Abstract
Male and female age are important factors that can influence mating and remating behavior. Females can discriminate against or prefer older males, but there have been relatively fewer studies on how female and male age influence female remating. Here we showed in wild flies of the Mexican fruit fly Anastrepha ludens (Loew), that when females were given a choice between males of different ages, younger females preferred to mate with younger males over older males, while older females were less selective. Also, when given a choice between males of different ages, older females had longer copulation durations than younger females. On the other hand, older males and females had lower mating success, compared with young and middle-aged flies under no choice conditions. However, middle-aged females mated faster compared to young females and young males mated faster compared to middle-aged males. Male age did not influence female remating, while female age strongly determined female remating, with no females remating when they were old. It is unclear if female receptivity mechanisms are switched off at older ages, or if females are reluctant to remate due to possible costs of mating. We discuss our results in terms of how male and female age can influence mating decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In all taxa in general, and specifically in insects, male age can influence reproductive success. Specially, it can affect copulation duration (Koref-Santibanez 2001; Pérez-Staples et al. 2008, 2010), mating success (Zuk 1988; Jones 2000; Jones and Elgar 2004; Fricke and Maklakov 2007; Pérez-Staples et al. 2010; Papanastasiou et al. 2011) and sperm traits (Taylor et al. 2001; Pérez-Staples et al. 2008; Papanastasiou et al. 2011; Costa et al. 2012; Decanini et al. 2013; Santhosh and Krishna 2013). Females may prefer to mate with young males as they have less deleterious mutations in the germline and are more fertile (Hansen and Price 1995, 1999; Beck and Powell 2000; Beck and Promislow 2007). However, female choice can also be tilted towards older males, who have demonstrated their ability to survive (Manning 1985; Kokko and Lindstrom 1996; Kokko 1998), or produce more honest signals (Proulx et al. 2002). In systems where males only provide sperm to their mates and no other direct benefits, females will be more discriminatory against older males (see Johnson and Gemmell 2012 for a review).
Female age also may influence their mating behavior. Young females are usually more selective as female choosiness can decline with age (Prosser et al. 1997; Gray 1999; Bonduriansky 2001; Xu and Wang 2009; Shelly et al. 2011). For example, in the crickets Acheta domesticus and Gryllus integer and the Mediterranean fruit fly Ceratitis capitata, young females are selective and able to discriminate their mating partners based on their age, while older females are not (Prosser et al. 1997; Gray 1999; Shelly et al. 2011). In the cockroach Nauphoeta cinerea, and the leafroller Choristoneura rosaceana older females have shorter mating latencies and mate earlier as female age increases (Delisle 1995; Moore and Moore 2001). Female choice will be conditioned not only by their sensory ability to distinguish the quality of their partner through courtship or pheromone blends, but also by the direct or indirect benefits gained by such a selection.
There have been fewer studies on how male or female age affects the post-copulatory behavior of females such as their remating propensity. In systems where males do not provide parental care, we expect older males to invest more in their ejaculate in terms of sperm or accessory gland products (AGPs) compared to younger males, as older males will have reduced remaining lifetime for future reproductions (Brooks and Kemp 2001; Benowitz et al. 2013). For example, in Drosophila pseudoobscura and Drosophila subobscura, females prefer to mate with older males and these females produce more offspring, a direct benefit possibly due to increased investment in sperm, seminal fluids or both (Avent et al. 2008; Verspoor et al. 2015). In many insects, both sperm and/or AGPs transferred during mating inhibit females from remating (Gillott 2003; Wedell 2005). If older males invest more in their ejaculate, then we could expect females mating with these males to have lower remating rates. On the contrary, if senescent males have lower ejaculate quality we could expect females to have a higher remating propensity when they mate with older males.
Various studies have reported an association between male age and female remating. For example, in C. capitata, middle-aged males (11 d-old) are more effective in reducing female sexual receptivity than younger (4 d-old), or older (18 d-old) males (Gavriel et al. 2009). Similarly, Shelly et al. (2007) found that middle-age males (5 or 10 d-old) are more effective in inhibiting female remating, compared to younger males (3–4 d-old). However, in another study on this same species, female remating was not influenced by male age (from 4 to 20 days-old) (Costa et al. 2012). Likewise, in the Queensland fruit fly Bactrocera tryoni, male age did not affect their ability to inhibit female remating (Pérez-Staples et al. 2008). Finally, in the butterfly Bicyclus anynana, females mated with old males had higher remating propensity and shorter refractory periods than females mated with young males (Karl and Fischer 2013). Females mating with older males may be more likely to remate if they can reduce costs from low sperm viability and increased mutations in the germ line associated with older sperm (Radwan 2003; Gasparini et al. 2010; reviewed in Johnson and Gemmell 2012).
There are few examples where the relationship between female age and remating behavior has been explored. Young females with better physical condition can perhaps better resist male manipulation and re-mate more frequently, if remating is advantageous for them. Indeed there are some examples where younger females are more likely to remate than older females such as in Drosophila nasuta nasuta, Drosophila nasuta albomicans and Drosophila sulfrigaster neonasuta (Shruti et al. 2012). Alternatively, males could invest less effort in manipulating older females, if they have lower fecundity compared to younger females and therefore older females would remate more often. In Drosophila nasuta kepulauana and Drosophila sulfurigaster sulfurigaster older females do not remate more often, but despite a longer sexual refractory period, are just as likely to remate as younger females. There is evidence that males allocate fewer resources to older females. In D. melanogaster, males allocate fewer sperm to older than younger females (Lüpold et al. 2011); and in the moth Ephestia kuehniella, males mate preferentially with younger females, allocate more sperm to younger and middle-age females but younger females remate more frequently (Xu and Wang 2009). Thus, overall it seems that there is more evidence for a decline in female remating propensity with age.
Here, we studied female choice of young and old females with young and old males of wild Anastrepha ludens (Diptera: Tephritidae). Additionally, we evaluated mating and remating behavior of young females mated with males of different ages and young males mated with females of different ages. We predicted that older males would be less efficient than young males in inhibiting female sexual receptivity, with younger females having higher remating rates compared to older females.
Anastrepha ludens is a long-lived species, with an average life expectancy of approximately 50 days under laboratory conditions (Carey et al. 2005). Older sexually experienced males (36 d-old) have increased mating success compared to younger males (18 d-old), although no benefits in terms of fecundity or fertility for their mates were found (Pérez-Staples et al. 2010). Male age did not affect copulation duration (Pérez-Staples et al. 2010), but female age did; older females had longer copulation durations than younger females (Pérez-Staples et al. 2014). Insemination success declines with male age (Harwood et al. 2015), sperm viability tends to decline with age in wild flies (Herrera-Cruz M. unpublished data) and older females (35 d-old) have a tendency to store more sperm (Pérez-Staples et al. 2014). Although the exact mechanism by which males inhibit female remating is unknown in this species, there seems to be a synergic effect of both sperm and AGPs (Abraham et al. 2014, 2016).
Materials and Methods
Insects
Wild flies were recovered from infested oranges (Citrus aurantium) collected at Tuzamapan (N 19° 24′ 125″, W 96° 52′ 567″), Xalapa, Veracruz, Mexico. Fruits were taken to the laboratory and placed in 30 x 50 x 15 cm plastic trays with soil. Larvae migrated from the fruits to the soil where they pupated. After 7–10 days, the soil was sieved and recovered pupae were placed in 30 x 30 x 30 cm cages at 26 ± 2 °C and 80 ± 10 % RH until adult emergence. Artificial light (fluorescent tubes) was provided under a 12:12 photoperiod, with half the lights going off or on at 6:00 and the other half at 6:30 to simulate dawn or dusk. Natural light was also provided from a window. Experiments were carried out during 2014 and 2015 at the Instituto de Biotecnología y Ecología Aplicada, Universidad Veracruzana, Xalapa, Veracruz, Mexico.
On the day of emergence, flies were sorted by sex and transferred to 30 x 30 x 30 cm cages in groups of 100–200 adults. Both sexes were provided with water and a diet consisting of sugar and hydrolyzed yeast (Yeast Hydrolyzated Enzymatic, ICN Biomedicals ®) in a ratio of 3:1. Fly ages used for the experiments are shown in Table 1.
Female Mate Choice
Two days before the experiment, males were marked with a dot of acrylic paint on the thorax (Politec®) in order to identify their age (older males: 65–74 d-old; young males: 19–23 d-old). This procedure does not affect the sexual performance of flies (Petit-Marty et al. 2004; Meza et al. 2005). The day of testing, one virgin female (65–74 d-old or 19–23 d-old) and two virgin males (one old and one young male) were placed in 250 mL plastic containers at approximately 16:00 h. Containers were checked for copulating pairs every five min for 6 h after releasing flies. Mating latency, copulation duration, the number of copulating pairs and the color of the mating male were recorded. After the end of copulation, flies were discarded. The experiment was conducted in a dark room lit with a dim red lamp to allow observation.
Mating and Remating Behavior under no Choice Conditions
On the day of the experiment, one virgin female and one virgin male of each age (see Table 1) were placed in 250 mL plastic containers at 16:00 h. Containers were checked for copulating pairs every five minutes for 6 h after releasing flies. Mating latency, copulation duration and the number of copulating pairs were recorded. After the end of copulation, the males were discarded and the females were kept singly in the plastic container with water and adult diet ad libitum. Two days later, one virgin young male (14–27 d-old) per female was offered at 16:00 h. Containers were checked for copulating pairs every five min for 6 h after releasing flies. Mating latency, copulation duration and the number of copulating pairs were recorded. Mating and remating experiments were conducted in a dark room lit with a dim red lamp to allow observation.
Statistical Analyses
The experimental unit was defined as the cup with a female and one/two males. All female-male age combinations were repeated at least twice and data were pooled for analysis.
Mating success was estimated as the proportion of pairs that mated out of all possible matings. Remating behavior was estimated as the proportion of mated females that remated out of all possible rematings. Mating latency was defined, as the time between the placement of the last pair in the cups and the beginning of each copulation. Copulation duration was recorded as the time the pair engaged in copulation and then separated.
Female preference under choice condition was analyzed with χ2-tests of goodness of fit. To analyze mating latency and copulation duration under choice conditions we applied an ANOVA with female age, male age and their interaction as independent variables and mating latency or copulation duration as response variables. A non-parametric Kruskal–Wallis was used when ANOVA assumptions (normality and homoscedasticity) were not met.
Under no choice conditions, the number of copulation pairs (mating success) was analyzed with χ2-tests of Homogeneity. For a comparison among more than two treatments, the sequential Bonferroni method (Rice 1989) was applied after χ2-tests. To analyze mating latency and copulation duration we applied a non-parametric Kruskal–Wallis test with the combination of female-male age as independent variables and mating latency or copulation duration as response variables. Multiple comparisons were carried out by Dunn’s test at a significance level of α = 0.05.
Results
Female mate choice (choice conditions)
Female Preference
More young females mated (67.8 %, 61/90) compared to old females (57.8 %, 52/90). Young females discriminated against older males (χ2 of goodness of fit, χ2 = 20.08; df = 1; P < 0.0001) while older females did not (χ2 = 3.77; df = 1; P = 0.151) (Fig. 1).
Mating percentage of young and old wild Anastrepha ludens females given a choice between one young or one old male. Numbers within bars represent sample size. Comparisons were done within each female age. Different letters over bars indicate significant differences (χ2-tests of goodness of fit, P < 0.0001)
Mating Latency
There was no significant effect of female age, male age nor their interaction on mating latency (ANOVA, F = 1.08; df = 1, 109; P = 0.30 female age; F = 3.82; df = 1, 109; P = 0.053 male age; F = 0.41; df = 3, 109; P = 0.523 female x male age) (Fig. 2a).
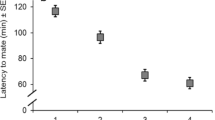
a Mating latency (min, mean + se) and b copulation duration (min, Q1-median-Q3) of different combinations of female and male ages under female choice conditions in wild Anastrepha ludens. Numbers within bars represent sample size. a There were no significant differences between the mating latency of young or old males or females (see text). b Different letters indicate significant differences after Dunn’s test
Copulation Duration
Female age affected copulation duration. Older females mated longer than younger females (Kruskal-Wallis, H = 13.00; df = 1, 109; P = 0.004) (Fig. 2b).
Mating and Remating Behavior under no Choice Conditions
Mating Success and Remating Behavior
Female mating success was significantly affected by male age (χ2 = 15.21; df = 2; P = 0.0015). Young females were less likely to mate with older males, while there was no significant difference in mating likelihood with middle age and young males (χ2 = 15.20; df = 1; P < 0.0001) (Fig. 3a). Similarly, male mating success was significantly affected by female age (χ2 = 27.17; df = 2; P < 0.0001). Young males were less likely to mate with older females, while there was no significant difference in mating likelihood with middle age and young females (χ2 = 26.20; df = 1; P < 0.0001) (Fig. 3b).
Mating percentage of young females mated with old, middle age or young males (a) and young males mated with old, middle age or young females (b) of Anastrepha ludens wild flies, under no choice (1♀:1♂ of each age per cage). To aid in comparison, the young male and female column is repeated in the data for young males. Numbers within bars represent sample size. Different letters over bars indicate significant differences (P < 0.0001) after the sequential Bonferroni method (Rice 1989) following χ2-tests
Female age determined remating frequency; female remating was higher in younger females, while no females remated when they were old (χ2 = 6.62; df = 1; P = 0.040). Male age did not influence female remating frequency (χ2 = 0.20; df = 2; P = 1.0) (Fig. 4).
Remating percentage of young Anastrepha ludens wild females mating originally with old, middle age or young males (a); remating of middle-aged and old females first mated with young males (b). Rematings with young males were observed 48 h after the initial copulation. To aid in comparison the young male and female column is repeated in the data for young males. Numbers within bars represent sample size. Different letters over bars indicate significant differences (P < 0.0001) after the sequential Bonferroni method (Rice 1989) following χ2-tests
The drop in mating success experienced by older males compared to young males was more pronounced when older males where under competition by younger rivals that when there was no competition (Fig. 5).
Mating Latency
Female and male age had a significant effect on mating latency (H = 31.24; df = 266, 4; P < 0.0001). Middle-age females mated earlier, followed by old females and young females. In contrast, young males mated earlier, followed by old males, while middle-age males mated last (Table 2).
Old females did not remate, thus only four combinations could be analyzed. The age of the first mate also affected the latency to mate for the second copulation (H = 8.47; df = 32, 3; P = 0.0372). Young females mated with older males mated later, compared to the other three combinations (Table 2).
Copulation Duration
Neither female nor male age affected copulation duration of the first or second copulation (H = 0.74; df = 266, 4; P = 0.940, first copulation; H = 3.87; df = 32, 3; P = 0.276, second copulation) (Table 2).
Discussion
Here, we showed using wild flies that when females were given a choice between differently aged males, younger females preferred younger males, whilst older females were less selective. Female age also had an effect on copulation duration; old females mated for longer compared to young females. Under no choice laboratory conditions, male age did not influence female remating, while female age strongly determined female remating; older females did not remate. On the other hand, older males and females had lower mating success, compared with young and middle-aged males and females. Middle-aged females and young males mated sooner.
One important issue that complicates comparisons among species and studies is that the terms “young” and “old” do not always mean the same ages. While in some cases the age differences are only a few days, in other cases flies are tested from emergence to senescence (Papanastasiou et al. 2011; Benelli et al. 2013; Harwood et al. 2015). On the other hand, time of sexual maturation differ among species. In A. ludens male reproduction (at 23 ± 3 °C) has been described in three phases: i) reproductive onset, from 0 to 20 days, ii) reproductive maturity between 25–30 days old (where the highest levels of insemination success are reached), and iii) reproductive senescence, after 30 days when insemination success begins to decline (Harwood et al. 2015). Here, we were interested in testing males and females that were already reproductively mature, and comparing them to older senescent males and females. Thus, our “young” category is already reproductively mature, while “middle-aged” and “old” flies correspond to the onset of senescence and senescence, respectively.
Female Mating Preferences under Choice or no Choice Conditions
Johnson and Gemmell (2012) in their review highlight the importance of competitive and non-competitive tests with males of different ages to distinguish between female choice with and without rival males. The difference between both situations could be interpreted as male-male interactions acting against female preferences (Verspoor et al. 2015). Here, we showed that when females were given a choice between differently aged males, younger females preferred younger males, whilst older females were less selective. Under no choice conditions, females were also more likely to mate with younger males, but older males had higher mating success than when females were given a choice. Similarly, in C. capitata, males experienced a drastic decrease in mating performance with age when competing against younger rivals. However, under no choice conditions, males as old as 50–70 d were able to obtain copulations as their younger counterparts (Papanastasiou et al. 2011).
Female discrimination against old males may occur because of a variety of factors, among which are an increase in sperm mutation and lower fertility (Johnson and Gemmell 2012). The mechanism by which females distinguish between young or old males is not known in Tephritidae. Our results suggest that not only are intrinsic male factors important, but that also some comparison between rivals aids females in discriminating between them. Courtship songs in the cricket Gryllus campestris and the fruit flies D. melanogaster and D. montana and pheromone blends in male boll weevil Anthonomus grandis may be used by females as cues to evaluate male age (Moulin et al. 2001; Spurgeon 2003; Jacot et al. 2007; Hoikkala et al. 2008). In the olive fly Bactrocera oleae young males (up to 11 d-old) secrete different components from their glands associated with the rectal ampulla, compared to older males. Although females secrete the same compound, this difference between males could perhaps be used by females as a signal of male age (Canale et al. 2012, cited in Benelli et al. 2013). Similarly, in the South American fruit fly A. fraterculus, the cuticular hydrocarbons (CHC) profile changes as both males and females age. The composition is similar between sexes until 5 days of age, then changes and becomes completely different in flies of 20 and 30 days old. Also, the absolute amount of CHCs tends to decrease with male age but not female age (Vanicková et al. 2012). Changes in CHCs profiles with male age have also been observed in the housefly Musca domestica and the malaria vector Anopheles gambiae (Mpuru et al. 2001; Caputo et al. 2005). CHCs are mixtures used by insects as cues for recognition of species and gender, thus, they could also be used by females to recognize the age of potential partners and aid in mate choice (Blomquist and Bagneres 2010).
Male Mating Latency, Copulation Duration and Mating Success
Wild middle-aged males took longer to mate compared with young or old males, while copulation duration was not affected by male age. This last finding is consistent with previous studies in this species (Pérez-Staples et al. 2010). Despite the fact that middle-aged males had longer latencies to mate, their mating success was higher compared to older males. A higher mating success for middle-aged males corresponds to previous field cage studies for this species were 36 d-old non-virgin males had higher mating success, compared with 18 d-old males (Pérez-Staples et al. 2010). However, in that study 36-d old males were the old males and no further ages were tested. In mass-reared A. ludens males, mating success increased progressively from 6 d, reaching a peak at 21 d, and then decreasing until 57–65 d-old (Reyes-Hernández M and Pérez-Staples D. Mating senescence and reproductive organ size in the Mexican fruit fly. Physiol. Entomol. as submitted). Similarly, in other species such as the leafroller Ch. rosaceana, the lekking sand-fly Lutzomyia longipalpis, the hide beetle Dermestes maculatus and the cabbage beetle Colaphellus bowringi, middle-aged males had higher mating success (Delisle 1995; Jones 2000; 2007; Jones and Elgar 2004; Hale et al. 2008; Liu et al. 2011).
Female Mating Latency, Copulation Duration and Mating Success
Older females were not as selective as young females. Because older females have reduced potential for future reproduction the cost of discriminating against an older male may be higher than for younger females (Manning 1985; Beck and Powell 2000). Alternatively, older females perhaps cannot distinguish between old and young males (Beck and Promislow 2007), implying that females have lost this ability with age.
Wild A. ludens middle-aged females mated faster, and young and middle-aged females had higher mating success compared to older females. Thus, the highest mating success coincided for both sexes when they reached middle age. Similarly, in mass-reared A. ludens females, mating success increases with age, has a peak at 21 d-old and then decreases until 65 d-old, when only ~65 % of females copulated (Reyes-Hernández and Pérez-Staples submitted). In D. ananassae, D. bipectinata and the cabbage beetle, Colaphellus bowringi middle-aged females also had significantly greater mating success, mated faster and copulated longer than younger or older females (Prathibha and Krishna 2010; Somashekar and Krishna 2011; Liu et al. 2014).
Female Remating Behavior
It has been suggested that female polyandry may serve to reduce the cost of mating with an old male (Radwan 2003; Pizzari et al. 2008; Gasparini et al. 2010). If mating with old males is costly for females because of lower sperm quality due to an accumulation of deleterious mutations, then females may reduce the risk that their eggs will be fertilized with old sperm via remating (Johnson and Gemmell 2012). Indeed in wild A. ludens sperm viability tends to decrease with age (Herrera-Cruz M. unpublished data). Thus, we expected higher female remating for females that had mated with older males. However, male age did not influence female remating. In mass-reared A. ludens flies, male age does influence female remating, but at younger ages that were not tested here. For example, females mated with 9 d-old mass-reared males were less likely to remate, compared with females mated with 21 d-old males (Reyes-Hernández and Pérez-Staples as submitted). These results suggest that 14 d-old males are sexually and reproductively mature, while at 9 days a male may be able to mate but may not be reproductively mature. At very young ages, the ejaculate could be of lower quality or quantity and thus affect female remating. Similarly, in C. capitata very young males were less likely to inhibit female remating (Shelly et al. 2007; Gavriel et al. 2009). Once males are reproductively mature, then male age may not have an impact on female remating. For example in B. tryoni, male age (8–28 d) did not influence female remating (Pérez-Staples et al. 2008). At these ages males are sexually mature, but the size of the testes and accessory glands suggests that they are also reproductively mature (Pérez-Staples et al. 2011).
We found that female age had a profound effect on their remating behavior. Female remating decreased as age increased. Similarly, in the moth E. kuehniella, younger females remate more often than middle-aged and old females (Xu and Wang 2009). However, we do not know if (i) older females had intrinsic characteristics that make them less receptive with age, (ii) older females have lower thresholds to resist male manipulation, or (iii) males invest more in inhibiting remating of older females. Maklakov et al. (2006) demonstrated that females of the seed beetle Acanthoscelides obtectus selected for early reproduction had increased remating rates as they aged, while females selected for late reproduction had decreased remating rates later in life. In A. fraterculus, females with higher fecundity also have higher remating rates while those with lower fecundity have lower remating rates (Abraham et al. 2011). Even though we did not evaluate female fecundity, it is possible that more fecund females, with a propensity to be polyandrous, died earlier; while females with late fecundity, with a tendency to be monandrous, lived longer. On the other hand, it is unlikely that males invest more in inhibiting remating of older females, due to in general, older females have less offspring viability and fecundity (Hercus and Hoffmann 2000; Moore and Moore 2001; Carey et al. 2005; Xu and Wang 2009; Lüpold et al. 2011). However, we cannot discard the possibility that male investment in the ejaculate and thus remating inhibition could be mediated by other female characteristics such as female size. In various insects, males deliver larger ejaculates to larger females (Wedell et al. 2002). Thus, bigger females could live longer and males could invest more in inhibiting receptivity in those females. However, in A. ludens males do not deliver more sperm to older, bigger or more fecund females (Pérez-Staples et al. 2014). Thus, it seems more likely that age effects on female remating are determined by intrinsic female factors rather than by males delivering a larger ejaculate and inhibiting older females.
Why would a female “choose” to be monandrous? The fact that no old females remated even when given the opportunity to remate with a younger male suggests that perhaps there is some cost to remating, or that receptors in older females do not allow a renewal of female receptivity. This opens interesting questions as to intrinsic and external factors that influence female sexual receptivity.
References
Abraham S, Goane L, Rull J, Cladera J, Willink E, Vera MT (2011) Multiple mating in Anastrepha fraterculus females and its relationship with fecundity and fertility. Entomol Exp App 141:15–24
Abraham S, Nuñez-Beverido N, Contreras-Navarro Y, Pérez-Staples D (2014) Female receptivity in Anastrepha ludens (Diptera: Tephritidae) is not modulated by male accessory gland products. J Insect Physiol 70:41–48
Abraham S, Contreras-Navarro Y, Núñez-Beverido N, Lara L, Ovruski S, Pérez-Staples D (2016) The male ejaculate as inhibitor of female remating in two tephritid flies. J Insect Physiol 88:40–47
Avent TD, Price TAR, Wedell N (2008) Age-based female preference in the fruit fly Drosophila pseudoobscura. Anim Behav 75:1413–1421
Beck CW, Powell LA (2000) Evolution of female mate choice based on male age: are older males better mates? Evol Ecol Res 2:107–118
Beck CW, Promislow DEL (2007) Evolution of female preference for younger males. PLoS One 2:e939
Benelli G, Bonsignori G, Stefanini C, Raspi A, Canale A (2013) The production of female sex pheromone in Bactrocera oleae (Rossi) young males does not influence their mating chances. Entomol Sci 16:47–53
Benowitz KM, Head ML, Williams CA, Moore AJ, Royle NJ (2013) Male age mediate reproductive investments and response to paternity assurance. Proc R Soc B 280:20131124
Blomquist GJ, Bagneres AG (2010) Insect hydrocarbons biology, biochemistry, and chemical ecology. University Press, New York
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Brooks R, Kemp DJ (2001) Can older males deliver the good genes? T Ecol Evol 16:308–313
Canale A, Carpita A, Conti B, Canovai R, Raspi A (2012) Effect of age on 1,7-dioxaspiro-[5.5]- undecane production in both sexes of olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). In: De Cristófaro A, Di Palma A (eds) Proceedings of the meeting "Integrated Protection of Fruit Crops". Vico del Gorgano, September 12-17-2010 IOBC Bulletin 74:219–225
Caputo B, Dani FR, Horne GL, Petrarca V, Turillazzi S, Coluzzi M, Priestman AA, Torre A d (2005) Identification and composition of cuticular hydrocarbons of the major Afrotropical malaria vector Anopheles gambiae s.S. (Diptera: Culicidae): analysis of sexual dimorphism and age-related changes. J Mass Spectrom 40:1595–1604
Carey JR, Liedo P, Muller H-G, Wang J-L, Senturk D, Harshman L (2005) Biodemography of a long-lived tephritid: reproduction and longevity in a large cohort of female Mexican fruit flies, Anastrepha ludens. Exp Gerontol 40:793–800
Costa AM, Anjos-Duarte CS, Roriz AKP, Dias VS, Joachim-Bravo IS (2012) Male diet and age influence to inhibit female remating in Ceratitis capitata (Diptera: Tephritidae). J Appl Entomol 136:456–463
Decanini DP, Wong BBM, Dowling DK (2013) Context dependent expression of sperm quality in the fruitfly. Biol Lett 9:20130736
Delisle J (1995) Effect of male and female age on the mating success of the obliquebanded leafroller Chroristoneura rosaceana (Lepidoptera: Tortricidae) under different ecological condition. J Insect Behav 8:781–799
Fricke C, Maklakov AA (2007) Male age does not affect female fitness in a polyandrous beetle, Callosobruchus maculatus. Anim Behav 74:541–548
Gasparini C, Marino IAM, Boschetto C, Pilastro A (2010) Effect of male age on sperm trait and sperm competition success in the guppy (Poecilia reticulata). J Evol Biol 23:124–135
Gavriel S, Gazit Y, Yuval B (2009) Remating by female Mediterranean fruit flies (Ceratitis capitata, Diptera: Tephritidae): temporal patterns and modulation by male condition. J Insect Physiol 55:637–642
Gillott C (2003) Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol 48:163–184
Gray DA (1999) Intrinsic factors affecting female choice in house cricket: time cost, female age, nutritional condition, body size and size-relative reproductive investment. J Insect Behav 12:691–700
Hale JM, Elgar MA, Jones TM (2008) Sperm quantity explains age related variations in fertilization success in the hide beetle. Ethology 114:797–807
Hansen TF, Price DK (1995) Good genes and old age: do old mates provide superior genes? J Evol Biol 8:759–778
Hansen TF, Price DK (1999) Age- and sex-distribution of the mutation load. Genetica 106:251–262
Harwood JF, Chen K, Liedo P, Müller H-G, Wang J-L, Morice AE, Carey JR (2015) Female access and diet affect insemination success, senescence and the cost of reproduction in the male Mexican fruit fly Anastrepha ludens. Physiol Entomol 40:65–71
Hercus MJ, Hoffmann AA (2000) Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc R Soc Lond B 267:2105–2110
Hoikkala A, Saarikettu M, Kotiaho JS, Liimatainen JO (2008) Age-related decrease in male reproductive success and song quality in Drosophila montana. Behav Ecol 19:94–99
Jacot A, Scheuber H, Brinkhof MWG (2007) The effect of age on a sexually selected acoustic display. Ethology 113:615–620
Johnson SL, Gemmell NJ (2012) Are old males still good males and can females tell the difference? BioEssays 34:609–619
Jones TM (2000) Adaptive female choice for middle-aged mates in a lekking sandfly. Proc R Soc B 267:681–686
Jones T, Elgar MA (2004) The role of male age, sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc R Soc Lond B 271:1311–1318
Karl I, Fischer K (2013) Old male mating advantage results from sexual conflict in a butterfly. Anim Behav 85:143–149
Kokko H (1998) Good genes, old age and life-history trade-offs. Evol Ecol 17:739–750
Kokko H, Lindstrom J (1996) Evolution of female preference for old mates. Proc R Soc Lond B 263:1533–1538
Koref-Santibanez S (2001) Effects of age and experience on mating activity in the sibling species Drosophila pavani and Drosophila gaucha. Behav Genet 31:287–297
Liu XP, Xu J, He HM, Kuang XJ, Xue FS (2011) Male age affect females mate preference and reproductive performance in the cabbage beetle, Colaphellus bowringi. J Insect Behav 24:83–93
Liu X-P, He H-M, Xue F-S (2014) The influence of female age on male mating preference and reproductive success in cabbage beetle, Colaphellus bowringi. Insect Science, 21: 515–522. doi:10.1111/1744-7917.12051
Lüpold S, Manier MK, Ala-Honkola O, Belote JM, Pitnick S (2011) Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav Ecol 22:184–191
Maklakov AA, Kremer N, Arnqvist G (2006) Ageing and the evolution of female resistance to remating in seed beetles. Biol Lett 2:62–64
Manning JT (1985) Choosy female and correlates of male age. J Theor Biol 116:349–354
Meza JS, Díaz-Fleischer F, Orozco D (2005) Pupariation time as a source of variability in mating performance in mass-reared Anastrepha ludens (Diptera: Tephritidae). J Econ Entomol 98:1930–1936
Moore PJ, Moore AJ (2001) Reproductive aging and mating: the ticking of the biological clock in females cockroaches. Proc Nat Ac Sci USA 98:9171
Moulin B, Rybak F, Aubin T, Jallon JM (2001) Compared ontogenesis of courtship song components of males from the sibling species, D. melanogaster and D. simulans. Behav Genet 31:299–398
Mpuru S, Blomquist GJ, Schal C, Roux M, Kuenzli M, Dusticier G, Clément JL, Bagneres AG (2001) Effect of age and sex on the production of internal and external hydrocarbons and pheromones in the house fly, Musca domestica. Insect Biochem Mol Biol 31:139–155
Papanastasiou SA, Diamantidis AD, Nakas CT, Carey JR, Papadopoulos NT (2011) Dual reproductive cost of aging in male medflies: dramatic decrease in mating competitiveness and gradual reduction in mating performance. J Insect Physiol 57:1368–1374
Pérez-Staples D, Harmer AMT, Collins SR, Taylor PW (2008) Potential for pre-release diet supplements to increase the sexual performance and longevity of male Queensland fruit flies. Agric For Entomol 10:255–262
Pérez-Staples D, Martínez-Hernández MG, Aluja M (2010) Male age and experience increases mating success but not female fitness in the Mexican fruit fly. Ethology 116:778–786
Pérez-Staples D, Weldon CW, Taylor PW (2011) Sex differences in developmental response to yeast hydrolysate supplements in adult Queensland fruit fly. Entomol Exp App 141:103–113
Pérez-Staples D, Córdova-García G, Aluja M (2014) Sperm dynamics and cryptic male choice in tephritid flies. Anim Behav 89:131–139
Petit-Marty N, Vera MT, Calcagno G, Cladera JL, Segura DF, Allinghi A, Rodriguero M, Gómez Cendra P, Viscarret MM, Vilardi JC (2004) Sexual behavior and mating compatibility among four populations of Anastrepha fraterculus (Diptera: Tephritidae) from Argentina. Ann Entomol Soc Am 97:1320–1327
Pizzari T, Dean R, Pacey A, Moore H, Bonsall MB (2008) The evolutionary ecology of pre- and post-meiotic sperm senescence. T Ecol Evol 23:131–140
Prathibha M, Krishna MS (2010) Greater mating success of middle-aged females of Drosophila ananassae. Zool Stud 49:806–815
Prosser MR, Murray AM, Cade WH (1997) The influence of female age on phonotaxis during single and multiple song presentation in the field cricket, Gryllus integer (Orthoptera: Gryllidae). J Insect Behav 10:437–449
Proulx SR, Day T, Rowe L (2002) Older males signal more reliably. Proc R Soc B 269:2291–2299
Radwan J (2003) Male age, germline mutations and the benefits of polyandry. Ecol Lett 6:581–586
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Santhosh HT, Krishna MS (2013) Relationship between male age, accessory gland, sperm transferred, and fitness traits in Drosophila bipectinata. J Insect Sci 13:159
Shelly TE, Edu J, Pahio E (2007) Age-dependent variation in mating success of sterile male Mediterranean fruit fly (Diptera: Tephritidae): implications for sterile insect technique. J Econ Entomol 100:1180–1187
Shelly TE, Edu J, Pahio E (2011) Females medfly mate selectively with young males but gain no apparent fitness benefits. J Insect Behav 24:55–66
Shruti B, Chayakumari, Ravi Ram K, Ramesh SR (2012) Influence of mating histories and age on female remating behavior in a few closely related species of Drosophila nasuta subgroup. Indian J Exp Biol 50:156–163
Somashekar K, Krishna MS (2011) Evidence of female preference for older males in D. bipectinata. Zool Stud 50:1–15
Spurgeon DW (2003) Age dependence of pheromone production by the boll weevil (Coleoptera: Curculionidae). Environ Entomol 32:31–38
Taylor PW, Kaspi R, Mossinson S, Yuval B (2001) Age-dependent insemination success of sterile Mediterranean fruit flies. Entomol Exp Appl 98:27–33
Vanicková L, Svatos A, Kroiss J, Kaltenpoth M, Do Nascimento RR, Hoskovec M, Brizová R, Kalinová B (2012) Cuticular hydrocarbons of the south American fruit fly Anastrepha fraterculus: variability with sex and age. J Chem Ecol 38:1133–1142
Verspoor LR, Cuss M, Price TAR (2015) Age-based mate choice in the monandrous fruit fly Drosophila subobscura. Anim Behav 102:199–207
Wedell N (2005) Female receptivity in butterflies and moths. J Exp Biol 208:3433–3440
Wedell N, Gage MJG, Parker GA (2002) Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol 17:313–320
Xu J, Wang Q (2009) Male moth undertake both pre- and in-copulation mate choice based on female age and weight. Behav Ecol Sociobiol 63:801–808
Zuk M (1988) Parasite load, body size, and age of wild-caught male field crickets (Orthoptera: Gryllidae): effects on sexual selection. Evolution 42:969–976
Acknowledgments
We wish to thank two anonymous referees for comments that improved the manuscript. We thank Evaristo Calihua Chipahua, Carlos Carmona and Nicolás Núñez-Beverido for colony maintenance and technical assistance with the experiments. Funding was provided by CONACyT Mexico grant number Ciencia Básica 179741 awarded to DPS. We thank CONACyT for a Master’s scholarship for YCN and CONICET Argentina for a postdoctoral scholarship for SA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abraham, S., Contreras-Navarro, Y. & Pérez-Staples, D. Female Age Determines Remating Behavior in Wild Mexican Fruit Flies. J Insect Behav 29, 340–354 (2016). https://doi.org/10.1007/s10905-016-9562-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-016-9562-4