided a better picture of field conditions. Considering both the wasp’s attack preferences and larvae mortality, M. anisopliae appeared as a less damaging entomopathogen for the generalist predator than B. bassiana.
The consequences of our study towards the use of entomopathogens for biocontrol heavily rely on their field persistence (Thompson et al. 2006). Castrillo et al. (2010) report a persistence of up to 28 days for B. bassiana conidia, while Vänninen et al. (2000) report a three-year persistence for M. anisopliae and at least a year for B. bassiana. In our experiments, every colony was exposed to a single infection event and even though larvae mortality increased immediately after the infection, no colony died; and they remained actively producing new individuals for six weeks up to when they were sacrificed. These data suggested that the two entomopathogens may have had a temporary effect on the populations and that these populations might be at risk if a pathogen is used on a regular basis. Field testing is required to assess these predictions.
Our study is in line with previous analyses that document the capacity of social insects to detect and deter pathogens, and in our case these pathogens also change the behavior of Polistes myersi. Our results pointed towards a non-target effect case for a generalist predator. However, the detection and survival depend on the pathogen; foragers rejected larvae infected with M. anisopliae and their colonies recorded lower larvae mortality compared with B. bassiana. Our results showed no capacity to detect larvae infected with B. bassiana, and consequently higher larvae mortality was recorded in their colonies. Even though time invested in grooming is similar for both pathogens, strong differences were recorded for the frequency and duration of each behavioral repertoire. These results pointed towards a pathogen, B. bassiana, that passes undetected, but the latter negatively affects the colony while the other pathogen, M. anisopliae, is readily detected and consequently has weaker effects on a colony’s survival. This study provided useful information for developing sustainable pest control, as native wasp species can be fundamental for controlling herbivore populations.
Data Availability
Authors are willing to provide data if required.
Code Availability
Not applicable.
References
Andrade FR, Prezoto F (2001) Horários de atividade forrageadora e material coletado por Polistes ferreri Saussure, 1853 (Hymenoptera, Vespidae), nas diferentes fases de seu ciclo biológico. Rev Bras Zool 3:117–128
Arathi HS, Burns I, Spivak M (2000) Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): behavioural repertoire of hygienic bees. Ethology 106:365–379
Babcock T, Borden JH, Gries R, Carroll C, Lafontaine JP, Moore M, Gries G (2019) Inter–kingdom signaling symbiotic yeasts produce semiochemicals that attract their yellowjacket hosts. Entomol Exp Appl 167:220–230
Baracchi D, Mazza G, Turillazzi S (2012) From individual to collective immunity: the role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J Insect Physiol 58:188–193
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Casida JE, Durkin KA (2013) Neuroactive insecticides: targets. Selectivity, Resistance, and Secondary Effects Annu Rev Entomol 58:99–117
Castrillo LA, Griggs MH, Liu H, Bauer LS, Vandenberg JD (2010) Assessing deposition and persistence of Beauveria bassiana GHA (Ascomycota: Hypocreales) applied for control of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), in a commercial tree nursery. Biol Control 54:61–67
Cheruiyot SK, Lattorff HMG, Kahuthia-Gathu R, Mbugi JP, Muli E (2018) Varroa-specific hygienic behavior of Apis mellifera scutellata in Kenya. Apidologie 49:439–449
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9.1.0. Persistent purl.oclc.org/estimates
Cruz JF, Durán T, Navarro V, Fierro J, Pardo D (2017) Field evaluation of Beauveria bassiana as biological control of ticks Rhipicephalus microplus in Colombia. Thünen Report 54:564–567
Danfa A, Van der Valk HCHG (1999) Laboratory testing of Metarhizium spp. and Beauveria bassiana on Sahelian non-target arthropods. Biocontrol Sci Techn 9:187–198
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859
de Conceição JP, de Lyra Neves CM, da Silva Sodré G, de Carvalho CAL, Souza AV, Ribeiro GS, de Campos Pereira R (2014) Susceptibility of Melipona scutellaris Latreille, 1811 (Hymenoptera: Apidae) worker bees to Beauveria bassiana (Bals.). Vuill Sociobiology 61:184–188
Elisei T, Vaz J, Junior CR, Junior AJF, Prezoto F (2011) Uso da vespa social Polistes versicolor no controle de desfolhadores de eucalipto. Pesqui Agropecu Bras 45:958–964
Ellis AR, Burchett WW, Harrar SW, Bathke AC (2017) Nonparametric inference for multivariate data: the R package npmv. J Stat Softw 76:1–18
Elmquist DC, Landolt PJ (2018) Associative learning of food odors by the European paper wasp, Polistes dominulus Christ (Hymenoptera, Vespidae). Environ Entomol 47:960–968
Espinosa-Ortiz E, Lara-Reyna J, Otero-Colina G, Alatorre-Rosas R, Valdez-Carrasco J (2011) Susceptibilidad de larvas, pupas y abejas adultas a aislamientos de Beauveria bassiana (Bals.) Vuill., Metarhizium anisopliae (Sorokin) y Paecilomyces fumosoroseus (Wize). Interciencia 36:148–152
Fox J, Weisberg S (2019) An R Companion to Applied Regression. 3rd edn. Sage, Thousand Oaks CA. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Harris RJ, Harcourt SJ, Glare TR, Rose EAF, Nelson TJ (2000) Susceptibility of Vespula vulgaris (Hymenoptera: Vespidae) to generalist entomopathogenic fungi and their potential for wasp control. J Invertebr Pathol 75:251–258
Hokkanen HMT, Zeng QQ, Menzler-Hokkanen I (2003) Assessing the impacts of Metarhizium and Beauveria on bumblebees. In: Hokkanen HMT, Hajek AE (eds) Environmental impacts of microbial insecticides. Springer, Dordrecht, pp 63–71
Joop G, Vilcinskas A (2016) Coevolution of parasitic fungi and insect hosts. Zoology 119:350–358
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: Back to the future. J Inv Path 132:1–41
Landolt PJ, Cha DH, Werle CT, Adamczyk JJ, Meagher RL, Gilbride RL, Clepper TS, Reed HC, Teal PEA, Sampson BJ (2014) Polistes spp. (Hymenoptera: Vespidae) orientation to wine and vinegar. Fla Entomol 97:1620–1630
Van Leeuwen, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Ins Biochem Mol Biol 40: 563–572
Lehner PN (1979) Handbook of ethological methods. Garland STPM Press, New York
Liu C, Bathke AC, Harrar SW (2011) A nonparametric version of Wilks’ lambda asymptotic results and small sample approximations. Stat Probabil Let 81:1502–1506
Lord JC (2001) Response of the wasp Cephalonomia tarsalis (Hymenoptera: Bethylidae) to Beauveria bassiana (Hyphomycetes: Moniliales) as free conidia or infection in its host, the Sawtoothed grain beetle, Oryzaephilus surinamensis (Coleoptera: Silvanidae). Biol Control 21:300–304
Mackenzie JK, Landolt PJ, Richard SZ (2008) Sex attraction in Polistes dominulus (Christ) demonstrated using olfactometers and morphological source extracts. J Entomol Soc Brit Columbia 105:35–44
Madden AA, Grassetti A, Soriano JN, Starks PT (2013) Actinomycetes with antimicrobial activity isolated from paper wasp (Hymenoptera: Vespidae: Polistinae) nests. Environ Entomology 42:703–710
Manfredini F, Dallai R, Ottaviani E (2008) Circulating hemocytes from larvae of the paper wasp Polistes dominulus (Hymenoptera, Vespidae). Tissue Cell 40:103–112
Masterman R, Ross R, Mesce K, Spivak M (2001) Olfactory and behavioral response thresholds to odors of diseased brood differ between hygienic and non-hygienic honey bees (Apis mellifera L.). J Comp Physiol A 187:441–452
Mburu DM, Ochola L, Maniania NK, Njagi PGN, Gitonga LM, Ndung’u MW, Hassanali A (2009) Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J Insect Physiol 55:774–780
Meikle M, Nansen G (2007) Duration and spread of an entomopathogenic fungus, Beauveria bassiana (Deuteromycota: Hyphomycetes), used to treat varroa mites (Acari: Varroidae) in honey bee (Hymenoptera: Apidae) hives. J Econ Entomol 100:1–10
Mesquita ALM, Lacey LA (2001) Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol Control 22:51–59
Meyling NV, Pell JK (2006) Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol Entomol 31:162–171
Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T (2010) Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 76:310–317
Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A (2005) Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73:3842–3850
Oi DH, Pereira RM (1993) Ant behavior and microbial pathogens (Hymenoptera: Formicidae). Fla Entomol 76:63–74
Otterstatter MC, Thomson JD (2017) Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS One 3:e2771
Poidatz J, Plantey RL, Thiéry D (2018) Indigenous strains of Beauveria and Metharizium as potential biological control agents against the invasive hornet Vespa velutina. J Invertebr Pathol 153:180–185
Poulsen M, Bot AN, Boomsma JJ (2003) The effect of metapleural gland secretion on the growth of a mutualistic bacterium on the cuticle of leaf-cutting ants. Naturwissenschaften 90:406–409
Prior C (1997) Susceptibility of target acridoids and non-target organisms to Metarhizium anisopliae and M. flavoviride, in: Krall, S., Peveling, R., and Diallo, B. D. (ed.), New strategies in locust control. Birkhäuser Basel, pp. 369–375
Rännbäck LM, Cotes B, Anderson P, Rämert B, Meyling NV (2015) Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J Invertebr Pathol 124:78–86
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357
Sadd BM, Barribeau SM (2013) Heterogeneity in infection outcome: lessons from a bumblebee–trypanosome system. Parasite Immunol 35:339–349
Sarmiento C (1997) Véspidos de Colombia (Hymenoptera: Vespidae) (Tesis dissertation, M. Sc. Universidad Nacional de Colombia, Instituto de Ciencias Naturales, Bogotá)
Sumana A, Starks PT (2004) Grooming patterns in the primitively eusocial wasp Polistes dominulus. Ethology 110:825–833
Thompson SR, Brandenburg RL, Arends JJ (2006) Impact of moisture and UV degradation on Beauveria bassiana (Balsamo) Vuillemin conidial viability in turfgrass. Biol Control 39:401–407
Vänninen I, Tyni-Juslin J, Hokkanen H (2000) Persistence of augmented Metarhizium anisopliae and Beauveria bassiana in Finnish agricultural soils. Biocontrol 45:201–222
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wagner-Ziemka A, Gonzalez-Szwacka A, Korczynska J, Kieruzel M, Fialkowska B, Godzinska EJ (2008) Comparison of the behavior of nurses and foragers of the carpenter ant, Camponotus melanocnemis, during dyadic Nestmate Reunion tests carried out after a period of social isolation (Hymenoptera: Formicidae). Sociobiology 52:667–702
Acknowledgements
The authors thank Juan José Lagos Oviedo, Andrea Carvajal, Angie Zuleidi Amézquita, André Rodríguez, and Angela Amarillo for help with field and colony management. Authors also thank Casa Buenos Aires for lodging and to the Universidad Nacional de Colombia for funding this study.
Funding
This project was founded by the Vicerrectoría of the Universidad Nacional de Colombia grant number Hermes 34846.
Author information
Authors and Affiliations
Contributions
DM-Ch.: conceptualization, data curation, formal analysis, research, methodology, validation, visualization, writing - original draft, writing - review and editing. NCCC: Colony maintenance and data curation. CR: conceptualization, data curation, formal analysis, funding acquisition, research, methodology. CES: conceptualization, formal analysis, Funding acquisition, methodology, project administration, resources, supervision, visualization, writing - original draft, writing - review and editing.
Corresponding author
Ethics declarations
Ethics Approval
All applicable national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain studies with human participants.
Consent to Participate
Not applicable.
Consent for Publication
All authors agree.
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mayorga-Ch, D., Castro-Cortés, N.C., Rodríguez, C. et al. Behavioral Responses of the Social Wasp Polistes myersi to Prey Infected with Fungi Used in Biological Control. J Insect Behav 34, 136–149 (2021). https://doi.org/10.1007/s10905-021-09775-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-021-09775-z