Abstract
Although mate preferences are most commonly examined in females, they are often found in both sexes. In the parasitoid wasp Urolepis rufipes, both female and male mating status affected certain aspects of sexual interactions. Female mating status mattered only in the later stages of mating. Males did not discriminate between virgin and mated females in terms of which they contacted or mounted first. However, once mounted, most virgin females were receptive to copulation, whereas very few mated females were. Whether a male’s mating status affected his own sexual response depended on the female’s ability to respond and the stage of mating. Examining male behavior toward dead females allowed elimination of the role of female behavior in how males responded. Virgin and mated males are both attracted to dead females as evidenced by their fanning their wings at such females. However, mated males were quicker than virgin males to contact and to mount in an experiment with dead females, whereas there was no such differential response in an experiment with live females. This difference is consistent with greater female sexual responsiveness to virgin males. Male mating status also affected female receptivity to copulate. Once mounted, live virgin females were less likely to become receptive to copulation by mated males than to virgin males, but only in a choice experiment, not in a no-choice experiment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mate preference is a differential sexual response to different types of reproductively mature conspecifics of the opposite sex (Bonduriansky 2001). Preference need not involve conscious choice and does not mean that the preferring sex is in complete control of whether mating occurs (Halliday 1983; King et al. 2005). One of the traits that males and females often prefer, besides larger size, is virginity (Bonduriansky 2001; Gaskett 2007; Wang et al. 2016; Avila et al. 2017). In addition to a particular mating status being a preferred trait in a prospective mate, an individual’s own mating status can influence its sexual response (Ortigosa and Rowe 2003; Judge et al. 2010; Ah-King and Gowaty 2016). Willingness to mate or remate and response to a potential mate’s mating status may vary at different stages of the mating process, e.g., at approach, mounting, courting or copulation.
The present study examines effects of mating status on sexual interactions at different stages of the mating process in the solitary parasitoid wasp Urolepis rufipes (Hymenoptera: Pteromalidae). By definition, solitary parasitoid wasps are those in which usually only one wasp develops per host, as opposed to gregarious species, where multiple wasps emerge from a single host. Solitary parasitoid wasps are one of only a few insect groups in which females are usually monandrous, i.e., mate only once (Ridley 1993). Males are typically polygynous in parasitoid wasps. Urolepis is of particular interest because it is a close relative of Nasonia (McAllister and Werren 1997). Nasonia’s behavior and genetics have been so well-studied that it has been called the Drosophila of the Hymenoptera (Pultz and Leaf 2003). Studies on the behavior of closely related species will facilitate future tests of the relative influence of current adaptation versus phylogenetic history on these behaviors.
Sexual responsiveness that varies with an individual’s own mating status may be adaptive. Sexual responsiveness can include releasing stimuli that attract or arrest the opposite sex; approaching or not fleeing the opposite sex; mounting and courting, or facilitating mounting and courting, e.g., by being still; providing stimuli that elicit genital opening or aedeagus protrusion and hence copulation; and releasing, or accepting and using, sperm and ejaculate. A lack of sexual responsiveness decreases the chance that successful mating occurs.
An already mated female may benefit from not remating if she gets sufficient sperm from her first mating and if remating would reduce her survival (Arnqvist and Nilsson 2000; McNamara et al. 2008), e.g., by compromising her immune functions (Fedorka and Zuk 2005; Gershman 2008), increasing predation on her (Kemp 2012), or reducing her time for other activities. On the other hand, a female may benefit from remating if her mate provides a limited resource, e.g., food, water or sperm. In most parasitoid wasps, including U. rufipes, males do not provide an obvious nuptial gift or paternal care (Godfray 1994). There is an effect of mating on longevity and/or fecundity in a subset of parasitoid wasp studies (King 2002 review; Reumer et al. 2007; Santolamazza-Carbone and Pestana 2010), but the effect may not be present in all studies of even a single species, e.g., in N. vitripennis (Geuverink et al. 2009; Boulton and Shuker 2015).
A mated male may benefit less than a virgin male from mating, particularly if the opportunity to remate is soon after the first mating. Temporary unresponsiveness by a mated male may provide time and energy for sperm or seminal fluids to replenish (Gerling and Legner 1968). Temporary unresponsiveness may also prevent his attempting to remate a female that he just mated, by providing time during which she may leave (Fischer and King 2008). Nevertheless, mated males often continue mating even when they are sperm-limited (Steiner et al. 2008; Boivin 2013; Chirault et al. 2016). Doing so may reduce the competition that a male’s daughters face if such mating reduces the female’s reproductive success with other males.
There may also be selection for sexual responsiveness to vary with the mating status of potential mates, e.g., for both females and males to prefer virgin mates (Bonduriansky 2001; Wedell et al. 2002). From a female’s perspective, a virgin male may transfer more sperm and other ejaculate components than an already mated male (e.g., Gerofotis et al. 2015; Chirault et al. 2016), although this is not always the case (Fischer and King 2008; Bressac et al. 2009). A lack of sperm does not prevent females from reproducing in wasps and other haplodiploid arthropods, because sons develop asexually from unfertilized eggs, whereas daughters develop sexually from fertilized eggs. But effects of mating on sex ratio may be important even if fecundity is unaffected. Parasitoid wasps often produce female-biased sex ratios (Clausen 1939; Hamilton 1967; Heimpel and Lundgren 2000), which appear to be adaptive (Hamilton 1967; Hardy 1994). Thus, if a female lacks sufficient sperm, she may be constrained to produce a sex ratio that is more male-biased than is optimal (Charnov 1982; West 2009). Males also may benefit from preferring virgins, because virgin females have more unfertilized eggs. For both sexes, mating with a virgin avoids the risk of acquiring a sexually transmitted disease. The benefit of mating with a virgin may depend on what alternative mates are available, because mating with a nonpreferred individual may be preferable to not mating at all (Dougherty and Shuker 2014).
If mating frequency is an adaptation, then a species being monandrous suggests that, at least recently, there has been little to no net benefit for females that remate or to males that overcome female resistance to remating. Under monandry, mating with a good quality male, e.g., a virgin male, is particularly important because he is the sole source of sperm. Once female reluctance to mate more than once begins to evolve, this will in turn decrease the benefit to males of attempting to mate with already mated females, by decreasing his chance of success and/or by increasing the time and energy needed for success.
The present study examines whether males preferentially mate with virgin over mated females when given a choice, whether females preferentially mate with virgin over mated males in both a choice and a no choice test, and the role of female behavior in female mate preference. Examining the role of female behavior is possible in U. rufipes because males respond sexually to dead females.
Materials and Methods
Biology of Urolepis rufipes
Urolepis rufipes Ashmead is polygynous and highly-monandrous. It parasitizes the pupae of brine flies along shore lines and the pupae of house flies and stable flies in and on decaying organic matter or manure (Rueda and Axtell 1985; Gibson and Floate 2004; Noronha et al. 2007). Offspring sex ratios are about 2.5 females per male, i.e., 71% female (Matthews and Petersen 1990), and males emerge before females and are generally smaller (Powell et al. 2003; personal observation). Males and females noticeably respond to each other only at fairly close range, a couple of wasp lengths (King and Kuban 2012; Cooper et al. 2013). In most cases, the response begins with the male running after the female as his antennae move up and down and/or he briefly fans his wings. Sometimes the female appears to have initiated the interaction by approaching him. Once the male contacts the female and begins to mount her dorsally, she stops walking. The pair then interact using their antennae and mouthparts. They copulate only if she opens her genital orifice, although occasionally he attempts copulation even when she has not opened.
In some confamilials, mating takes place at the natal site prior to females dispersing in search of hosts (Nasonia: King 1993; Leonard and Boake 2006; Grillenberger et al. 2009; Steiner and Ruther 2009; Spalangia endius: King 2002). However, in U. rufipes and some of these confamilials, both sexes can fly, and males may also encounter females at host sites. In the confamilial S. cameroni, males are attracted to host and host environment odors (Myint and Walter 1990).
Insect Rearing
The U. rufipes were a Canadian strain that originated from cattle feedlots in southern Alberta (K. Floate personal communication). The wasps have been maintained on Musca domestica pupae. The M. domestica were reared as described in King et al. (2014). Wasps were reared at approximately 25 °C with a photoperiod of 12 h light: 12 h dark. Parasitized host pupae were individually isolated in glass test tubes prior to the wasps’ emergence in order to obtain virgin wasps. Wasps used in experiments were 0–1 d old and had received a drop of honey prior to testing.
General Methods of Bioassays
Each experiment compared two treatments, virgin versus mated; but the experiments varied in whether a virgin of the opposite sex had a choice of these two treatments or no choice. In no choice experiments, the two treatments (virgin versus mated) were run in temporal pairs (i.e., performed simultaneously or nearly so) with wasps in a pair matched for age to the nearest day and visually matched for size. Wasps were assigned to treatments randomly. Mated wasps had mated only once, in a test tube. Wasps were never tested with a wasp with which they had previously mated. When both a virgin and a mated individual were presented simultaneously, a separate observer kept track of each.
Tests were performed in sand dishes, plastic dishes about half full of sand that had been dampened to keep humidity high, which reduces static. Dishes were 5.7 cm diameter, 1 cm height, except where noted, with a glass cover. Clean containers and sand were used for each test. The behaviors recorded were contact, mount and copulation. Tests were terminated at 10 min if the male had not copulated with one of the females by then.
Male Choice: Virgin and Mated Female with a Virgin Male
A virgin male was tapped from his test tube into a dish containing a virgin female and a mated female (n = 46). Which female had been added first to the dish was alternated. Mated females had mated within about 5 min of testing. Which female the male first contacted, first mounted and first copulated with were recorded, as well as when the first mount and first copulation occurred.
Female Choice: Virgin and Mated Male with a Live Virgin Female
This experiment was the same as the previous one except that females replaced the males and vice versa (n = 39). Mated males had mated within 5 min of testing. Which male was first to contact the female, which was first to mount and which was first to copulate were recorded, as well as when the first mount and first copulation occurred.
Female no Choice: Virgin or Mated Male with a Live Virgin Female
This experiment was the same as the previous one except that the virgin female was not given a choice between the two treatments (virgin, mated). Instead, the two types of males (mated, virgin) were each in a separate dish with a virgin female. Observations of the pair of dishes (n = 19) were simultaneous by two different observers. Each observer recorded the duration until her male first contacted the female, first mounted, and first attempted copulation. Which treatment each observer collected data from was alternated.
Virgin or Mated Male with a Dead Female
This experiment was similar to the previous one except that the female was dead, allowing examination of how a male’s mating status affects his behavior independent of female response to it. We also used a smaller dish (1.5 cm diameter, 1 cm height) than in the previous experiments because in preliminary trials with a larger dish, males were not contacting the dead female within 10 min. Prior to placing a mated male (n = 27) or a virgin male (n = 27) into a dish, a freeze killed female was placed dorsal side up near the edge of the dish away from the male. Freeze killed females were frozen for 5 min in a -86 °C freezer and then left at room temperature (23 °C ± 2 °C) for at least 2 min before being used in the experiment.
Data Analyses
All statistical tests were two-tailed. Tests of independence of categorical data were by G tests, which are also called likelihood ratio chi square tests. Each mean is presented with its standard error, minimum and maximum. Means were compared with t-tests, except when the assumption of normality was violated strongly (P < 0.001), in which case a Mann-Whitney U test was used.
Virgin and mated treatments were temporally paired even in the no choice experiment; however, independent sample, rather than paired, tests were used because there were no significant correlations of pairs, e.g., between duration to mount for a virgin versus for the mated individual that was tested at the same time.
In the Female No Choice experiments, duration until first contact, first mount, and first attempted copulation were each compared between the virgin and mated treatments with survival analysis, specifically Cox’s regression. Survival analysis accounts for the possibility that if allowed a longer testing period, some behaviors that did not occur within the 10 min test period eventually would (reviewed in van Alphen et al. 2003). Within each experiment, alpha was set at 0.05 for each stage of mating (contact, mount, and copulation) for each response variable (e.g., which individual was first, duration until a mating stage). Alpha was controlled separately for each stage because mating status can have different effects at different stages (e.g., King et al. 2005). Where sample sizes differ for different behaviors within an experiment, a particular behavior was absent or unobservable for some replicates.
In comparing mated treatments to virgin treatments, one concern is that not all individuals assigned to the mated treatment then mate, in which case mated individuals, but not virgins, represent a filtered group, a self-selected group, one that excludes uneager or inactive individuals. Such a filtering effect would result in greater “eagerness” or quickness in the mated than the virgin treatment. Our results, however, were largely in the opposite direction, in which case our reported effects may be underestimates. The exception was in the dead female experiment; but even in this experiment, conclusions were unaffected by omitting the 11% least eager virgins (i.e., those slowest to contact the female) to counterbalance the exclusion of that 11% of males that had been assigned to the mated treatment but failed to mate and so were not used.
Results
Male Choice: Virgin and Mated Female with a Live Virgin Male
Males showed no preference for virgin females versus mated females in terms of which female they first contacted and mounted (Table 1). When the female was virgin, the male’s first mount was after 57.84 ± 9.84 s, 18–175 s, n = 19; whereas when the female was mated, the male’s first mount was after 65.25 ± 11.45 s, 9–207 s, n = 24 (t = 0.48, df = 41, P = 0.64). Among females that copulated during the 10 min test, when the virgin female was the first to copulate, it was after 173.47 ± 23.17 s, 20–547 s, n = 36; and when the mated female was the first, copulation was after 236.50 ± 93.50 s, 143–330 s, n = 2 (Mann-Whitney U = 43.00, P = 0.48).
Whether the male mounted the virgin or the mated female first depended on which he contacted first (X21 = 48.08, P < 0.001). Males almost always first mounted the female that they first contacted: 90% of males that contacted the virgin first (n = 21) then mounted her; and 100% of males that contacted the mated female first (n = 24) then mounted her.
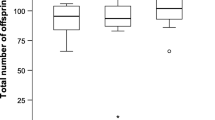
Whether the first female that was mounted was receptive (opened her genital orifice) depended on her mating status: 84% of virgin females that were mounted first were receptive, whereas only 7% of mated females that were mounted first were receptive (Table 2). As a result, most males copulated with the virgin female first regardless of whether they had first mounted the virgin female or the mated female. Specifically, 94% that mounted the virgin first proceeded to copulate with her (n = 18); likewise, 95% that mounted the mated female first then copulated first with the virgin (n = 20). Males that mounted the virgin female first then copulated sooner than males that first mounted the mated female (Fig. 1).
Female Choice: Virgin and Mated Male with a Live Virgin Female
Females showed no preference for virgin males versus mated males in terms of which male first contacted and first mounted (Table 3). The first mount occurred after 47.66 ± 10.28 s, 11–147 s, n = 13 when it was by the virgin male and after 69.19 ± 12.01 s, 1–244 s, n = 23 when it was by the mated male (t = 1.21, df = 34, P = 0.24).
Once mounted, the female was more likely to become receptive if the first male to mount was virgin than if he was mated (Table 4). Similarly, looking just at females that copulated within the 10 min trial, time from mounting to copulation was quicker when the female had been first mounted by the virgin male versus by the mated male (Fig. 2). Two of the three longest durations from mounting to copulation were the only cases in which the first copulation was with a different male than the male that first mounted; the mated male was first to mount, but the copulation was with the virgin male. However, the duration was still greater when those cases were excluded (Mann-Whitney U = 42.50, P = 0.03). The females that did not copulate within the 10 min trial had all been mounted first by a mated male. Because of females being more receptive to virgin males than mated males, females that were mounted first by the virgin male were more likely to copulate within 10 min than females that were mounted first by the mated male (100%, n = 15 versus 73%, n = 22; X21 = 7.02, P = 0.008).
In 28% of trials (11 of 39), there were two males mounted on the female at some point, i.e., double mounts. We use the term “direct male” to refer to the male that was directly on the female (i.e., had the most contact with her dorsal surface), and we use the term “indirect male” to refer to the male that mounted the direct male. In 5 of 11 cases both males copulated. Among the 8 cases in which the female copulated at least once, the first or only male to copulate was the direct male in 7 cases and the indirect male in 1 case (binomial test 2-tailed P = 0.07). In the 3 cases where the female did not copulate at least once, it was because the female left in the ensuing jumble. In addition to cases of two males on a female, in another 2 cases, a male mounted another male, but neither male was mounted on the female.
Female no Choice: Virgin or Mated Male with a Live Virgin Female
When a live virgin female was with only one type of male, virgin males were not significantly quicker than mated males in their first contact, mount, or copulation (Table 5). In contrast to the female choice experiment, virgin females were no more likely to be receptive after being mounted when the male was virgin than when he was already mated (44%, n = 18 versus 50%, n = 18; X21 = 0.11, df = 1, P = 0.74).
Virgin or Mated Male with a Dead Female
Mated males were significantly quicker than virgin males in duration until their first contact and first mount of the dead female, by about 2 min; but mated males were not quicker in their first attempt to copulate (Table 5; Fig. 3). Male mating status had no significant effect on the duration from contact to mounting (Mann-Whitney U = 277.5, P = 0.13), and the pattern for duration until contact was similar to that shown for mounting in Fig. 3. After mounting, mated males were no more likely than virgin males to attempt copulation within the duration of the trial (77%, n = 26, versus 85%, n = 20; X21 = 0.48, df = 1, P = 0.49).
Discussion
Sexual interactions of U. rufipes were affected by both female and male mating status at several stages of mating. The effect of female status was that virgin and mated females were contacted and mounted about equally by males. However, after being mounted, virgin females were usually receptive to copulation, whereas mated females seldom were (84% versus 7%). The effect of male status was that mounted virgin-females were more likely to become receptive to copulation by a virgin male than by a mated male in the choice experiment, although not in the no choice experiment. This last result is consistent with a metaanalysis by Dougherty and Shuker (2014), which found that female mating preferences are generally stronger in choice experiments than in no-choice experiments. Female behavior may differ in choice versus no choice situations. In addition, choice tests allow interactions between males, including interference of one male by another. Male-male interactions in U. rufipes include lunging at and chasing each other before mounting (Cooper and King 2015), as well as attempts to mount, stay on, and copulate with a female that is already mounted by another male (King and Kuban 2012).
Male-male physical interactions cannot explain all aspects of female preference for virgin males. Females also prefer substrate-borne pheromone marks by males that have mated fewer times (Wittman 2016), as is also seen in N. vitripennis (Ruther et al. 2009). The lack of a preference for the virgin male in the female no-choice experiments may be adaptive; the benefits of mating with a previously mated male may outweigh the costs of not mating at all. In the presence of choice, female U. rufipes may benefit from preferentially copulating with a virgin male, at least relative to a male that has just mated four times; a female mated to a four-time-mated male produces a substantially lower proportion of daughters (Wittman 2016). Whether females benefit with once-mated males remains to be seen. It also remains to be seen whether females discriminate against males that mated longer ago.
There are at least two explanations for why males readily chased, mounted and courted already mated females even though mated females rarely become receptive again (King and Kuban 2012). Perhaps males are not time- or energy-limited, in which case failed attempts are inconsequential; or perhaps male encounters with mated females are infrequent, e.g., if females usually disperse after mating.
Although male mating status had no effect on first contact and mounting when a male was with a live female, there was an effect with a dead female, i.e., when female response was held constant. With dead females, mated males were quicker than virgin males to contact and mount. Mated males may have walked more directly to the female or more quickly (King and Owen 2012). There may be a rewarding aspect to mating which mated males learn to associate with female pheromones. Mates are an effective reward for associative learning in the confamilial N. vitripennis (Baeder and King 2004). Among confamilials, whether having recently mated increases or decreases quickness of male sexual response varies. For example, there is no effect in N. vitripennis (King, unpublished data), and the effect is opposite that of U. rufipes in S. endius (King et al. 2005). That U. rufipes mated males were quicker than virgin males to contact and mount in the experiment with dead females but not in the experiment with live females would also be consistent with greater female sexual responsiveness to virgin males.
Across insects, when mating status affects sexual interactions, there tends to be a preference by males and by females for virgins (Bonduriansky 2001; Wedell et al. 2002) and greater sexual responsiveness by virgins, even in polyandrous and polygynous species (Ortigosa and Rowe 2003; Judge et al. 2010). However, there are cases where males prefer already mated females (Salehialavi et al. 2011) and where neither males or females prefer virgin or mated individuals as mates in choice or in no-choice tests (Cheng et al. 2004). The effects of mating status may depend on factors such as the stage of mating (e.g., Brent 2010; the present study), duration since the previous mating (Kant et al. 2012), and the number of previous matings (Fleischman and Sakaluk 2004; Steiner et al. 2008). U. rufipes was fairly typical in that when there was a quicker response, or a greater percent responding, it was by or with virgins, and for both males and females. The exception was that recently mated males were quicker than virgin males to contact and mount dead females.
References
Ah-King M, Gowaty PA (2016) A conceptual review of mate choice: stochastic demography, within-sex phenotypic plasticity, and individual flexibility. Ecol Evol 6:4607–4642
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Avila GA, Withers TM, Holwell GI (2017) Courtship and mating behaviour in the parasitoid wasp Cotesia urabae (hymenoptera: Braconidae): mate location and the influence of competition and body size on male mating success. Bull Entomol Res 107:439–447
Baeder JM, King BH (2004) Associative learning of color by males of the parasitoid wasp Nasonia vitripennis (hymenoptera: Pteromalidae). J Insect Behav 17:201–213
Boivin G (2013) Sperm as a limiting factor in mating success in hymenoptera parasitoids. Entomol Exp Appl 146:149–155
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Boulton RA, Shuker DM (2015) The costs and benefits of multiple mating in a mostly monandrous wasp. Evolution 69:939–949. https://doi.org/10.1111/evo.12636
Brent CS (2010) Reproduction of the western tarnished plant bug, Lygus hesperus, in relation to age, gonadal activity and mating status. J Insect Physiol 56:28–34
Bressac C, Thi Khanh HD, Chevrier C (2009) Effects of age and repeated mating on male sperm supply and paternity in a parasitoid wasp. Entomol Exp Appl 130:207–213
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Cheng LI, Howard RW, Campbell JF, Charlton RE, Nechols JR, Ramaswamy SB (2004) Mating behavior of Cephalonomia tarsalis (Ashmead) (hymenoptera: Bethylidae) and the effect of female mating frequency on offspring production. J Insect Behav 17:227–245
Chirault M, Van de Zande L, Hidalgo K, Chevrier C, Bressac C, Lecureuil C (2016) The spatio-temporal partitioning of sperm by males of the prospermatogenic parasitoid Nasonia vitripennis is in line with its gregarious lifestyle. J Insect Physiol 91-92:10–17
Clausen CP (1939) The effect of host size upon the sex ratio of hymenopterous parasites and its relation to methods of rearing and colonization. J N Y Entomol Soc 47:1–9
Cooper JL, King BH (2015) Substrate-borne marking in the parasitoid wasp Urolepis rufipes (hymenoptera: Pteromalidae). Environ Entomol 44:680–688
Cooper JL, Burgess ER IV, King BH (2013) Courtship behavior and detection of female receptivity in the parasitoid wasp Urolepis rufipes. J Insect Behav 26:745–761
Dougherty LR, Shuker DM (2014) The effect of experimental design on the measurement of mate choice: a meta-analysis. Behav Ecol. https://doi.org/10.1093/beheco/aru125
Fedorka K, Zuk M (2005) Sexual conflict and female immune suppression in the cricket, Allonemobious socius. J Evol Biol 18:1515–1522
Fischer CR, King BH (2008) Sexual inhibition in Spalangia endius males after mating and time for ejaculate replenishment. J Insect Behav 21:1–8
Fleischman R, Sakaluk SK (2004) Sexual conflict over remating in house crickets: no evidence of an anti-aphrodisiac in males' ejaculates. Behavior 141:633–646
Gaskett AC (2007) Spider sex pheromones: emission, reception, structures, and functions. Biol Rev 82:26–48
Gerling D, Legner EF (1968) Developmental history and reproduction of Spalangia cameroni, parasite of synanthropic flies. Ann Entomol Soc Am 61:1436–1443
Gerofotis CD, Yuval B, Ioannou CS, Nakas CT, Papadopoulos NT (2015) Polygyny in the olive fly: effects on male and female fitness. Behav Ecol Sociobiol 69:1323–1332
Gershman SN (2008) Sex-specific differences in immunological costs of multiple mating in Gryllus vocalis field cricket. Behav Ecol 19:810–815
Geuverink E, Gerritsma S, Pannebakker BA, Beukeboom LW (2009) A role for sexual conflict in the evolution of reproductive traits in Nasonia wasps? Anim Biol 59:417–434. https://doi.org/10.1163/157075509x12499949744261
Gibson GAP, Floate KD (2004) Filth fly parasitoids; on dairy farms in Ontario and Quebec, Canada. Can Entomol 136:407–417
Godfray HCJ (1994) Parasitoids. Princeton University Press, Princeton
Grillenberger BK, Gadau J, Bijlsma R, Van De Zande L, Beukeboom LW (2009) Female dispersal and isolation-by-distance of Nasonia vitripennis populations in a local mate competition context. Entomol Exp Appl 132:147–154
Halliday T (1983) The study of mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 3–32
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Hardy ICW (1994) Sex ratio and mating structure in the parasitoid hymenoptera. Oikos 69:3–20
Heimpel GE, Lundgren JG (2000) Sex ratios of commercially reared biological control agents. Biol Control 19:77–93. https://doi.org/10.1006/bcon.2000.0849
Judge KA, Tran K-C, Gwynne DT (2010) The relative effects of mating status and age on the mating behaviour of female field crickets. Can J Zool 88:219–223
Kant R, Trewick SA, Sandanayaka WRM, Godfrey AJR, Minor MA (2012) Effects of multiple matings on reproductive fitness of male and female Diaeretiella rapae. Entomol Exp Appl 145:215-221Kemp DJ (2012) costly copulation in the wild: mating increases the risk of parasitoid-mediated death in swarming locusts. Behav Ecol 23:191–194. https://doi.org/10.1093/beheco/arr173
Kemp DJ (2012) Costly copulation in the wild: mating increases the risk of parasitoid-mediated death in swarming locusts. Behav Ecol 23:191–194. https://doi.org/10.1093/beheco/arr173
King BH (1993) Flight activity in the parasitoid wasp Nasonia vitripennis (hymenoptera: Pteromalidae). J Insect Behav 6:313–321
King BH (2002) Breeding strategies in females of the parasitoid wasp Spalangia endius: effects of mating status and body size. J Insect Behav 15:181–193
King BH, Kuban KA (2012) Should he stay or should he go: male influence on offspring sex ratio via postcopulatory attendance. Behav Ecol Sociobiol 66:1165–1173
King BH, Owen MA (2012) Post-mating changes in restlessness, speed and route directness in males of the parasitoid wasp Spalangia endius (hymenoptera: Pteromalidae). J Insect Behav 25:309–319
King BH, Saporito KB, Ellison JH, Bratzke RM (2005) Unattractiveness of mated females to males in the parasitoid wasp Spalangia endius. Behav Ecol Sociobiol 57:350–356
King BH, Colyott KL, Chesney AR (2014) Livestock bedding effects on two species of parasitoid wasps of filth flies. J Insect Sci 14:185
Leonard JE, Boake CRB (2006) Site-dependent aggression and mating behaviour in three species of Nasonia (hymenoptera: Pteromalidae). Anim Behav 71:641–647
Matthews JR, Petersen JJ (1990) Effects of host age, host density and parent age on reproduction of the filth fly parasite Urolepis rufipes (hymenoptera: Pteromalidae). Med Vet Entomol 4:255–260
McAllister BF, Werren JH (1997) Phylogenetic analysis of a retrotransposon with implications for strong evolutionary constraints on reverse transcriptase. Mol Biol Evol 14:69–80
McNamara K, Elgar M, Jones T (2008) A longevity cost of re-mating but no benefits of polyandry in the almond moth, Cadra cautella. Behav Ecol Sociobiol 62:1433–1440
Myint WW, Walter GH (1990) Behaviour of Spalangia cameroni males and sex ratio theory. Oikos 59:163–174
Noronha C, Gibson GAP, Floate KD (2007) Hymenopterous parasitoids of house fly and stable fly puparia in Prince Edward Island and New Brunswick, Canada. Can Entomol 139:748–750
Ortigosa A, Rowe L (2003) The role of mating history and male size in determining mating behaviours and sexual conflict in a water strider. Anim Behav 65:851–858
Powell JR, Graham LC, Galloway TD (2003) Development time of Urolepis rufipes (hymenoptera: Pteromalidae) and effect of female density on offspring sex ratio and reproductive output. Proc Entomol Soc Manitoba 59:16–20
Pultz MA, Leaf DS (2003) The jewel wasp Nasonia: querying the genome with haplo-diploid genetics. Genesis 35:185–191
Reumer BM, Kraaijeveld K, van Alphen JJM (2007) Selection in the absence of males does not affect male–female conflict in the parasitoid wasp Leptopilina clavipes (hymenoptera: Figitidae). J Insect Physiol 53:994–999. https://doi.org/10.1016/j.jinsphys.2007.05.002
Ridley M (1993) Clutch size and mating frequency in parasitic hymenoptera. Am Nat 142:893–910
Rueda LM, Axtell RC (1985) Guide to common species of pupal parasites (hymenoptera: Pteromalidae) of the house fly and other muscoid flies associated with poultry and livestock manure, technical bulletin 278. In: North Carolina Agricultural Research Service, North Carolina State University. http://www.nhm.ac.uk/resources/research-curation/projects/chalcidoids/pdf_Y/RuedaAx985.pdf
Ruther J, Matschke M, Garbe LA, Steiner S (2009) Quantity matters: male sex pheromone signals mate quality in the parasitic wasp Nasonia vitripennis. Proc R Soc B Bio 276:3303–3310
Salehialavi Y, Fritzsche K, Arnqvist G (2011) The cost of mating and mutual mate choice in 2 role–reversed honey locust beetles. Behav Ecol 22:1104–1113
Santolamazza-Carbone S, Pestana M (2010) Polyandry increases male offspring production in the quasi-gregarious egg parasitoid Anaphes nitens. Ethol Ecol Evol 22:51–61. https://doi.org/10.1080/03949370903515984
Steiner S, Ruther J (2009) How important is sex for females of a haplodiploid species under local mate competition? Behav Ecol 20:570–574
Steiner S, Henrich N, Ruther J (2008) Mating with sperm-depleted males does not increase female mating frequency in the parasitoid Lariophagus distinguendus. Entomol Exp Appl 126:131–137
van Alphen J, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
Wang D, Lu L, He Y, Shi Q, Tu C, Gu J (2016) Mate choice and host discrimination behavior of the parasitoid Trichogramma chilonis. Bull Entomol Res 106:530–537
Wedell N, Gage M, Parker G (2002) Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol 17:313–320
West SA (2009) Sex allocation. Princeton University Press, Princeton
Wittman TN (2016) Behavioral and chemical ecology of a male produced substrate borne pheromone in Urolepis rufipes. Thesis, Northern Illinois University
Acknowledgements
Thanks to K. Floate for U. rufipes; to A. Coletta, J. Niew and A. van Pelt for assistance with experiments; to J. Cooper. M. King, and W. Nichols for assistance with colony maintenance; and to A. Kremer for feedback on the writing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
King, B.H., Miller, K.A. Mating Status Effects on Sexual Response of Males and Females in the Parasitoid Wasp Urolepis rufipes. J Insect Behav 31, 144–157 (2018). https://doi.org/10.1007/s10905-018-9667-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-018-9667-z