Abstract
Tranosema rostrale (Brischke) (Hymenoptera: Ichneumonidae) is an important parasitoid of low-density spruce budworm Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) populations. To investigate the effectiveness of this parasitoid in attacking low-density spruce budworm populations, we conducted a detailed laboratory study on its reproductive biology and behavior including mating behavior, potential fecundity, longevity, host searching, and oviposition behavior. We found that the occurrence of mating increases with the number of males present in the cage but that it drastically decreases when females mated previously. Females may mate multiple times with different males in one breeding session and mating duration is significantly longer when a male mates the second time with the same female. Dissections of T. rostrale’s oviducts showed that it is a synovigenic species emerging with about 17 % of its lifetime egg load and develops most of its eggs in the first three days after emergence at 20 °C. Sugar, but not pollen, significantly increased the insect’s longevity compared to water. Spruce budworm larvae, silk from larvae and damaged balsam fir foliage triggered probing in T. rostrale females significantly more often than larval feces. The sequence of the parasitoid’s behaviors leading to successful attack is described as antennation, probing, insertion of the ovipositor, oviposition, and subsequent disinterest or resuming of the sequence. Defense mechanisms of the host larva such as vigorous movements, biting, and/or regurgitation and behavioral countermeasures by T. rostrale are described in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important outbreaking insects in boreal coniferous forests of eastern North America is the spruce budworm Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) (USDA Forest Service 2009; NFD 2013). It reaches outbreak levels in more or less regular intervals of 30–40 years (Blais 1965; Morin 1994). Several factors influencing its population dynamics have been identified, one of which are natural enemies, in particular parasitoids (Royama 1984; Régnière et al. 1995; Régnière and Nealis 2007). Spruce budworm parasitoid communities change in terms of species occurrence and relative importance as mortality factors depending on their host’s population densities (Eveleigh et al. 2007). It is thus important to not only study parasitoids attacking spruce budworm during outbreaks, but also those causing high mortality at low spruce budworm population densities. This will help to better understand conditions that lead to natural control, and to predict when and where pests may escape such control and reach outbreak levels (Nealis 1991).
Tranosema rostrale (Brischke) (Hymenoptera: Ichneumonidae) is an important parasitoid of low-density spruce budworm populations. Parasitism by this species can exceed 90 %, inflicting considerable mortality on spruce budworm larvae (Cusson et al. 1998; Seehausen et al. 2014), and helping to maintain populations at low levels for many years (Régnière et al. 2013). Besides spruce budworm, T. rostrale attacks several other tortricid species and is believed to have one or two additional generations per year on unknown alternate hosts (Cusson et al. 1998). Like most parasitoid wasps, T. rostrale is arrhenotokous, with fertilized eggs developing into female wasps and unfertilized eggs becoming males (Flanders 1965; Quicke 2014). Although some aspects concerning the basic biology of T. rostrale have been described (Cusson et al. 1998), several key life history traits related to its reproductive biology and behavior (e.g., mating, female fecundity, oviposition behavior) remain unknown. This limits our understanding of the role T. rostrale plays in spruce budworm population dynamics and our ability to manipulate it for further laboratory experiments.
Previous work has shown that female T. rostrale can mate at least twice in their lifetime (Cusson et al. 1998), but it is unclear whether females mate with multiple males or whether male competition and mate guarding is important in this process (Thornhill 1984). Similarly, it is unknown whether T. rostrale is proovigenic (full egg complement upon adult emergence) or synovigenic (mature eggs during their adult life), yet these oogenesis strategies are important in determining essential life-history traits such as preoviposition period, oviposition, and foraging habits that ultimately determine parasitoid success (Flanders 1950; Jervis and Kidd 1986). For synovigenic species, the longer a female lives, the more eggs she can produce and the more hosts she can attack. Although it is well established that feeding (either on the host (Jervis and Kidd 1986) or on carbohydrate and protein sources such as flower nectar, pollen, or honeydew (Jervis et al. 1993)) increases parasitoid longevity (Syme 1975; Berndt and Wratten 2005; Wäckers et al. 2008), it is not clear whether different diets influence the longevity of T. rostrale, nor how they may affect biological traits determining parasitoid success.
Successful parasitism by T. rostrale is a function of a number of factors, including the parasitoid’s ability to locate hosts and effectively overcome their defenses during attack. Many parasitoids locate their hosts in the environment through volatiles emitted by the host itself or from its host plant (Godfray 1994). Because T. rostrale is a parasitoid that successfully attacks low-density spruce budworm populations, it is likely that it has a very effective host location mechanism, although the olfactory cues for this are unknown. Once a host is found, host defenses influence the attack and oviposition success of a parasitoid. Such host defenses can be behavioral (e.g., concealed feeding, commensalism, violent movements) or morphological (e.g., thick cuticle, hairs) or physiological (e.g., immune response); in all three cases, parasitoids have co-evolved countermeasures that overcome mechanisms that hinder their success (reviewed in Godfray 1994). In this study, we investigated the behavioral host defenses and countermeasures by T. rostrale.
Here, we examine key life-history traits of T. rostrale that play a role in its success as a parasitoid attacking low-density spruce budworm populations. Through behavioral and experimental observations, we describe: (1) mating behavior, (2) fecundity, (3) longevity, (4) host-searching behavior, and (5) oviposition behavior of the parasitoid. The overall goal is to provide insight into the successful reproduction of T. rostrale in order to better understand how this parasitoid reaches such high parasitism levels during low-density spruce budworm populations (Cusson et al. 1998). In practice, the information can be used to rear this parasitoid under laboratory conditions for further experiments.
Material and Methods
Adult T. rostrale were obtained by implanting laboratory-reared spruce budworm larvae into two field sites in Quebec near Armagh (46°46′ N, 70°39′ W, 312 m) and Petit-lac-à-l’Épaule (47°18′ N, 71°12′ W, 725 m). The physical environment, vegetation, and climate for these sites is described by Lethiecq and Régnière (1988). A detailed description of the implantation technique is provided by Seehausen et al. (2013). After recovery from the field, implanted spruce budworm larvae were placed individually into 37-ml plastic cups in a growth chamber at 20 °C and a L16:D8 h diel period, and checked daily until parasitoid or moth emergence.
Mating Behavior
One virgin or mated female and one to three males were introduced into a meshed sleeve cage (24 × 41 × 32 cm) placed next to a window providing natural light. Males were aged between <24 h and 25 days, and females between <24 h and 36 days. The number and duration of couplings were recorded in 469 trials. Individuals were separated when no mating occurred after a 30 min observation period. To investigate whether females were mono- or polyandrous, one female was released into a cage with three color-marked males. We used blue, yellow, and red non-toxic paint (SCHOLA Inc., Marieville, Quebec) to mark males on their thorax. When mating occurred, the color code of the male was noted to test whether a specific color biased the probability of mating. Mating behavior was observed and the number of couplings with one or more males and duration of mating were recorded for 97 trials. Male and female age, as well as the length of the hind tibia of males, were recorded to test the influence of age and male size on the occurrence of coupling. To test whether females would re-mate, a total of 71 females were presented within 24 h after the first coupling to one (n = 20), two (n = 29) or three (n = 22) males, and the success and number of couplings were recorded.
Potential Fecundity
Tranosema rostrale virgin females were held without hosts for 2, 5, 7, 10, 15 or 20 days at 20 °C and a L16:D8 h diel period, and provided with a 20 % sucrose water solution. At the end of each time period, 10 individuals were frozen and dissected in a saline buffer solution under a binocular microscope. Female reproductive organs were isolated, ovarioles were separated from the two lateral oviducts, and eggs in the oviducts were counted.
Longevity
Virgin and unfed T. rostrale males and females were transferred within 24 h of emergence into 237-ml transparent plastic cages with a screened window on the top for ventilation. Cages were placed into a growth chamber at 20 °C, 70 % relative humidity, and a L16:D8 h diel period. They were offered one out of four different diets ad libitum, provided through soaked cotton rolls inserted into glass vials placed at the bottom of the cage. The diets were tap water, 20 % sucrose water solution as a source of carbohydrates, water containing 1 % of commercially available multifloral pollen mix (Prince-Leclerc & Ass., Saint-Agapit, Quebec) as a source of protein, vitamin C and iron, or a mix of 20 % sucrose water solution and 1 % pollen. Diet vials were replaced every two days until the death of the parasitoid. Replicates of 15–18 males and 15 females were followed for each diet. Parasitoids were checked daily for survival.
Host-Searching Behavior
Mated and fed females aged between 11 and 24 days (mean 17.25) were placed into the 237-ml transparent plastic cages described above in a closed box lit from below to avoid light from any other particular direction. A small window at the side of the box, facing away from the light sources in the room, allowed the female to be observed. Five cues related to the host (C. fumiferana) were presented in random order to each of 20 females. The host cues were: (1) an unparasitized fifth-instar spruce budworm larva feeding on balsam fir (Abies balsamea (L.) Miller) foliage in its feeding tunnel, containing silk and feces (hereafter referred to as ‘whole host system’); (2) an unparasitized fifth-instar larva; (3) silk from a spruce budworm larva rolled on a piece of paper; (4) fresh feces from spruce budworm larvae; and (5) balsam fir foliage with feeding damage from a spruce budworm larva and carefully cleaned of other cues (feces and silk) using a microscope. Two behaviors related to host location and recognition were evaluated: antennation and probing. Antennation was defined as bringing antennae together in front of the head and drumming them against the presented cue or surface. Probing was defined as bending of the abdomen, extracting the ovipositor from its shield and pushing it forward several times while walking over the substrate. Cues were presented on the bottom of the plastic cage for 5 min, or until both antennation and probing occurred. Control females were observed for 5 min in the cage with no host cues. The number of samples triggering one or both behaviors and the time until the two behaviors occurred were analyzed.
Oviposition Behavior
A total of 135 mated (n = 98) and virgin (n = 37) T. rostrale females of different ages were released individually 1–33 times during their adult life into 237-ml transparent plastic cages (described above) containing an 8-cm balsam fir twig with one fifth-instar spruce budworm larva having fed at least 24 h on the shoot. Females were observed 5 min or until an attack took place. A successful attack was defined as the insertion of the ovipositor through the larva’s cuticle. Female behavior prior to the attack was noted, such as the position of ovipositor insertion into the host larva (dorsal, lateral or ventral; anterior, mid- or posterior section of the larva; observed for a subsample, n = 248), and the age of the female (n = 930). Host behavior before and during the attack was also noted. The success of oviposition was evaluated by dissecting 58 larvae after the attack to determine whether an egg had been laid.
Statistical Analysis
Success of coupling, occurrence of multiple matings, occurrence of antennation and probing, and success of attack were all analyzed using binomial logistic regression (PROC GLIMMIX; SAS Institute Inc. 2015). To analyze success of coupling, the number of males, previous mating status (mated, virgin), and female and male age were used as explanatory variables. The occurrence of multiple matings was related to the number of males present in the cage. The effect of the host cues, and of their order of presentation, on the frequency of antennation and probing were determined in separate analyses. Females were introduced into the model as repeated measures because all cues were presented to each female. Females were also taken as repeated measures in the analysis of attack success, because each female was tested several times during the experiment. The explanatory variable in this case was female age.
Analyses of variance (ANOVA) were used to analyze mating duration, longevity, potential fecundity, and time before host-searching behavior occurred (PROC GLM; SAS Institute Inc. 2015). Two separate mating time analyses were performed: one where the sequence of matings (1- > 5) was the explanatory variable, and one specifically for mating duration of color-coded males where the sequence of mating (first and second) and the identity of the male (same, different) were the explanatory variables. Mating durations were log transformed in the first analysis to meet the assumption of residual normality. For the analysis of longevity, the explanatory variables diet, sex, and an interaction term were used, and longevity was rank-transformed because of non-normality of residuals. Female age was the explanatory variable of potential fecundity. Two separate analyses were performed for time until antennation and probing (log transformed), with host cue as the explanatory variable and females used as repeated measures.
Separate chi-square tests were used to analyze the influence of male color code (blue, yellow, red) and male size class (small, medium, large) on the probability of coupling, and the frequency with which different body parts of host larvae were attacked by the parasitoid (PROC FREQ; SAS Institute Inc. 2015).
In all analyses, differences between means of significant effects were tested using Tukey’s range test and all requirements of residual dispersion were met for all models unless otherwise stated.
Results
Mating Behavior
Occurrence of coupling significantly increased from 42 to 57 % with the number of males present in the cage (F1,399 = 8.56; P = 0.0036). Occurrence of coupling was almost ten times lower among previously-mated females (F1,399 = 29.32; P < 0.0001). Male (F1,399 = 0.72; P = 0.3963) or female age (F1,399 = 0.53; P = 0.4675) had no influence on the success of coupling.
About 25 % of females mated more than once during the experiments (n = 184). The mean number of matings per female during one mating session was 1.66 (±0.13 SEM, maximum 18 times over 4 h 48 min). The number of males present in the cage did not significantly influence the occurrence of multiple matings with one female (F2,181 = 0.02; P = 0.9815). From the experiment with differently colored males, 24 % (n = 54) of females mated more than once, most of those with different males (69 %; n = 13). Four females mated twice with the same male. Male hind tibia length (size) did not significantly influence success of coupling (χ2 2 = 2.5336; P = 0.2817). Color coding of males did not significantly influence the probability of mating (χ2 2 = 0.059; P = 0.9710).
Mating duration varied significantly between first (or only) and subsequent matings in multiple mating series (Fig. 1; F7,77 = 3.22, P = 0.0048). The first mating was shortest (420 s ± 10 SEM). The second was longest (999 s ± 97 SEM), but duration decreased gradually with subsequent matings down to 563 s (±93 SEM) after more than 5 matings in a single mating session. Results from the male-coloration experiment showed that mating durations were significantly influenced by an interaction between the individual male and the sequence in which males were mating with the female (F3,18 = 61.17; P < 0.0001). Mating duration was significantly longer when a female mated a second time with the same male during one mating bout. However, when mating for a second time with a different male, mating duration did not differ from the first mating (Fig. 2).
Potential Fecundity
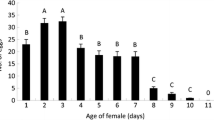
The oviducts of T. rostrale contained an average of 9.06 (±1.44 SEM) mature eggs within 24 h after emergence. This number increased significantly over the first three days after emergence (F6,68 = 25.92; P < 0.0001), and reached a mean maximum of 52.10 (±5.25 SEM) eggs after 7 days. No further increase or significant decrease was found thereafter until day 20 of adulthood (Fig. 3).
Longevity
Diet had a significant influence on longevity of T. rostrale (F3,118 = 135.9; P < 0.0001). Differences between males and females were small and non-significant (F1,118 = 3.59; P = 0.0607). There was no significant interaction between diet and sex (F3,118 = 1.99; P = 0.1185). Adult T. rostrale lived significantly longer on a 20 % sucrose water solution, with or without 1 % pollen, than on water alone or water with 1 % pollen (Fig. 4).
Longevity (days) and SEM of Tranosema rostrale males (dark grey) and females (light grey) on different diets (water, water with 1 % pollen, water with 20 % sucrose, and water with 20 % sucrose and 1 % pollen). Bars with the same letter do not differ significantly at P < 0.05 according to post hoc comparisons with Tukey’s range test
Host-Searching Behavior
The order in which host cues were presented to females did not influence the probability of antennation (F1,113 = 0.38; P = 0.5401) or probing (F1,113 = 3.34; P = 0.0701). But the cues themselves influenced both the probability of antennation (F5,113 = 3.57; P = 0.0049) and probing (F5,113 = 2.98; P = 0.0144). The probability of antennation seemed unaffected by the presence or absence of host cues (none were significantly different from cue-free controls). However, significantly fewer females responded with antennation to feces than to silk, damaged foliage or the whole host system (Fig. 5A). No female responded with probing in the absence of host cues and the probability of probing was significantly lower in the presence of feces alone when compared to other cues, except foliage (Fig. 5B; F4,94 = 3.10; P = 0.0191). The duration before occurrence of the behavior was not significantly influenced by host cues, whether for antennation (F5,72 = 0.64; P = 0.6689) or for probing (F4,45 = 1.25; P = 0.3045).
Oviposition Behavior
Tranosema rostrale’s oviposition behavior can be described as a sequence of five behaviors. (1) Antennation while searching for a host on the substrate (e.g., foliage). (2) Probing while searching and antennating. Once T. rostrale gets closer to a host larva, both antennation and probing become more frequent and insistent. (3) When the host is found by antennation, the parasitoid bends its abdomen under the thorax in front or deep into the foliage and pushes the ovipositor towards the host. When the larva is found by probing, the ovipositor is simply pushed forward under the cuticle of the host. (4) The oviposition itself only takes approximately 0.5–1.0 s, during which the parasitoid is immobile or tracks the host if it is attempting to escape. (5) Immediately after the attack, most females walk away but some resume oviposition behavior and attack the same host again. Even if some host larvae “bleed” after oviposition, no host feeding has been observed in more than 1000 observations done during this study.
Aside from being cryptic feeders, hidden from natural enemies in a silk-feeding tunnel, spruce budworm larvae exhibited several defensive behaviors against attack by T. rostrale. When the larva was approached or touched by the parasitoid, it responded with one or a combination of the following behaviors: vigorous movements, biting, and/or regurgitation. In some cases, larvae fell out of their feeding tunnel on a silk thread when the parasitoid approached. While this may lead to avoidance of parasitism in some cases, T. rostrale females were observed following the fresh silk thread to the bottom of the cage through antennation, where they found and attacked the larva.
Significant differences were found in the frequency of parasitoid attacks on different positions of the host’s body (Table 1; χ2 8 = 112.23; P < 0.0001). Dorsal attacks were more frequent (62.5 %) than ventral (26.6 %) or lateral (10.9 %) attacks.
The overall success of attack (defined above) was 32 % (n = 943), and there was no difference between mated (32 %; n = 835) and virgin (32 %; n = 108) females. There was a significant positive correlation between the female’s age and the success of attack (F1,928 = 35.13; P < 0.0001). However, even newly-emerged females successfully attacked spruce budworm larvae. Tranosema rostrale’s eggs were found in 83 % (n = 58) of spruce budworm larvae dissected after successful attack.
Discussion
Mating Behavior
Our laboratory results show that female T. rostrale are more likely to mate when there are more males present in a cage. In nature, female parasitoids can attract several males that compete for mating (Goh and Morse 2010), however, it is unknown whether this occurs in T. rostrale. One factor known to play an important role for both intra- and intersexual selection in parasitoids is male size (Grant et al. 1980; Charnov et al. 1981; Jones 1982). While we did observe fights between males for a single female, we found no relationship between male size and mating success in T. rostrale. Flight movements that take more space than provided by the cages in this experiment might be an important factor influencing mating success in the field.
The probability of mating was 10× higher for virgin T. rostrale females than for females that had mated on a previous day. However, among receptive T. rostrale females, 25 % mated multiple times in one mating session, in most cases with different males. Polyandrous females (mating multiple times) are thought to be relatively rare among hymenopteran parasitoids, one mating being generally sufficient to provide enough sperm to fertilize all eggs (Gordh and DeBach 1978; van Assem 1986; Ridley 1993; Godfray 1994).
To ensure paternity, males of some species have evolved morphological, physiological, and behavioral adaptations (Parker 1970; Knowlton and Greenwell 1984; Simmons 2001). Behavioral paternity enhancement mechanisms include prolonged mating duration, increased mating frequency (Thornhill 1984), post-copulatory interactions or restricting access to the female (called ‘mate guarding’) (Parker 1974; Gwynne 1984; Alcock 1994). When T. rostrale males mated a second time with the same female, mating duration was significantly increased. This prolonged mating for the second mating may be a post-copulatory ritual that prevents the female from mating with other males. In some parasitoid species, males remove the sperm from the precedent male before inseminating the female with their own (Simmons 2001). One indicator of sperm removal is that the mating time with the second male is considerably longer than with the first (Thornhill and Alcock 1983; Waage 1984). We found that in T. rostrale, mating duration is short the first time a male mates with a specific female, even if another male had previously mated with that female. Therefore, sperm of different males is most probably mixed in the female’s spermatheca.
Potential Fecundity
Our results demonstrate that T. rostrale is synovigenic, with an index of approximately 0.17 at 20 °C, meaning that its initial egg load at emergence is about 17 % of its potential life time fecundity (Jervis et al. 2001). This rate is most likely overestimated, as females in our experiments were not allowed to lay eggs, which may reduce the total number of eggs produced during a female’s lifetime due to capacity limits in the female’s oviduct. Most egg production occurred within the first three days of emergence, after which no further increase in the number of eggs was observed. This plateau in potential fecundity may be due to capacity limits of the oviducts, once again because females were not allowed to lay eggs.
Longevity
Adult parasitoids are known to be dependent on food sources in their habitat such as nectar and pollen from flowers (Jervis et al. 1993). Several laboratory studies have shown that longevity and fecundity of parasitoids are increased by access to flowers, nectar, honey or sugar-water (e.g., Syme 1975; Dyer and Landis 1996; Mathews and Stephen 1997; Fidgen and Eveleigh 1998). In T. rostrale, the addition of pollen to water as a source of protein, vitamin C, and iron did not increase longevity, but 20 % sucrose water solution increased longevity almost 6× for males and more than 11× for females, highlighting the importance of carbohydrates for longevity of this species. Natural sources of sugar in forest habitats are diverse and include flowers in the understory or trees and honeydew from sap-sucking insects (Wäckers et al. 2008). It remains unknown whether the fecundity of T. rostrale is also increased by an availability of carbohydrate- or protein-based food.
Host-Searching Behavior
It is well-established that parasitoids use chemical, visual, tactile and auditory cues from the host’s microhabitat and host plant, as well as indirect (derived from the activity of the host) and direct (derived from the host itself) cues from the host for host location (Vinson 1976; Godfray 1994). Volatile chemical cues are often used by the parasitoid for long-range host or host-habitat location, while contact chemicals and other cues are used for short-ranged host location (Vinson 1976). Our findings are restrained to short-range host location, as experiments were done in confined plastic cages. While T. rostrale’s antennation was not triggered by a specific host cue, probing occurred most often when a female encountered direct cues from the host, i.e. silk and the host larva itself, but also when damaged foliage was found and less frequently when larval feces were encountered. As spruce budworm larvae are more or less concealed in their feeding tunnel, probing into the foliage triggered by direct or indirect host cues might be the most effective way for T. rostrale to find and oviposit into the host. Females were observed probing for several minutes into empty feeding tunnels, while the host larva was just outside of the tunnel, suggesting that visual cues might not be important for this species at short range. In the field, T. rostrale does not exhibit any host instar preference (Seehausen et al. 2016). Therefore, it can be assumed that the host searching behavior of T. rostrale we describe here for fifth-instar spruce budworm larvae is applicable to other instars.
Oviposition Behavior
Insects have developed numerous morphological and behavioral defense mechanisms against parasitoids (Gross 1993). The relatively short duration of oviposition for T. rostrale that we observed might be necessary for the parasitoid to avoid host defensive behaviors such as biting, spitting or escaping, as also described for other parasitoid species (Prop 1960; Tripp 1960; Goff and Nault 1974). A defense mechanism that T. rostrale has apparently learned to circumvent is the retreat of the larvae on a silk thread. We show that silk can be detected by the parasitoid and that it triggers probing. Therefore, spruce budworm larvae “spinning-down” on a silk thread to escape parasitism can be easily tracked and parasitized by T. rostrale. Such behavior has also been reported for other parasitoid species (Yeargan and Braman 1986, 1989).
Some parasitoid species oviposit in specific locations of their host to avoid active removal of eggs (Herrebout 1968, Danks 1975; Martin et al. 1989), sclerotized host cuticles (Shaw and Huddleston 1991), or aggressive host defense behavior. Spruce budworm larvae are most frequently attacked by T. rostrale in the dorsal part of the body. This may simply be related to the position of the host larva in its feeding tunnel relative to T. rostrale search behavior. Tranosema rostrale crawls on top of the foliage when searching for hosts, probing between balsam fir needles to find the larva. However, by ovipositing through the foliage, T. rostrale avoids aggressive host defense behavior.
Mated and virgin T. rostrale females attacked spruce budworm larvae at similar rates. This behavior is consistent with the biology of haplodiploid parasitoids, where arrhenotokous females lay viable eggs (male) without mating. We observed female wasps readily attacking hosts within 24 h of emergence, and the probability of attack increased with female age. These behaviors may also be related to the reproductive biology of T. rostrale because females emerge with mature eggs available for oviposition and can continue to develop more during their life time regardless of mating status. It appears therefore that T. rostrale has neither an obligatory nor facultative preoviposition period in captivity.
Conclusion
Our work provides critical insight into key reproductive biology and behavior of T. rostrale. It is clear that a number of traits contribute to the success of this parasitoid in attacking low-density spruce budworm populations, namely: (1) its lack of a pre-mating or preoviposition period; (2) the relatively rapid maturity of its eggs soon after emergence despite being synovigenic; and (3) its efficacy in host searching and oviposition behavior that appears to successfully circumvent basic host defenses. The results reported here were obtained under laboratory conditions, using small experimental arenas, mating, host searching, and attack success in nature may differ. Our work also includes advanced methodological insights for rearing this important spruce budworm parasitoid in the laboratory, including: (1) its mating success is increased when more males are present; (2) mating and oviposition can take place immediately after emergence; and (3) sucrose water solution is sufficient to significantly increase parasitoid longevity, when compared to water only.
References
Alcock J (1994) Postinsemination associations between males and females in insects: the mateguarding hypothesis. Annu Rev Entomol 39:1–21
Berndt LA, Wratten SD (2005) Effects of alyssum flowers on the longevity, fecundity, and sex ratio of the leafroller parasitoid Dolichogenidea tasmanica. Biol Control 32:65–69
Blais JR (1965) Spruce budworm outbreaks in the past three centuries in the Laurentide Park, Quebec. For Sci 11:130–138
Charnov E, Los-den Hartogh RL, Jones WT, van den Assem J (1981) Sex ratio evolution in a variable environment. Nature 289:27–33
Cusson M, Barron JR, Goulet H, Régnière J, Doucet D (1998) Biology and status of Tranosema rostrale rostrale (hymenoptera: Ichneumonidae), a parasitoid of the eastern spruce budworm (Lepidoptera: Tortricidae. Ann Entomol Soc Am 91:87–93
Danks HV (1975) Factors determining levels of parasitism by Winthemia rufopicta (Diptera: Tachinidae), with particular reference to Heliothis spp. (Lepidoptera: Noctuidae) as hosts. Can Entomol 107:655–684
Dyer LE, Landis DA (1996) Effects of habitat, temperature, and sugar availability on longevity of Eriborus terebrans (hymenoptera: Ichneumonidae. Environ Entomol 25:1192–1201
Eveleigh ES, McCann KS, McCarthy PC, Pollock SJ, Lucarotti CJ, Morin B, McDougall GA, Strongman DB, Huber JT, Umbanhower J, Faria LDB (2007) Fluctuations in density of an outbreak species drive diversity cascades in food webs. P Natl Acad Sci USA 104:16976–16981
Fidgen JG, Eveleigh ES (1998) Life history characteristics of Elachertus cacoeciae (hymenoptera: Eulophidae), an ectoparasitoid of spruce budworm larvae, Choristoneura fumiferana (Lepidoptera: Tortricidae. Can Entomol 130:215–229
Flanders SE (1950) Regulation of ovulation and egg disposal in the parasitic hymenoptera. Can Entomol 82:134–140
Flanders SE (1965) On the sexuality and sex ratios of hymenopterous populations. Am Nat 99:489–494
Godfray HCJ (1994) Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press, Princeton
Goff AM, Nault LR (1974) Aphid cornicle secretions ineffective against attack by parasitoid wasps. Environ Entomol 3:565–566
Goh MZ, Morse DH (2010) Male mate search for female emergence sites by a parasitic wasp. Anim Behav 80:391–398
Gordh G, DeBach P (1978) Courtship behavior in the Aphytis lingnanensis group, its potential usefulness in taxonomy, and a review of sexual behavior in the parasitic hymenoptera (Chalcidoidea: Aphelinidae. Hilgardia 46:37–75
Grant B, Burton S, Contoreggi C, Rothstein M (1980) Outbreeding via frequency-dependent mate selection in the parasitoid wasp, Nasonia (= Mormoniella) vitripennis Walker. Evolution 34:983–992
Gross P (1993) Insect behavioral and morphological defenses against parasitoids. Annu Rev Entomol 38:251–273
Gwynne DT (1984) Male mating effort, confidence of paternity, and insect sperm competition. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Orlando, pp. 117–144
Herrebout WM (1968) Some aspects of host selection in Eucarcelia rutilla Vill. (Diptera: Tachinidae). Neth. J Zool 19:1–104
Jervis MA, Kidd NAC (1986) Host-feeding strategies in hymenopteran parasitoids. Biol Rev 61:395–434
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 27:67–105
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NA (2001) Life-history strategies in parasitoid wasps: a comparative analysis of ‘ovigeny. J Anim Ecol 70:442–458
Jones WT (1982) Sex ratio and host size in a parasitoid wasp. Behav Ecol Sociobiol 10:207–210
Knowlton N, Greenwell SR (1984) Male sperm competition avoidance mechanisms: the influence of female interests. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Florida, pp. 62–83
Lethiecq J-L, Régnière J (1988) CFS spruce budworm population studies: site descriptions. Information Report LAU-X-83. Canadian Forest Service, Sainte-Foy, Quebec, Canada
Martin WR Jr, Nordlund DA, Nettles WC Jr (1989) Ovipositional behavior of the parasitoid Palexorista laxa (Diptera: Tachinidae) on Heliothis zea (Lepidoptera: Noctuidae) larvae. J Entomol Sci 24:460–464
Mathews PL, Stephen FM (1997) Effect of artificial diet on longevity of adult parasitoids of Dendroctonus frontalis (Coleoptera: Scolytidae. Environ Entomol 26:961–965
Morin H (1994) Dynamics of balsam fir forests in relation to spruce budworm outbreaks in the boreal zone of Quebec. Can J For Res 24:730–741
National Forestry Database (NFD) (2013) http://nfdp.ccfm.org/data/compendium/html/comp_41e.html. Accessed 29 September 2015
Nealis VG (1991) Natural enemies and forest pest management. The Forest Chron 67:500–505
Parker GA (1970) Sperm competition and its evolutionary consequences in insects. Biol Rev Camb Philos 45:525–567
Parker GA (1974) Courtship persistence and female guarding as male time investment strategies. Behaviour 48:157–184
Prop N (1960) Protection against birds and parasites in some species of tenthredinid larvae. Arch Néerl Zool 13:380–447
Quicke DL (2014) The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics. Evolution and Ecology. John Wiley & Sons, Ltd, Chichester
Régnière J, Nealis VG (2007) Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol Entomol 32:461–477
Régnière J and Lysyk TJ (1995) Population dynamics of the spruce budworm, Choristoneura fumiferana. In: Armstrong JA an Ives WGH (eds) Forest Insect Pests in Canada National Resources of Canada, Canadian Forest Service, Ottawa, Canada, pp 95–105
Régnière J, Delisle J, Pureswaran DS, Trudel R (2013) Mate-finding allee effect in spruce budworm population dynamics. Entomol Exp Appl 146:112–122
Ridley M (1993) Clutch size and mating frequency in parasitic hymenoptera. Am Nat 142:893–910
Royama T (1984) Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr 54:429–462
SAS Institute Inc (2015) SAS Studio 3.4: User's Guide. SAS Institute Inc, Cary
Seehausen ML, Bauce E, Régnière J (2013) Does spruce budworm (Lepidoptera: Tortricidae) rearing diet influence larval parasitism? Can Entomol 145:539–542
Seehausen ML, Bauce E, Régnière J, Berthiaume R (2014) Influence of partial cutting on endemic spruce budworm (Lepidoptera: Tortricidae) populations. Environ Entomol 43:626–631
Seehausen ML, Régnière J, Martel V, Smith SM (2016) Seasonal parasitism and host instar preference by the spruce budworm (Lepidoptera: Tortricidae) larval parasitoid Tranosema rostrale (Hymenoptera: Ichneumonidae). Environ Entomol. doi:10.1093/ee/nvw081
Shaw MR, Huddleston T (1991) Classification and biology of braconid wasps. In: Dolling WR, Askew RR (eds) Handbooks for the identification of British insects, Vol 7, Part 11. Royal Entomological Society, London, pp. 1–126
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton
Syme PD (1975) The effects of flowers on the longevity and fecundity of two native parasites of the European pine shoot moth in Ontario. Environ Entomol 4:337–346
Thornhill R (1984) Alternative hypotheses for traits believed to have evolved by sperm competition. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Orlando, pp. 151–176
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Cambridge University Press, Cambridge
Tripp HA (1960) Spathimeigenia spinigera Townsend (Diptera: Tachinidae), a parasite of Neodiprion swainei Middleton (hymenoptera: Tenthredinidae. Can Entomol 92:347–359
Unites States Department of Agriculture (USDA) Forest Service (2009) Major Forest Insect and Disease Conditions in the United States 2007. . FS-919, Washington
van den Assem J (1986) Mating behaviour in parasitic wasps. In: Waage JK, Greathead D (eds) Insect Parasitoids. Academic Press, London, pp. 137–167
Vinson SB (1976) Host selection by insect parasitoids. Annu Rev Entomol 21:109–133
Waage JK (1984) Alternative hypotheses for traits believed to have evolved by sperm competition. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems Academic Press, Orlando, pp 251–290
Wäckers FL, van Rijn PC, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal? Biol Control 45:176–184
Yeargan KV, Braman SK (1986) Life history of the parasite Diolcogaster facetosa (weed) (hymenoptera: Braconidae) and its behavioral adaptation to the defensive response of a lepidopteran host. Ann Entomol Soc Am 79:1029–1033
Yeargan KV, Braman SK (1989) Life history of the hyperparasitoid Mesochorus discitergus (hymenoptera: Ichneumonidae) and tactics used to overcome the defensive behavior of the green cloverworm (Lepidoptera: Noctuidae. Ann Entomol Soc Am 82:393–398
Acknowledgments
We are grateful to P. Bolduc, S. Ouellet, M. Leblanc, A.-M. Dion, S. Trudeau, S. Parent, M. Moisan, and H. K. Nenzén for help in the field and laboratory and to P. Therrien, D. Simoneau, G. Trudel, and C. Dussault of the Ministère des Forêts, Faune et Parcs Québec (MFFPQ) for insect rearing. Many thanks also to L. Royer and M. Cusson from the Canadian Forest Service for helpful discussions. Financial and in-kind support was granted through SERG-International by the provincial governments of Ontario, Quebec, Newfoundland, Nova Scotia, Saskatchewan and the Société de protection des forêts contre les insectes et maladies (SOPFIM), as well as by the Atlantic Canada Opportunities Agency and Natural Resources Canada (Canadian Forest Service). Funding for MLS was provided by the Ontario Trillium Scholarship through the University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Seehausen, M.L., Labrecque, M., Martel, V. et al. Reproductive Biology and Behavior of Tranosema rostrale (Hymenoptera: Ichneumonidae), a Parasitoid of Low-Density Spruce Budworm (Lepidoptera: Tortricidae) Populations. J Insect Behav 29, 500–514 (2016). https://doi.org/10.1007/s10905-016-9576-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-016-9576-y