Abstract
Male courtship behavior and displays influence female mating decisions, and therefore affect mating success in a diverse range of organisms. While there is substantial evidence confirming that females prefer males who invest more in courtship, less attention has been paid to the relative importance of individual behaviors, and the discrete sequences of courtship that result in mating success. The small hairy maggot blow fly, Chrysomya varipes, performs stereotyped courtship behaviors, involving orienting, tapping, waving, arching, wing vibration and mounting. This study aimed to quantify male investment in specific courtship behaviors, and compare courtship investment and behavioral transitions between males who gained mating success (successful males) and those that did not (unsuccessful males). Our results show that mating success was influenced by the behaviors orienting, tapping, arching and mounting. Behavioral transitions revealed a distinct pattern of behaviors leading to a mating attempt, and some differences were observed between successful and unsuccessful males. Overall, our findings suggest that female mating decisions were based on differences in specific male courtship behaviors. This detailed observational study has quantified multiple courtship behaviors for the first time in C. varipes, and highlights the importance of considering multiple behavioral traits when exploring the influence of male courtship on mating success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male courtship displays consist of specific signals conveyed and perceived by potential mates, and are characterized by repetitive or stereotyped behaviors that are directed at the female prior to mating (Andersson 1994). Courtship displays can play a crucial role in both female mating decisions and mate preferences, and, therefore, the evolution of male courtship behavior can be driven by female mate choice (Andersson 1994; Darwin 1859, 1871). There is substantial evidence confirming that males with higher courtship intensity, or a higher investment in the time spent courting, are preferred by females in a wide range of taxa (reviewed by Beyers et al. 2010), including birds (Patricelli et al. 2002), mammals (Longpre et al. 2011), fishes (Karino 1995), amphibians (Vinnedge and Verrell 1998) and insects (Callander et al. 2012; Shamble et al. 2009). However, while female preference for male courtship investment has received extensive empirical attention, almost no attention has been paid to the relative importance of individual behavioral components of courtship, and the discrete sequences of courtship behaviors that result in a higher probability of mating success (but see Chelbi et al. (2012) and Lasbleiz et al. (2006)). Female choice may act on multiple behavioral traits, and selection may operate on different traits either in concert or antagonistically (Candolin 2003). In addition, courtship displays are often complex, involving multiple components, each of which may provide different signals. Therefore, in order to gain a greater understanding of the relationship between male courtship behavior and female mating decisions, there is a need for studies that quantify multiple courtship behaviors. Disentangling these aspects of courtship behavior could provide key insights into indicators of male quality and advance our understanding of the associations between display traits and female preferences, thus improving our capacity to predict the influence of male phenotype on mating success.
There are three main hypotheses that explain the use of multiple male secondary sexual characteristics in mate choice: 1) the multiple messages hypothesis, which proposes that different traits reflect different qualities and evaluated together provide a more accurate assessment of mate quality (Johnstone 1996; Møller and Pomiankowski 1993); 2) the back-up signal hypothesis, which proposes that multiple traits reflect the same aspect of male quality to protect against signal redundancy (Johnstone 1996; Møller and Pomiankowski 1993); and 3) the species recognition hypothesis, which proposes that different traits are required to ensure effective species recognition (Pfennig 1998). In addition, hypotheses relating to the use of multiple signals may not necessarily be mutually exclusive; for example, a study in the eland, Tragelaphus oryx, found that male display traits provided simultaneous support for the multiple messages hypothesis and for the back-up signal hypothesis (Bro-Jørgensen and Dabelsteen 2008).
Empirical studies have begun to explore the use, function and interaction of multiple male secondary sexual characteristics (Hebets et al. 2011; Jones et al. 2014; Kekäläinen et al. 2010; Kodric-Brown and Nicoletto 2001; Lehtonen et al. 2007; Patricelli et al. 2003). However, these types of investigations have almost invariably focused on examining the combination of male ornamental traits and courtship displays (in terms of overall investment) on male mating success and female mating preferences. Very few studies have attempted to divide courtship behavior into its multiple components, and examine the influence of individual courtship behaviors on mating success (but see Bray and Hamilton 2007; Chelbi et al. 2012). Owing to the paucity of work on the use and importance of specific courtship behaviors for mating success, there is a need for studies that thoroughly analyze the importance of specific courtship components in a wider range of species. Such research will enhance our understanding of the complex relationship between female mate choice and male courtship, as the interpretation of courtship behaviors by behavioral ecologists is often oversimplified (Johnstone 1996).
Studies in invertebrates, such as fruit flies Drosophila spp., and wolf spiders Schizcosoa spp., are unraveling the signaling function of multiple courtship behavioral components (see Gleason et al. 2012; Hebets 2005). For example, studies of Drosophila spp. have investigated the function of individual courtship behaviors, and analyzed the relevance of the temporal structure of behavioral sequences (Liimatainen and Hoikkala 1998; Markow 1987; Markow and Hanson 1981; Saarikettu et al. 2005). Markow (1987) found slight differences in the temporal patterns of male courtship between successful and unsuccessful males; most notably that successful males had reduced locomotion and movement compared with unsuccessful males. However, no particular behavior was identified as an absolute predictor of mating success (Markow 1987). Manning (1967) suggested that courtship behaviors deliver different stimuli that, in combination, gradually raise the receptively of a female until a certain threshold is attained and the female accepts the courting male. Therefore, it may be that a combined effect of multiple behaviors is required for mating success in Drosophila (rather than individual behaviors being crucial). In addition, there is evidence that the use of multiple signals in Drosophila (including the exchange of visual, acoustic, olfactory, gustatory and tactile signals) informs each fly about the identity of the potential mate, thereby providing support for the species recognition hypothesis (Greenspan and Ferveur 2000; Lasbleiz et al. 2006; Ritchie et al. 1999). Furthermore, these signals have also been correlated with male condition and genetic quality (Hoikkala et al. 1998), thereby also supporting the multiple messages hypothesis. However, further research is required to test the generality of these findings, and more thoroughly explore the hypotheses regarding the use of multiple behavioral traits in Drosophila and other species.
The small hairy maggot blow fly, Chrysomya varipes, provides an excellent model for a detailed examination of male courtship behavior, and the importance of individual behaviors. Chrysomya varipes is an obligate inhabitant of vertebrate carrion. After forming leks, males typically engage in courtship behavior on vegetation adjacent to a carcass. The work presented in this paper builds upon data from a recent study in which we described courtship behavior in C. varipes for the first time. We reported that courtship consists of a number of discrete behaviors, including: orienting, tapping, waving, wing vibration, arching and mounting, and found that mating success was significantly influenced by investment in courtship behavior (defined as the total time spent courting a female) (see Jones et al. 2014). However, the relative importance of individual behaviors, and the sequence of behaviors leading to copulation, were not explored and remain unknown. The aims of this study were to: 1) characterize male courtship behavior through the analysis of stereotyped pre-copulatory behaviors; 2) determine which behaviors were most important in determining mating success in this species; and 3) compare the temporal patterns of courtship behaviors between males that gained mating success (successful males) and males that did not (unsuccessful males). We predict that female mating decisions will be based on differences in specific male courtship behaviors, and that investment in the different behaviors will influence male mating success. Addressing the aforementioned aims will also allow us to begin evaluating hypotheses relating to the use of multiple traits (i.e. multiple messages, back-up signals and species recognition).
Methods
Fly collection, culturing and mating experiments
Flies were trapped in January 2013 at the University of Wollongong Australia, and then kept in a constant temperature room (23 °C ± 1 °C) with a 12:12 h light/dark cycle. Flies were allocated a constant supply of raw sugar and water, as well as approximately 100 g of kangaroo mince which provided a food source and oviposition medium (Luddenham Pet Meats, Sydney, Australia). After eggs were laid, an additional 200 g of meat was provided and the meat and eggs were removed and placed in a plastic rearing container (130 × 190 mm and 70 mm high). The bottom of the container was covered in wheaten chaff as a pupation substrate, and upon pupation the container was placed in a larger cage (300 × 500 mm and 250 mm high) to permit free movement of adult flies after eclosion. To ensure that all flies used in experiments were virgins, the flies were sexed within 24 h of emergence, and males and females were kept in separate cages.
Mating trials commenced when the F1 generation of flies were aged 12 (± 2) days. Two flies were randomly selected and placed in mixed-sex pairs into a sealed transparent petri dish (85 × 15 mm), containing approximately 0.5 g of kangaroo mince positioned centrally. The behavior of flies was recorded with Samsung SCB-2000P digital cameras and a CCTV recording system under fluorescent lights and constant temperature conditions (23 °C ± 1 °C). Eight behavioral trials ran concurrently, with each trial recorded using a separate video camera. The experimental arenas were surrounded with cardboard barriers to ensure that flies in different trials were unable to see each other. Behavioral trials ran until mating occurred, or for a maximum of 20 min. All trials took place between four and eleven hours since lights on, and between one and seven hours until lights out, over three consecutive days in January, 2013 (i.e. trials were conducted between 1000 and 1700). The experimental trials described here are the same as those previously published by Jones et al. (2014), but the present study uses new behavioral analysis methods to address completely different aims.
Behavioral analysis
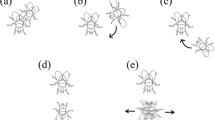
Analysis was performed for trials in which courtship behavior occurred (n = 73) using the behavioral analysis software package EthoLog 2.2 (Ottoni 2000). Male courtship behavior was described and quantified from the time of courtship initiation until successful mating or cessation of the trial at 20 min (whichever occurred first). This approach was used because the focus of this study was in analyzing courtship duration and the behaviors in this period (time from first contact until mating), and is consistent with other studies of courtship behavior (Birge et al. 2010; Dyakonova and Krushinsky 2008). There were six distinct courtship behaviors, and these were categorized as: orienting, tapping, waving, arching, wing vibration and mounting (Jones et al. 2014). Orienting occurred when the male followed and circled around a female. Tapping occurred when the male touched the female using his forelegs. Waving occurred when the male raised straightened forelegs (one or both legs, usually alternating). Arching was when a male stood directly in front of a female with straightened forelegs and an arched back. Arching was often accompanied by rocking backwards and forwards and an occasional slow wing flick. Wing vibration was rapid wing beating and also usually (but not always) occurred while the male was arched and standing directly in front of a female.
Mounting involved a male attempting to mate with the female by jumping onto her back. Males that mounted females were either rejected (pushed away by the female) or accepted (not pushed away by the female). Acceptance was followed by copulation (engagement of the male and female genitalia). At the end of a 20-min trial, males that achieved copulation were deemed to have gained mating success and were categorized as ‘successful males’, while males that did not achieve copulation were categorized as ‘unsuccessful males’. (see video footage of behaviors in Online Resource 1).
The behaviors orienting, wing vibration and arching were recorded as ‘state’ events as defined by the program EthoLog, and quantified as the proportion of time spent by the male in that behavior (quantified from the time of courtship initiation until mating, or completion of the trial). The behaviors orienting, wing vibration and arching will hereafter be referred to in terms of relative duration. Tapping, waving and mounting were recorded as discrete events, and quantified as the number of taps, waves or mountings per min (averaged from the time of courtship initiation until mating, or completion of the trial). The behaviors tapping, waving and mounting will hereafter be referred to in terms of occurrence per unit time (i.e. frequency).
Statistical analysis
Descriptive statistics, including means, standard deviations and range (minimum – maximum value), were calculated for each behavior. In addition, to determine the degree of variability in courtship behaviors, the coefficient of variation (CV) was quantified. The CV was calculated by dividing the standard deviation by the mean. Pairwise correlations were conducted to determine if there were significant associations between individual courtship behaviors. These correlations were conducted on the full set of data (n = 73) as well as separately for successful males (n = 25) or unsuccessful males (n = 48). All correlations were tested for significance using nonparametric methods (Spearman’s rank correlation), as not all behaviors satisfied assumptions of normality.
To determine if there were differences in courtship behaviors between successful and unsuccessful males, t-tests were conducted for each courtship behavior, courtship initiation latency, and for time spent on non-courtship behaviors. To determine which male courtship behaviors were predictive of mating success, a generalized linear regression model (GLM) with binomial errors and a logit link was used. Male mating success (successful or unsuccessful) was the response variable, and the predictor variables were: proportion of time spent orienting, wing vibrating, arching, and frequency of taps, waves and mountings. Data were analyzed using JMP V11 (SAS Institute, USA). The appropriate diagnostic procedures were implemented, including residual inspection and testing for overdispersion, and all assumptions of GLM analysis were satisfied. Statistical significance was accepted when p < 0.05.
To complement significance testing, we also calculated effect sizes with confidence intervals for the GLM. These were determined in order to gauge the magnitude of effect (Hebets et al. 2011; Nakagawa and Cuthill 2007). For all of our models we calculated the statistic ϕ (phi) from the chi-square values generated for each of the predictor variable in our models. We calculated ϕ and its confidence intervals (CI) using freely available software (es calculator; Wilson (2001)).
To test for temporal associations between courtship behavioral patterns, we used a first order Markov chain model (where the probability of a given act depends on the identity of the act immediately preceding it) (Colgan 1978; Gottman and Roy 1990). Sequential data for both successful and unsuccessful males were used to construct two separate transition matrices, by tabulating all instances in which one behavior led to another (excluding non-courtship behaviors, following Chelbi et al. (2012)). Each matrix was compared to a randomly distributed matrix, using likelihood-ratio tests (G statistics) to evaluate whether certain transitions were more likely to occur than others (Chen et al. 2002). Williams correction was performed as part of the analysis. Freeman-Tukey deviates (cell-wise examinations) were used to identify which cells were statistically more likely to precede other behaviors. In addition, conditional probabilities were calculated as the total number of each transition from one behavior to the subsequent behavior, divided by the total number of the antecedent behavior in question (Saarikettu et al. 2005). These matrices, Freeman-Tukey deviates, conditional probabilities, as well as direct observations of courtship pairs, were used to construct a flow diagram of courtship behaviors for successful males and unsuccessful males (Bray and Hamilton 2007; Chelbi et al. 2012; Chen et al. 2002). These analyses were all performed using freely available Java applets (http://caspar.bgsu.edu/~software/java).
Results
Variation and correlations of courtship behavior in C. varipes
Males varied considerably in the time that they invested in each courtship behavior, with wing vibration and mounting having the largest amount of variation (Table 1). In total, 25 of the 73 (33%) male-female pairs achieved a successful mating. Both successful and unsuccessful males invested the largest amount of time during the mating trial in orienting themselves. Orienting and tapping were displayed by every male, while waving, wing vibration, arching and mounting were not always observed (indicated by % occurrence in Table 1).
Pairwise correlations between behaviors revealed a number of significant associations (Table 2). When pooled, orienting was positively correlated with all other behaviors except for wing vibration. In addition, waving and arching were each positively correlated with mounting, and arching and wing vibration were positively correlated with each other. All other correlations were not significant. There were several key differences when comparing correlations of behaviors for successful versus unsuccessful males. Notably, all of the associations between orienting and other behaviors were not significant in the successful mating group (Table 2).
Courtship behavior and mating success
Successful males invested more time in the behaviors arching, tapping and mounting compared to unsuccessful males (Table 1). The differences were not significant for the behaviors orienting, waving and wing vibration. However, when an outlier was removed the difference for wing vibration became significant, whereby successful males invested more in this behavior (t-test: t = 2.2, DF = 25, p = 0.03). There was no significant difference between courtship latency between successful and unsuccessful males (t test: t = −1.1, DF = 42, p = 0.27). This result is consistent when one outlier from each of the groups was removed (t test: t = −1.3, DF = 41, p = 0.20).
When pooled, male courtship behaviors had a significant effect on mating success (GLM: χ2 = 51.08; df = 6 p = <0.0001, ϕ = 0.84). Mating success was dependent on time invested in orienting and arching, and frequency of taps and mounting (Table 3). Mounting occurred more often in the trials of successful males (Table 3, Fig. 1b). The frequency of tapping and the proportion of time spent orienting were also higher for successful males (Table 3, Fig. 1a and b). Arching was always present in the trials of successful males (with successful males spending at least 16 s or 3% of the time arching), and time invested in arching had a highly significant effect on mating success (Table 3). The magnitude of the difference in time invested in arching for successful and unsuccessful males is shown in Fig. 1a. Mating success was not influenced by the number of waves or by time invested in wing vibration. Unsuccessful males spent more time on non-courtship behaviors, while successful males spent more time on courtship (t-test: t = −2.9, DF = 35, p = 0.007).
Flow diagrams of courtship behaviors in C. varipes derived from the analysis of (a) 25 males that mated successfully; and (b) 48 males that were unsuccessful at mating. The figures represent a first-order Markov chain analysis of transition matrices, and the sequences of behaviors are depicted when transitions occur significantly more than predicted by chance. The sizes of the boxes represent the relative frequency of occurrence of a particular behavior. The size of the arrows and the numbers beside the arrows indicate the conditional probability for each transition (or the degree to which particular transitions are over-represented). See attached files ‘Fig1a.JPG’ and ‘Fig1b.JPG’
Sequence of behaviors during courtship
The flow diagrams (Fig. 1) were derived from the transition matrices (Table 4 and Table 5), and suggest the following simplified model of C. varipes courtship behaviors. Male flies initiated courtship by orienting themselves. The male then alternated between orienting and tapping (male tapped the female, usually on the abdomen or legs) and waving (slowly raised his forelegs). Following orienting, the male then stood directly in front of the female (arching). During arching, the male often slowly raised and lowered his wings. Arching could also lead to wing vibration, where the male rapidly vibrated his wings (which then led back to arching). In successful males, arching only progressed to wing vibration or mounting (Fig. 1a and Table 4). By comparison, in unsuccessful males, although arching also led to wing vibration and mounting, more often it reverted to orienting, or progressed to the behavior waving (Fig. 1b). Thus, mounting featured more prominently in the post-arching behavior of successful males than of unsuccessful males. Overall, the two flow diagrams highlighting the differences between successful and unsuccessful males (Fig. 1a and b) are very similar, with only subtle differences with respect to the sequence and frequency of transitions.
Discussion
The results of our study demonstrate that variation in relative amounts and sequences of courtship behavior influenced male mating success in C. varipes. In particular, mating success was significantly influenced by the relative duration of orienting and arching, and by the frequency of tapping and mounting. While the sequence of behavioral events appeared to follow a pattern, there were some subtle differences observed between successful and unsuccessful males in the progression of behavioral sequences leading to mating. The progression from arching to mounting was particularly characteristic of successful males, while unsuccessful males more often displayed orienting or waving after arching, rather than mounting. In addition, the differences between courtship behaviors between individual flies were highly variable with respect to their duration and frequency. Overall, our findings support our prediction that female mating decisions are based on differences in the male’s courtship behaviors. In addition, this study has provided the first step towards understanding the role played by these multiple behavioral traits in female mate choice.
Orienting was performed by every courting male; it comprised a very large proportion of male courtship behavior and time spent orienting had a significant effect on mating success. Drosophila melanogaster displays a similar behavior (also termed orienting) and in these flies orienting is used by the male to initiate courtship (Greenspan and Ferveur 2000). Similarly, this appears to be the case in C. varipes. However, in D. melanogaster, orientation is characterized by the male facing the female, whereas orientation in C. varipes was often accompanied by a distinct movement by the male around the female, which is not seen in D. melanogaster (Greenspan and Ferveur 2000). However, males from other species of Drosophila that possess male-specific wing spots, such as D. suzukii and D. biarmipes, engage in a circling behavior around the female in order to increase the visibility of their ornamented spotted wings (Kopp and True 2002; Mazzoni et al. 2013; Revadi et al. 2015). Thus, similarly, orienting behavior in C. varipes may be to improve visibility of their ornamented forelegs. There were positive associations between orienting and all other courtship behaviors in unsuccessful males (except for wing vibration), but no associations in successful males. The reason for this difference is unknown, but it may be that orienting is uninformative in regard to male quality (or at least not directly reflecting quality). The positive associations between orienting and other courtship behaviors in unsuccessful males may be a reflection of a male’s motivation to mate, rather than male quality per se (Guevara-Fiore et al. 2010).
In between periods of orienting, males proceeded to the behaviors tapping and waving. Male tapping behavior (where the male touched the female using his forelegs) was present in every trial in which males displayed courtship behaviors, with contact most often made on the female’s legs or abdomen. The frequency of taps performed by a male significantly influenced his mating success. The most likely explanation for this association is that tapping is a tactile signal, as is seen in many other insects including Drosophila (Colyott et al. 2016; Giglio and Dyer 2013; Narda 1966). It is also possible that this behavior may facilitate the transfer of pheromone signals, such as cuticular hydrocarbons. Chemical signals are extremely important in the mating behavior of various insect species (Barry et al. 2010; Billingham et al. 2010; Howard and Blomquist 2005). For example, in D. melanogaster, tapping is used to detect chemical substances through contact with gustatory receptors which are predominately located on the tarsi of their forelegs (Bray and Amrein 2003). Chrysomya varipes may therefore also use chemical signals in recognizing and even discriminating between potential mates, and these may be transmitted when physical contact is made. However, we cannot disentangle the exact function of this behavior based only on observation; further research is therefore needed to quantify other modalities.
Waving did not significantly influence male mating success, which may indicate that this behavior does not signal male quality to prospective mates. However, analysis of the sequence of behavioral transitions revealed that unsuccessful males sometimes reverted back to waving after arching, which was not the case for successful males. Waving may be used as a signal to gain the initial attention of females, or to re-engage unreceptive females. Once a female is engaged, other behaviors may then be more important for female mating decisions and male mating success.
After males oriented, they also proceeded to the behavior arching. Arching appears to be crucial for mating success as it always preceded mounting, and mating success was highly influenced by this behavior. Arching involved the male and female facing each other for long periods (up to 4 min) with the male occasionally lifting his wings. Arching may be similar to the behavior ‘face-off’ in the flesh fly Phrosinella aurifacies (Spofford and Kurczewski 1985) or ‘facing’ in the sand fly Phlebotomus papatasi (Chelbi et al. 2012). In P. papatasi, Chelbi et al. (2012) stated that this apparent waiting behavior during courtship might indicate that a physiological change needs to occur in either partner prior to mating. In addition, at times it appeared as if contact between the mouthparts was made in C. varipes, which might allow for the transfer of substances required to stimulate mating, such as pheromones or nuptial gifts. However, a more detailed behavioral investigation involving close-up video recordings from multiple angles will be required to ascertain the exact nature and function of this behavior. Despite this uncertainty, there appears to be a minimum threshold above which a male must arch in order to mate: males that achieved mating success spent at least 16 s (or 3%) of the trial time arching. This finding supports the notions of Bastock and Manning (1955), who proposed that female receptivity in D. melanogaster is enhanced by a gradual summation of courtship, and that once a certain threshold is attained, a female will accept the courting male (Bastock and Manning 1955). However, other than the present study, there is limited evidence for specific individual behavioral traits playing a crucial role in determining mating success (but see Bray and Hamilton (2007) and Budriene and Budrys (2004)).
In between periods of arching, males often proceeded to wing vibration (49% of successful males and 36% of unsuccessful males) (Figs 1a and b, respectively). Occurrence of this behavior did not always lead to mating, and there was no significant effect of wing vibration on mating success. The function of this presumably energetically expensive display is therefore unknown. It has been hypothesized by Bray and Hamilton (2007) that the function of ‘wing-flapping’ in the sand fly Lutzomyia longipalpis is to disperse sex pheromones. In addition, wing-flapping produces an auditory signal that is predicted to function in mate recognition (Bray and Hamilton 2007). Similarly, wing vibration in D. melanogaster produces an acoustic signal known as their ‘courtship song’ which is important in species recognition (Kyriacou and Hall 1982; Ritchie et al. 1999). A more detailed analysis is required to quantify auditory and chemical signals produced and/or released by wing vibration in order to detect an effect of wing vibration on mating success in C. varipes. In addition, there is likely to be variation in the actual motor performance in terms of vigor and skill between males that cannot be detailed with data only considering the amount of time spent on the action (Beyers et al. 2010). For example, two males may wing vibrate for the same length of time, but one of these males could potentially be beating at a higher frequency, and this may be a more honest indicator of quality (Zahavi 1975). For example, in the olive, fruit fly Bactrocera oleae, males who mated successfully had a significantly higher frequency and pulse duration of wing vibration during courtship (Benelli et al. 2012). Further research will therefore be necessary to uncover the exact function of wing vibration.
Although this study has illuminated potential influences of the different courtship behaviors on female mating preferences, the use of these multiple behaviors remains unknown (i.e. it is unknown whether the use of multiple behaviors is for conveying multiple messages, providing back-up signals, or permitting species recognition). Disentangling support for the aforementioned hypotheses is difficult when considering only time invested in a particular action, and also not quantifying the multi-modal aspect of each behavior (e.g. chemical and acoustic signals). To clarify the exact role of each behavior, future studies may need to specifically examine the relationship between courtship behaviors and other reproductive traits, including aspects of male quality such as the fertility and reproductive potential of individual males.
Importantly, while the focus of this study was on female mate choice and male courtship behaviors, it must be recognized that male courtship investment may vary depending on the frequency and intensity of female cues received during the courtship process. Males have been shown to adjust their courtship investment depending on their reception of different female visual, auditory and olfactory signals (Appelt and Sorensen 2007; Bonduriansky 2001; Galán and Price 2000; Guevara-Fiore et al. 2010; Semple and McComb 2000). In addition, specific female behaviors can alter a male’s courtship investment. For example, in the eastern fence lizard, Sceloporus undulates, the way in which a female approached or retreated from a male was found to have an important influence on male courtship behavior (Swierk et al. 2013). Swierk et al. (2013) suggested that such behaviors may advertise a female’s willingness to mate, which in turn, could increase a male’s motivation, and courtship investment in receptive females. The influence of female behavior in C. varipes is an area we are currently investigating, and preliminary results suggest that there are indeed associations between female behaviors and male courtship (unpublished data). Therefore, incorporating measures of female behavior in C. varipes might show that some of the variation in male behavior, and mating success, is explained by the degree of female receptivity.
Furthermore, studying isolated interactions between single male and female pairs may be difficult to evaluate when lekking species interact in more complex social environments (Fiske et al. 1998; McGhee et al. 2007). Specifically, male behaviors can be influenced by the presence of other males, and male rivalry can play important roles in dictating mating outcomes (Wong and Candolin 2005). From our observations in nature, while males interact and display courtship behaviors towards each other, there is no overt physical male-male combat in this species. Nevertheless, future studies in C. varipes may benefit from examining the effect of competition on mating success.
In conclusion, this detailed observational study, the first in blow flies, allowed us to quantify courtship behaviors in C. varipes. As predicted, we found evidence for the importance of individual behaviors on mating success, particularly the behavior ‘arching’ which appears to be critical for mating. We also analyzed the temporal patterns of courtship behaviors, and found there to be a progression of behaviors during courtship that precedes a mating attempt. Additionally, courtship behaviors between individuals were extremely variable with respect to their duration and frequency. An important next step will be to examine and incorporate measures of female behavior, as well as quantifying the relative importance of different sensory cues for mating success, including chemical and acoustic signals. Nevertheless, the present study underscores the importance of considering multiple behavioral traits when attempting to determine the influence of male courtship investment on male mating success.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Appelt CW, Sorensen PW (2007) Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim Behav 74:1329–1338. doi:10.1016/j.anbehav.2007.02.032
Barry K, Holwell G, Herberstein M (2010) Multimodal mate assessment by male praying mantids in a sexually cannibalistic mating system. Anim Behav 79:1165–1172
Bastock M, Manning A (1955) The courtship of Drosophila melanogaster. Behaviour 8:85–111
Benelli G, Canale A, Bonsignori G, Ragni G, Stefanini C, Raspi A (2012) Male wing vibration in the mating behavior of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae). J Insect Behav 25:590–603. doi:10.1007/s10905-012-9325-9
Beyers J, Hebets E, Podos J (2010) Female mate choice based upon male motor performance. Anim Behav 79:771–778
Billingham ZD, Chapple DG, Sunnucks P, Wong BBM (2010) Chemical cues and group association preferences in a subsocial cockroach, Panesthia australis. Aus J Zool 57:385–390. doi:10.1071/ZO09066
Birge LM, Hughes AL, Marshall JL, Howard DJ (2010) Mating behavior differences and the cost of mating in Allonemobius fasciatus and A. socius. J Insect Behav 23:268–289. doi:10.1007/s10905-010-9213-0
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Bray S, Amrein H (2003) A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39:1019–1029
Bray DP, Hamilton JGC (2007) Courtship behaviour in the sandfly Lutzomyia longipalpis, the New World vector of visceral leishmaniasis. Med Vet Entomol 21:332–338. doi:10.1111/j.1365-2915.2007.00700.x
Bro-Jørgensen J, Dabelsteen T (2008) Knee-clicks and visual traits indicate fighting ability in eland antelopes: multiple messages and back-up signals. BMC Biol 6:1–8. doi:10.1186/1741-7007-6-47
Budriene A, Budrys E (2004) Behavioural elements influencing mating success of Symmorphus allobrogus (Hymenoptera: Eumeninae). Acta Zool Lit 14:39–47
Callander S, Jennions M, Backwell PY (2012) The effect of claw size and wave rate on female choice in a fiddler crab. J Ethol 30:151–155. doi:10.1007/s10164-011-0309-6
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev Camb Philos Soc 78:575–595. doi:10.1017/S1464793103006158
Chelbi I, Bray DP, Hamilton JG (2012) Courtship behaviour of Phlebotomus papatasi the sand fly vector of cutaneous leishmaniasis. Parasit Vectors 5:1756–3305
Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA (2002) Fighting fruit flies: a model system for the study of aggression. PNAS 99:5664–5668. doi:10.1073/pnas.082102599
Colgan PW (1978) Quantitative Ethology. Wiley, New York
Colyott K, Odu C, Gleason JM (2016) Dissection of signalling modalities and courtship timing reveals a novel signal in Drosophila saltans courtship. Anim Behav 120:93–101. doi:10.1016/j.anbehav.2016.07.015
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. J. Murray, London
Darwin C (1871) The descent of man, and selection in relation to sex. J. Murray, London
Dyakonova V, Krushinsky A (2008) Previous motor experience enhances courtship behavior in male cricket Gryllus bimaculatus. J Insect Behav 21:172–180. doi:10.1007/s10905-008-9117-4
Fiske P, Rintamäki PT, Karvonen E (1998) Mating success in lekking males: a meta-analysis. Behav Ecol 9:328–338. doi:10.1093/beheco/9.4.328
Galán P, Price AH (2000) Females that imitate males: dorsal coloration varies with reproductive stage in female Podarcis bocagei (Lacertidae). Copeia 2000:819–825. doi:10.1643/0045-8511(2000)000[0819:ftimdc]2.0.co;2
Giglio EM, Dyer KA (2013) Divergence of premating behaviors in the closely related species Drosophila subquinaria and D. recens. Ecol Evol 3:365–374. doi:10.1002/ece3.477
Gleason JM, Pierce AA, Vezeau AL, Goodman SF (2012) Different sensory modalities are required for successful courtship in two species of the Drosophila willistoni group. Anim Behav 83:217–227. doi:10.1016/j.anbehav.2011.10.029
Gottman JM, Roy AK (1990) Sequential analysis: A guide for behavioral researchers. Cambridge University Press, Cambridge
Greenspan RJ, Ferveur J (2000) Courtship in Drosophila. Annu Rev Genet 34:205–232
Guevara-Fiore P, Stapley J, Watt P (2010) Mating effort and female receptivity: how do male guppies decide when to invest in sex? Behav Ecol Sociobiol 64:1665–1672. doi:10.1007/s00265-010-0980-6
Hebets EA (2005) Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav Ecol 16:75–82. doi:10.1093/beheco/arh133
Hebets A, Stafstrom J, Rodriguez R, Wilgers DJ (2011) Enigmatic ornamentation eases male reliance on courtship performance for mating success. Anim Behav 81:963–972
Hoikkala A, Aspi J, Suvanto L (1998) Male courtship song frequency as an indicator of male genetic quality in an insect species, Drosophila montana. Proc R Soc B 265:503–508
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393. doi:10.1146/annurev.ento.50.071803.130359
Johnstone RA (1996) Multiple displays in animal communication:`Backup Signals' and `Multiple Messages. Philos Trans R Soc B 351:329–338. doi:10.1098/rstb.1996.0026
Jones SD, Byrne PG, Wallman JF (2014) Mating success is predicted by the interplay between multiple male and female traits in the small hairy maggot blowfly. Anim Behav 97:193–200. doi:10.1016/j.anbehav.2014.09.022
Karino K (1995) Male-male competition and female mate choice through courtship display in the territorial damselfish Stegastes nigricans. Ethology 100:126–138. doi:10.1111/j.1439-0310.1995.tb00320.x
Kekäläinen J, Valkama H, Huuskonen H, Taskinen J (2010) Multiple sexual ornamentation signals male quality and predicts female preference in minnows. Ethology 116:895–903. doi:10.1111/j.1439-0310.2010.01802.x
Kodric-Brown A, Nicoletto P (2001) Female choice in the guppy (Poecilia reticulata): the interaction between male color and display. Behav Ecol Sociobiol 50:346–351. doi:10.1007/s002650100374
Kopp A, True JR (2002) Evolution of male sexual characters in the Oriental Drosophila melanogaster species group. Evol Dev 4:278–291. doi:10.1046/j.1525-142X.2002.02017.x
Kyriacou CP, Hall JC (1982) The function of courtship song rhythms in Drosophila. Anim Behav 30:794–801
Lasbleiz C, Ferveur J-F, Everaerts C (2006) Courtship behaviour of Drosophila melanogaster revisited. Anim Behav 72:1001–1012. doi:10.1016/j.anbehav.2006.01.027
Lehtonen TK, Rintakoski S, Lindström K (2007) Mate preference for multiple cues: interplay between male and nest size in the sand goby, Pomatoschistus minutus. Behav Ecol 18:696–700. doi:10.1093/beheco/arm032
Liimatainen J, Hoikkala A (1998) Interactions of the males and females of three sympatric Drosophila virilis-group species, D. montana, D. littoralis, and D. lummei, (Diptera: Drosophilidae) in intra- and interspecific courtships in the wild and in the laboratory. J Insect Behav 11:399–417. doi:10.1023/a:1020906815133
Longpre KM, Koepfinger ME, Katz LS (2011) Female goats use courtship display as an honest indicator of male quality. Horm Behav 60:505–511. doi:10.1016/j.yhbeh.2011.07.019
Manning A (1967) The control of sexual receptivity in female Drosophila. Anim Behav 15:239–250. doi:10.1016/0003-3472(67)90006-1
Markow TA (1987) Behavioral and sensory basis of courtship success in Drosophila melanogaster. PNAS 84:6200–6204
Markow TA, Hanson SJ (1981) Multivariate analysis of Drosophila courtship. PNAS 78:430–434
Mazzoni V, Anfora G, Virant-Doberlet M (2013) Substrate vibrations during courtship in three Drosophila species. PLoS One 8:e80708. doi:10.1371/journal.pone.0080708
McGhee KE, Fuller RC, Travis J (2007) Male competition and female choice interact to determine mating success in the bluefin killifish. Behav Ecol 18:822–830. doi:10.1093/beheco/arm051
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176. doi:10.1007/bf00173774
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. doi:10.1111/j.1469-185X.2007.00027.x
Narda RD (1966) Analysis of the stimuli involved in courtship and mating in D. Malerkotliana (Sophophora, drosophila). Anim Behav 14:378–383. doi:10.1016/S0003-3472(66)80101-X
Ottoni EB (2000) EthoLog 2.2 - a tool for the transcription and timing of behaviour observation sessions. Behav Res Methods Instrum Comput 32:446–449
Patricelli GL, Uy JAC, Walsh G, Borgia G (2002) Sexual selection: male displays adjusted to female's response. Nature 415:279–280
Patricelli GL, Uy JAC, Borgia G (2003) Multiple male traits interact: attractive bower decorations facilitate attractive behavioural displays in satin bowerbirds. Proc R Soc B 270:2389–2395. doi:10.1098/rspb.2003.2530
Pfennig KS (1998) The evolution of mate choice and the potential for conflict between species and mate–quality recognition. Proc R Soc B 265:1743–1748. doi:10.1098/rspb.1998.0497
Revadi S, Lebreton S, Witzgall P, Anfora G, Dekker T, Becher P (2015) Sexual behavior of Drosophila suzukii. Insects 6:183–196. doi:10.3390/insects6010183
Ritchie MG, Halsay EJ, Gleason JM (1999) Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster song. Anim Behav 58:649–657
Saarikettu M, Liimatainen JO, Hoikkala A (2005) Intraspecific variation in mating behaviour does not cause sexual isolation between Drosophila virilis strains. Anim Behav 70:417–426. doi:10.1016/j.anbehav.2004.12.008
Semple S, McComb K (2000) Perception of female reproductive state from vocal cues in a mammal species. Proc R Soc B 267:707–712. doi:10.1098/rspb.2000.1060
Shamble P, Wilgers D, Swoboda K, Hebets E (2009) Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav Ecol 20:1242–1251
Spofford MG, Kurczewski FE (1985) Courtship and mating behavior of Phrosinella aurifacies Downes (Diptera: Sarcophagidae: Miltogramminae). Proc Entomol Soc Wash 87:273–282
Swierk L, Myers A, Langkilde T (2013) Male mate preference is influenced by both female behaviour and morphology. Anim Behav 85:1451–1457. doi:10.1016/j.anbehav.2013.03.042
Vinnedge B, Verrell P (1998) Variance in male mating success and female choice for persuasive courtship displays. Anim Behav 56:443–448
Wilson D (2001) Meta-analysis macros for SAS, SPSS, and Stata. http://mason.gmu.edu/~wdwilsonb/ma.html. Accessed 7 Feb 2014
Wong BB, Candolin U (2005) How is female mate choice affected by male competition? Biol Rev Camb Philos Soc 80:559–571. doi:10.1017/s1464793105006809
Zahavi A (1975) Mate selection - a selection for a handicap. J Theor Biol 53:205–214. doi:10.1016/0022-5193(75)90111-3
Acknowledgements
We acknowledge financial assistance towards this work from UOW’s Centre for Sustainable Ecosystem Solutions, and the assistance of 360 Degree Films with capturing the supplementary video footage of the behavior of Chrysomya varipes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All work described here complies with ethical guidelines for the use of invertebrates, and the authors have no conflicts of interest to declare.
Electronic supplementary material
Online Resource 1
(MP4 30125 kb)
Rights and permissions
About this article
Cite this article
Jones, S.D., Byrne, P.G. & Wallman, J.F. Exploring the influence of individual courtship behaviors on male mating success in a blow fly. J Insect Behav 30, 528–543 (2017). https://doi.org/10.1007/s10905-017-9633-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-017-9633-1