Abstract
Doped with high-quality neodymium ions, poly(methyl methacrylate) (PMMA) [(PMMA)-x wt% Nd3+] by using the traditional solution casting method, polymeric composite films with [x = 0, 0.025, 0.1, 0.25, 1.25, and 2.5 wt%] were formed. The XRD, SEM, UV–Vis–NIR, and electrical characteristics were used to characterize the films. According to XRD research, some interactions between PMMA and Nd2O3 result in a decrease in composite crystallinity. According to SEM, Nd2O3 particles were uniformly distributed throughout the host matrix. With Nd2O3 filler rose, the size of the particles also increased, leading to rising agglomeration. Incorporating Nd3+ ions into the polymer chains of (PMMA) significantly changed the optical energy gaps, causing the indirect optical gap to fall from 4.82 to 4.47 eV and the direct optical gap to drop from 4.98 to 4.85 eV. The behavior of the Urbach energy is the opposite of that of the bandgap energy. Additionally, the measured dielectric constant of the investigated composites showed a very alluring dielectric constant for the dielectric media. The examined films’ dielectric constant and dielectric loss both decline exponentially as the incident frequency rises, while their AC electrical conductivity enhances as the Nd3+-ions content rises. The produced sheets were then used in the artificial UV-induced photodegradation of Methylene Blue. They demonstrated good potential for immobilizing Nd3+ for the photodegradation of contaminants typically present in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polymer materials are frequently utilized through numerous devices as insulators and optoelectronic products. This is because they have special qualities such as being lightweight, highly flexible, and able to be produced at low cost and temperatures. Polyimide, poly(methyl methacrylate) (PMMA), polyvinyl phenol (PVP), and other organic dielectric compounds have all been researched. Poly(methyl methacrylate, or PMMA) is a well-known thermoplastic polymer with an amorphous structure. It is produced by polymerizing methyl methacrylate [1].
The PMMA can be utilized as a dielectric material in organic field effect transistors (OFET) and organic thin film transistors (OTFT) because it has superior insulating qualities, including a low dielectric constant and low dielectric loss over a wide frequency range [2, 3]. In addition to having a low dielectric permittivity and dielectric loss, the device performance is also influenced by the surface morphology of the dielectric. Because it influences the electrical transport properties, which depend on the location of the film’s flaw and grain boundaries, the surface morphology must be homogeneous and smooth [4,5,6,7]. PMMA offers remarkable optical and mechanical characteristics. It has outstanding dimensional stability and is resistant to acid and alkali conditions because of the stiff polymer chains in its chains [1]. Commercially known as acrylic glass, PMMA can replace glass in various high-wear items like anti-riot vehicles. It can be used for various technological purposes like optical devices, optical lenses, gratings, and waveguides [8, 9].
Due to their unique features coming from a 4f-orbital that is only partially protected, rare earth compounds have received a lot of attention. One significant rare earth element with many uses is neodymium (Nd), which belongs to the lanthanide series. in various applications, such as magnetic and luminous devices, catalysts, dielectrics, and protective coatings [10,11,12,13,14,15]. Its absorption properties are essentially independent of the nearby atoms in the host lattice due to transition within a well-protected inner shell. It is a strong contender for gamma-ray dosimetry due to its favorable thermoluminescence (TL) characteristics [16,17,18]. Nd2O3 can be used to enhance a material’s electrical characteristics [19], as gate dielectrics [20], and in photocatalytic applications [21]. Nd2O3 is a powerful doping agent in Nd: YAG laser apparatus. In Nd: YAG laser systems, Nd2O3 performs the function of a doping agent effectively [22]. To navigate in inclement weather, glasses containing Nd2O3 are utilized as a vision aid. Studying the characteristics of Nd2O3 particles with a size in the nanometer range is intriguing since the physical properties of nanoparticles are frequently substantially different from those of their bulk counterparts. More people are interested in creating nanocrystalline Nd2O3 due to its enhanced luminous features, longer emission lifespan, higher quantum efficiency, attractive optical nonlinearity, outstanding chemical stability, enhanced catalytic activity, and natural refractoriness.
The host materials selected do not greatly affect the RE ions’ energy levels. As a result, any materials with low absorption at the pump and emission wavelengths can be employed. The characteristics of RE-doped ions with various materials have been covered in several articles up to this point. Glasses, optical crystals (Al2O3, Y2O3), and semiconductors (Si, SiGe) are common photonics matters used to provide lasing action [23]. Various characteristics have recently made polymers desirable hosts for RE ions, with great transparency in the visible and near-infrared spectra, well-controlled refractive indices, good thermal stabilities, simple production processes, and low cost [24, 25].
Rapid industrialization around the globe in recent decades has had a huge negative influence on the environment and aquatic system. Heavy organic pollutants like azo dyes and other dangerous compounds have accumulated in water sources due to the rise of the textile, leather, paper, and food industries, particularly [26,27,28,29,30,31]. Polluting the environment and posing a major health danger is dye waste. A typical cationic dye used in the printing, pharmaceutical, cosmetic, and textile industries is methylene blue (MB). Cancer, allergic dermatitis, genetic disorders, and skin rashes can all be brought on by MB [32].
Complete removal of the organic pigment from wastewater via conventional, physical, and chemical approaches is difficult [33]. Adsorption [34], ozonation [35], photocatalytic degradation [36], sonochemical degradation [37], photo-Fenton degradation [38], and UV/H2O2 process [39] are just a few of the studies done for the elimination of MB by progressive oxidation process. To help semiconductor photocatalysts resolve environmental issues, organic pollutants are now removed utilizing UV and visible light [40]. Due to the use of sun irradiation, photocatalysis technology may be applied widely and uses less energy [41]. The latest studies have intensive on the application of advanced oxidation processes (AOPs) that can degrade industrial effluents and are found in semiconductors. Among the several semiconductors [30], Nd2O3, a rare earth oxide, increases PMMA photocatalysis’s effectiveness for MB photodegradation in the presence of light at a lower cost [42].
This study used a simple solution casting technique to make Nd2O3 with PMMA composite films. The structural confirmations were examined using X-ray diffraction (XRD). Surface morphological examination for the samples was done using SEM. From UV–Vis–NIR spectra, the energy bandgap for the material was determined. Additionally, the samples’ dielectric and electrical conductivity characteristics are reviewed in detail. For the photocatalytic degradation of MB and the CUT-OFF laser filter applications, optical limiting was done.
2 Experimental Work
2.1 Samples Preparation
PMMA was supplied by Sigma-Aldrich. Polymeric composites of PMMA dispersed with Nd2O3 were created using the solution casting process and are denoted by the abbreviations S0, S1, S2, S3, S4, and S5, depending on the weight amount of PMMA utilized. Initially, 15 mL of ultra-pure chloroform was used for each sample to dissolve 2 g of PMMA. The necessary amount of Nd2O3 was first dissolved in 10 mL of chloroform. The PMMA solution and the Nd2O3 dispersion solution were blended, and a homogeneous solution was produced by magnetic stirring for 1 h.
To further uniformly distribute the filler particles, this viscous solution underwent ultrasonication. To create the free-standing PNC film, the solution was then cast into a Petri dish that had been thoroughly cleaned and allowed to dry there. Using the same procedures, the other PNC films with different Nd2O3 concentrations were created concurrently. After drying in a vacuum oven at 40 °C for 24 h, the PMMA-x wt% Nd2O3 films were finally cut into 2 × 2 cm2 squares for further research.
2.2 Characterizations and Devices
Shimadzu LabX-XRD-6000 model with Cu Kα radiation (λ = 1.5406 Å) was operated to record the XRD patterns of films made of Nd2O3 and PMMA-x wt% Nd2O3 in reflection mode at a scan rate of 0.05°/s. The XRD tube was performed at 30 kV; the current was 30 mA in the 2θ range (5–70).
Surface morphology was employed for the samples under study in a scanning electron microscope (SEM), model (JSM-6360), operating at 20 kV.
To evaluate the linear optical characteristics of T(λ) and Abs(λ), the JASCO V-570 double-beam spectrometer was used in the 190–2500 nm wavelength range. At room temperature, all of the measurements were completed.
Through the use of a modified Z-scan configuration with fixed samples, the optical limitation was determined. Using undoped PMMA film and PMMA polymer composite dielectrics doped with Nd2O3, the optical limiting effect investigation has been recorded using two laser beams at 532 & 632.8 nm. Utilizing an optical laser power meter coupled with a photodetector, the applied He–Ne laser’s input/output power meters were monitored (Model: Newport 1916-R). The carrying holder for composite films was created to work with them.
The AC electrical conductivity/dielectric characteristics were measured via an automated LCR meter (FLUKE-PM6306 Model). At room temperature, measurements were taken in the frequency range of 100 Hz to 1 MHz. Two copper plates were sandwiched between the samples to make an ohmic contact before taking measurements. At each excitation frequency, the Nd2O3/PMMA film’s capacitance (C), resistance (R), and loss tangent (tanδ) values were noted.
The photocatalytic validity of each generated sample has been assessed using a wood photoreactor. Yahia and his team developed the photoreactor at NLEBA, Ain Shams University (ASU) [43]. At room temperature, 200 mL of MB (0.01 g/L) aqueous mixture and 1 mL of H2O2 were combined with Nd2O3/PMMA membranes. To create the adsorption–desorption equilibrium, the produced solution was left with Nd2O3/PMMA in the dark for 30 min. The experiment involved irradiating the solution using UV light. Every 10 min, 3 mL of the suspension was taken out of the mixture after irradiation. The absorbance at a maximum wavelength of 665 nm was a marker for the change in dye absorbance during photodegradation. By measuring absorbance data/plots, the degradation of MB was determined by a UV–Vis spectrophotometer.
3 Results and Discussion
3.1 Structural Analysis and Surface Morphology of Nd2O3/PMMA Polymeric Composite Films
3.1.1 XRD Analysis of Nd2O3/PMMA Films
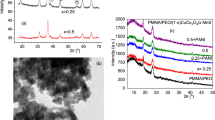
To determine how the presence of Nd2O3 affects the amorphous PMMA polymer chain, the XRD spectra for Nd2O3/PMMA polymeric films have been analyzed and are depicted in Fig. 1. The two broad peaks at 2θ values of 14.9° and 31.5° in the measured diffraction pattern for pure PMMA are characterized by a regular decrease in intensity, which supported the polymer’s amorphous nature [44, 45]. The first peak’s shape reflects how extended polymer chains are packed, while the second peak shows how large chains are ordered within them and how their intensity is gradually dwindling [46].
The XRD patterns of the Nd2O3/PMMA polymeric films examined here show no peaks corresponding to Nd2O3 crystalline peaks, indicating that the Nd2O3 is totally dissociated in the solutions created for the creation of the films. According to other studies [43, 47], the Nd2O3 completely dissociates when the carbonyl group C=O of PMMA forms strong complexes with the cation Nd3+ of Nd2O3. The inclusion of filler alters the first peaks’ initial intensity and width in the polymeric host. Increased width or decreased intensity indicates that the pristine polymer’s crystallinity has changed (decrease). It might imply that specific interactions between the polymer chains and the filler are possible [47].
3.1.2 SEM Analysis of Nd2O3/PMMA Polymeric Films
To prevent the formation of crack initiatives in the PMMA film, homogeneous Nd2O3 filler dispersion is preferred. Using SEM, it was possible to see how the distribution and dispersion of the Nd2O3 filler in the polymer PMMA matrix changed as it was added (0–2.5 wt%). The Nd2O3/PMMA polymeric composite films’ SEM images are shown in Fig. 2. The PMMA polymer surface under SEM analysis (see Fig. 2a) was discovered to be clear and smooth [46], indicating that the PMMA matrix is transparent [46]. The Nd2O3/PMMA composite samples with varying wt% ratios of Nd2O3 in the PMMA matrix are shown in Fig. 2b–f. These photos demonstrated how evenly distributed the Nd2O3 particles were throughout the PMMA matrix. When Nd2O3 is present in PMMA matrices at a concentration of 0.25 wt%, the homogenous distribution was seen. As Nd2O3 content rises, Nd2O3 particle size also rises, and the Nd2O3 particles clump together [48]. This phenomenon suggests that large concentrations of Nd2O3 can impact Nd2O3 agglomeration. The surface area of the particles decreases as the particle size rises [49], increasing the contact between the particles and raising the conductivity of the Nd2O3/PMMA polymeric composite. This concurs with Vishal and Sharma’s findings [50, 51].
3.2 Optical Characteristics of Nd2O3/PMMA Composite Films
Figure 3 displays the UV–Vis results for samples of pure and doped PMMA. Understanding the optoelectronic properties of materials depends heavily on these observations. Figure 3a shows the transmittance data as a function of concentration for all investigated samples. As noticed in Fig. 3, the microstructure, which is influenced by the distribution of Nd3+-contents in the polymer matrix, governs the behavior of the spectra. As seen, pure PMMA exhibits transmittance that is around 90% in the visible spectrum. In this region, the optical transmittance of all samples is decreased by including Nd3+ in the PMMA matrix. According to Fig. 3b, pure PMMA-prepared samples have a distinctive absorption peak at 250 nm. These characteristics have been reported in a number of papers on doping PVDF polymeric material with Nd2O3 ions [52], and they can be discussed in terms of the disorders inside the polymeric samples. This is caused by the electronic excitation in the carbonyl chromophores (C=O) of the PMMA structure. For Nd2O3/PMMA polymeric composite, including Nd3+ improves optical absorbance. Because of this, the amount of Nd3+ doped in PMMA affects the transmission and absorbance spectroscopy [T(λ) and Abs(λ)] of the films as they are formed. The optical absorption coefficient was calculated using Beer’s Lambert’s expression [52] from measurements of optical absorbance (A) (see Fig. 3b):
where d is the thickness of the sample. The outcomes for all compositions are displayed in Fig. 4. The outcomes for pure PMMA show an absorption edge located at 251 nm. Yahia and associates discovered the absorption edge at almost 250 nm, demonstrating agreement between the two observations [43, 45, 46, 53].
The optical absorption coefficient α was employed to calculate the absorption edge values of these films, and the extinction coefficient (k) was calculated as follows:
where λ is the wavelength of the incident photon and k: is the extinction coefficient; the most critical characteristics for optoelectronic applications tell us how easily an incident photon beam penetrates the sample. The extinction coefficient represents the energy lost due to the scattering or absorption induced by the molecules of the composites [54]. The relation between the extinction coefficient and wavelength is seen in Fig. 5a. The fact that the prepared samples are still transparent causes the extinction coefficient to have minimal values, which is visible [55]. The wavelength and Nd3+ percentage both affect the extinction coefficient, which rises as they do. According to the data, the k value rapidly decreases up to 230 nm and then swiftly increases as λ increases. Islam cited a connection between the optical absorption at grain boundaries and the behavior indicated by the k coefficient [56]. The same could happen in the PVDF/Nd samples due to the optical absorption of the Nd grain boundary and PVDF spherulites [56, 57].
For both pure and doped PMMA samples. The fluctuation in skin depth or depth penetration (d) as a function of photon energy is seen in Fig. 5b. The incident photon’s exponential decay in a material with finite electrical conductivity is represented by the δ parameter, which is considered by:
As the Nd3+ % content rises, the skin depth value decreases. The estimated outcomes demonstrate that for all prepared samples, δ decreases as a function of photon energy. Since the skin depth depends on optical transmittance (T), the behavior displayed by the PMMA matrix is caused by a decrease in the parameter T [58]. The following equation describes how the absorption coefficient depends exponentially on photon energy at the photon energy lower the optical band gap (tail absorption) [59]:
where constant is \({\alpha }_{o}\), photon energy is (\(h\nu\)), and the EU, which is related to the amorphous properties of the composites, is the band tail energy of localized states in the forbidden energy bandgap.
The calculated EU, values are detailed in Table 1 using the estimated values slope from Fig. 6 and the next relation (d/lnα)(dhυ)−1 [59]. The EU, Urbach energy gives data on the width of the localized states’ tails in the restricted bandgap, which develops because amorphous materials include structural flaws and inhomogeneities known to cause disorders. As shown in Fig. 6, the optical EU values in the composite films were greater than those in pure PMMA film. Several transitions from bands to tails and tails to tails are made feasible due to this increase in the redistribution of states from the band to the tail [60]. Indirectly, this suggests an increase in the amorphous phase in polymer films by introducing disorder and defects into the band structure of the Nd2O3/PMMA polymeric composite, which may enhance the local states within the optical bandgap. Cluster size increases due to the formation of dopant aggregates as Nd2O3 loading in composites increases, raising EU [61, 62].
Additional research on the absorbance spectrum has determined the optical energy gap and other characteristics [63, 64]. To calculate the optical band gap \({E}_{g}\) of the non-crystalline materials, Tauc equation [65] was utilized.
where B, hυ constant, and Eg are constant, the photon energy and the optical energy gap, respectively. m = ½ or 2 for indirect and directly allowed transitions, respectively. Previously, it was established that the photon energy-absorbing material undergoes both indirect and direct transitions close to the fundamental band edge [66, 67]. With the Davis and Mott approach [67], which involves graphing (αhυ)1/2 and (αhυ)2 values via the incident photon energy (hυ) values, the band boundaries corresponding to these transitions can be identified. Figure 7a, b shows the plots of (αhυ)1/2 and the (αhυ)2 against hν for the polymeric composite made of Nd2O3 and PMMA metrics for the indirect optical band gap. Extrapolating the linear portion of the high energy curves to the photon energy axis at zero absorbance, as shown in the corresponding plots of Fig. 7, Egind and Egdir of these films were estimated. The gained values of these band gaps of the films as a function of wt% of Nd2O3 are informed in Table 1. The Egdir values are found to be higher than the Egind values for all the samples under study. With the presence of Nd3+ ions, the band gap is seen to be decreasing in Fig. 7a, b [68]. These, however, also demonstrate the presence of energy levels produced in the Nd3+/PMMA mobility band gap, facilitating the passage of electrons. The optical transitions from the HOMO band to the LUMO band can be used to explain the variance of the energy gaps. Based on the charge transfer complexes (CTCs) produced between the filler atoms and the functional groups of the host polymer lattice, as mentioned earlier in Table 1 indicates that the optical band gap values of these materials steadily drop with an increase in Nd3+ concentrations [69].
Another approach based on the absorption spectra fitting (ASF) model can be used to determine the band gap [70, 71]:
By extrapolating the linear component of the \({\left(A/\lambda \right)}^{1/2}\) via \(1/\lambda\) plot to \({\left(A/\lambda \right)}^{1/2}\), as shown in Fig. 8, one may determine the wavelength \({\lambda }_{g}\) corresponding to the band gap Eg in eV. Using the relation, the band gap may then be determined:
Table 1 lists the basic absorption edge and direct and indirect optical gaps for Nd2O3/PMMA polymeric composite compared to the band gap calculated using the ASF model. The results for pure PMMA film are in excellent agreement with the previous findings [72]. The values of the band gaps calculated using the ASF method and those determined from Tauc’s relation are nearly equal, reflecting the homogeneity and good dispersion of Nd3+ ions in the PMMA polymer backbone. As the amount of Nd3+ ions increases, it can be seen from Table 1 that the values of band gaps and absorption edges dramatically decrease. These results suggest that the degree of crystallinity significantly influences the optical energy gaps. In the sample doped with 2.5 wt% Nd3+ ions, the band gap values drop from 4.81 eV for pure PMMA film to 4.65 eV as the Nd3+ ions content increases. These findings might be explained by incorporating Nd3+ in the amorphous portion of the polymer matrix, which leads to localized states in the optical band gap and disordered accumulation in the (PMMA) matrix.
3.3 The Optical Limitation of Nd2O3/PMMA Films
The optical apparatus described above was utilized to gauge the optical limiting properties of the samples under investigation. Figure 9a, b shows a plot of the output and the normalized power for Nd2O3/PMMA polymeric composites as a function of the Nd3+ concentration utilizing two distinct laser beams (He–Ne laser of 632.8 nm and laser diode of 532 nm). The power intensity for the two laser sources is limited to a minor extent in pure PMMA [46]. It is remarkable to note from these plots that the output power and the normalized power tend to drop significantly with rising Nd3+ concentration, indicating that the content of Nd3+ plays a crucial role in the optical limiting behavior [73]. The examined samples can control laser strengths, and increasing the Nd3+ concentration strengthens the optical limiting effect in response to reduced output power. As a result of Nd3+ taking part in the interaction during the nonlinear absorption processes, these results can be attributed to an increase in the molecular density (number of molecules per unit volume) of the polymer Nd3+ composite films. Therefore, compared to samples of films with high Nd3+ content, the response of the film samples with low Nd3+ content to optical limiting action is relatively modest. So, PMMA films doped with many Nd3+ have improved optical limiting performance [74].
3.4 Dielectric and Electrical Properties
3.4.1 Dielectric Spectra
It is generally known that the induced polarization as a function of the external AC field is the primary factor that affects the samples’ dielectric characteristics. Furthermore, based on the structure of the analyzed samples, they are essentially predicted. The following straightforward formulas have been used to compute the dielectric constant, \({\varepsilon }_{1}\), and the dielectric loss, \({\varepsilon }_{2}\) [75]:
In this equation, C is the film capacitance, d is the film thickness, \({\varepsilon }_{o}\) is the vacuum’s permittivity (8.85 × 10−12 F/m), and A is the electrodes’ surface area. Tanδ is the loss tangent, often known as the dissipation factor in an AC field. The dielectric loss \({\varepsilon }_{2}\) (ω) represents the energy lost due to Joule’s heating effect, and the actual part \({\varepsilon }_{1}\) (ω) is the dielectric constant \({\varepsilon }_{1}\), which describes the stored charge and energy inside the film. Figure 10a, b depict how the frequency affects the behavior of the Nd2O3/PMMA polymeric composite parameters \({\varepsilon }_{1}\) and \({\varepsilon }_{2}\). Due to the tendency of dipoles to be orientated towards the applied field, the dielectric constant \({\varepsilon }_{1}\) is large in the lower frequency zone and drastically drops with increasing the applied frequency. Additionally, it has been noted that the \({\varepsilon }_{1}\) rises when Nd2O3 doping concentrations rise. The interfacial or Maxwell–Wagner–Sillars (MWS) polarization is thought to be the main polarization type in this frequency range and to be the cause of the \({\varepsilon }_{1}\) diminishing with frequency, based on the high values of \({\varepsilon }_{1}\) at lower frequencies. Numerous researchers [75,76,77] have noted this phenomenon in polymer composites. It is noted that \({\varepsilon }_{1}\) has slightly decreased at higher frequencies because the dipoles have a reduced response to field fluctuations [77]. According to Fig. 10b, the dielectric loss \({\varepsilon }_{2}\) reduces as the frequency rises owing to an accumulation of free charges in the polymeric matrix close to the electrodes, which would explain the large values of \({\varepsilon }_{2}\) at the lower frequency. The enhancement of the free charge motion inside the matrix and the rise in the dipole charge cause the values of \({\varepsilon }_{2}\) to rise at a lower frequency as Nd2O3 doping contents are increased. While, at the higher frequency, the Nd2O3 doping and tiny fluctuations in \({\varepsilon }_{2}\) are caused by the electric field’s quick direction change, which leaves little time for the dipoles to orient themselves [78].
The complex modulus formalism is an important and valuable tool for detecting, deciphering, and understanding the dynamics of the material’s electrical transport process, such as the carrier/ion hopping rate, conductivity relaxation durations, etc., by lowest dielectric capacity. The dielectric modulus is given by a reciprocal relative permittivity complex, as previously mentioned in ε* [79]. The complex modulus formalism is an extremely important and useful tool for identifying, analyzing, and interpreting the dynamics of the material’s electrical transport process, such as the carrier/ion hopping rate, conductivity relaxation durations, etc., by minimal dielectric capacity. A reciprocal relative permittivity complex is what gives the dielectric modulus, as previously stated in ε* [79]:
where M′ and M″ are the electric modulus’s real and imaginary components, respectively. Figure 11a shows the fluctuation of the real (M′) complex electric modulus of the investigated composites as a function of frequency at ambient temperature. M′ is quite low in the low-frequency zone. The maximum constant value of \({M}_{\infty }=\frac{1}{{\varepsilon }_{\infty }}\) for all prepared films at higher frequencies is reached as the frequency rises. These results might result from the absence of restoring forces controlling carriers’ movement in an electrically produced field. These properties demonstrated that electrode polarization has very little impact on the material [75].
For Nd3+/PMMA films, a relaxation peak was observed in Fig. 11b, which shows the maximum fluctuation of the imaginary portion of the electric modulus (M″) dependent on the frequency at room temperature. With the help of this pattern, we may learn more about the charging procedure, the electrical transport mechanism, conductivity relaxation, and ion dynamics as a frequency function. As the Nd3+ content rises, the process’ peak shifts to the higher frequency side. According to the peak’s side with the low frequency, ions can successfully move between two locations. The peak’s side with low frequency indicates a successful ion hop from one site to another. The side of this high-frequency peak shows that ions can move locally within their potential wells and have spatially constrained themselves to those wells. The peak region shows this as the change from long to short range occurs more frequently. A definite sign of conductivity relaxation is provided by the peak’s emergence in the modulus spectrum [68, 80].
Jonscher’s power law yields the following relation, which is used to calculate the total conductivity of a dielectric polymeric material:
where σDC denotes the conductivity at zero or very low frequencies, often known as the limiting zero-frequency conductivity. The \({\sigma }_{AC}=\omega {\varepsilon }_{o}{\varepsilon }^{{\prime}{\prime}}\) [81] denotes the AC conductivity [80]. Thus, there is \(\omega =2\pi f\) angular frequency. The Nd2O3/PMMA polymeric composite’s AC conductivity (σAC(ω)) variation with applied frequency is shown in Fig. 12 at room temperature. Two zones might be distinguished from this frequency response: A low-frequency plateau comes first, followed by a high-frequency dispersive area. Charge carriers were thought to be hopping through grain boundaries more quickly at high frequencies, while charge diffusion was thought to be responsible for the plateau at low frequencies. There is a power law that governs this behavior.
where s is an exponent often equal to or less than unity and is typically used to quantify the ac conduction in various semiconducting materials, A is a constant that depends on both temperature and angular frequency, and \({\sigma }_{DC}\) is the DC conductivity (the extrapolation of the plateau zone to zero frequency). The power exponent was determined by plotting the slope of ln\({\sigma }_{AC}\) vs. lnω [82]. It should be noted that Elliot’s [83] indicated that the value of (s) for amorphous materials is between 0.91 and 1.00. Furthermore, it can be seen from Fig. 12 that the majority of films exhibit a similar tendency, where the exponent (s) lowers by raising the Nd3+ contents. The correlated barrier hopping (CBH) model is the conduction mechanism based on the behavior of (s) [84].
3.5 Efficiency and Kinetics of Photocatalysis
The degradation of MB dye in an aqueous solution under UV-light irradiation was used to assess the photocatalytic efficiency of Nd2O3/PMMA polymeric composites. The photocatalytic performance of PMMA and the polymeric Nd2O3/PMMA composite was also evaluated for comparison. The UV–Visible absorption spectra of MB dye over the recently prepared photocatalyst are shown concerning exposure time in Fig. 13. In the absorption spectrum, the pure MB dye’s absorption peak can be seen at 664 nm (π to π*). The color of the dye solution shifted from blue to colorless when exposed to sunlight; however, the dye solution in complete darkness displayed essentially no color change [85]. It is clear from (Fig. 13) that all samples’ intensities decrease over time, indicating that the effectiveness of dye photodegradation increased over time. The percentage of dye degradation is computed using the equation:
where Ao & A are absorptions at 0 min illumination and irradiation time, respectively. After 60 min of UVc light exposure, so degraded by 16.4%, whereas the S4 catalyst had the maximum photocatalytic efficiency, up to 99.23%. The first-order Langmuir–Hinshelwood equation is obeyed by the kinetics model of MB photodegradation over the polymeric film catalysts [86]:
where K is the kinetic rate (min−1). According to (Fig. 13), the Nd2O3 content had the following effects on the photodegradation rate: S0 (0.0033 min−1), S1 (0.0733 min−1), S2 (0.0685 min−1), S3 (0.0511 min−1), S4 (0.0822 min−1), and S5 (0.0338 min−1), respectively. Within 60 min of UV-light irradiation, the entire dye degradation was demonstrated, showcasing the excellent catalytic activity of S4. The optical studies are consistent with the photocatalytic output. Due to the superior heterostructure that is formed between the Nd2O3 particles and the PMMA matrix [43], the reaction rate is increased for S4 by 24.8 times more than it was for S1 (see Table 2).
3.6 Photocatalytic Degradation Reaction Mechanisms
At the catalyst’s surface, during the photocatalytic process, electron–hole pairs are produced if the light intensity is greater than or equal to the catalyst energy bandgap (Eg). The photoinduced electron and hole stimulation of the adsorbed oxygen and water molecules on the catalyst surface can result in the formation of reactive oxygen species such O2−· and HO·. These reactive oxygen species can initiate catalytic activities through particular reaction pathways that result in organic pigment oxidation [90]. Free radical trapping assays were used to identify the active oxidative species responsible for the photocatalytic degradation of MB over the Nd2O3/PMMA polymeric composites. Ascorbic acid (ASC), isopropanol (IPA), sodium nitrate (NaNO3), and sodium chloride (NaCl) were used as O2−·, HO·, electron (e−), and hole (h+) scavengers, respectively. The PMMA, which has good carrier transport, contributed to lowering e/h+ pair recombination rates and extending the life of the photogenerated center [91]. It improves the resistance of Nd2O3 fillers to environmental factors (see Figs. 14, 15). As a result of its optical transparency, PMMA was employed as a matrix membrane [92]. Compared to not using a scavenger test, adding NaCl inhibits MB’s photodegradation. Therefore, the photoinduced holes play a part in the MB’s degradation when UV light is shone on a Nd2O3/PMMA film. The following equations provide a concise summary of the potential photocatalytic reaction pathways for the breakdown of MB dye using Nd2O3/PMMA film:
4 Conclusion
A [(PMMA)-x wt% Nd3+] was created using the solution casting technique, resulting in composite films with [x = 0, 0.025, 0.1, 0.25, 1.25, and 2.5 wt%] content. PMMA and Nd2O3 can interact in various ways that reduce the crystallinity of the composite, according to an XRD study. The PMMA polymer matrix contained Nd2O3 particles that were evenly dispersed, according to SEM. As a function of Nd3+ concentration, the optical absorption coefficient of the PMMA/Nd3+ samples was determined. It was found that as the Nd3+ content rises, the absorption edge also grows. The extinction coefficient’s behavior may be connected to the optical absorption of spherulites and the Nd3+ grain boundary. Skin depth has been decreasing due to the reduction of the optical transmittance. The bandgap energy (direct and indirect) of these films rapidly reduces when the Nd3+ content rises due to the addition of Nd3+. The bandgap energy behavior of the Urbach energy was analyzed as a function of the Nd3+ content, supporting the earlier findings. As the applied frequency was raised, ε1 and ε2 both fell. The behavior of M′ demonstrated that the material is very slightly polarized by the electrodes. A definite indicator of conductivity relaxation is provided by an occurrence of the relaxation peak in Mʺ. The rise in Nd3+-ion content has improved AC electrical conductivity, and the s values have slightly decreased as Nd3+-ion concentration has increased. With a 0.0822 min−1 reaction rate, the S4 film demonstrated good photocatalytic activity. The trapping research demonstrated that the photoinduced holes play a significant part in MB deterioration in the presence of S4 film. Future photocatalyst superiority should be given to the created materials.
References
M.A. Habeeb et al., Effect of high density polyethylene (HDPE) on structural and optical properties of (PP/PMMA) blends. Int. Lett. Chem. Phys. Astron. 60, 94–106 (2015)
D. Dorranian et al., Structural and optical characterization of PMMA surface treated in low power nitrogen and oxygen RF plasmas. J. Non-Oxide Glasses 1(3), 217–229 (2009)
H.M. Ahmed et al., Dielectric properties of commercial non-polar polymers. J Zankoy Sulaimani A 11(1), 1–8 (2008)
D. Knipp et al., Influence of organic gate dielectrics on the performance of pentacene thin film transistors. Synth. Met. 155(3), 485–489 (2005)
M. Mukherjee et al., Investigation of organic n-type field-effect transistor performance on the polymeric gate dielectrics. Synth. Met. 160(5–6), 504–509 (2010)
C. Feng et al., Influence of trapping states at the dielectric–dielectric interface on the stability of organic field-effect transistors with bilayer gate dielectric. Org. Electron. 12(8), 1304–1313 (2011)
L.N. Ismail et al., Influence of doping concentration on dielectric, optical, and morphological properties of PMMA thin films. Adv. Mater. Sci. Eng. (2012). https://doi.org/10.1155/2012/605673
M.S. Hamdy et al., Optical constants, photo-stability and photo-degradation of MB/PMMA thin films for UV sensors. Optik 127(12), 4959–4963 (2016)
M. Kutz et al., Handbook of Materials Selection (Wiley, Hoboken, 2002)
G. Dhamale et al., Synthesis and characterization of Nd2O3 nanoparticles in a radiofrequency thermal plasma reactor. Nanotechnology 27(8), 085603 (2016)
G. Bonnet et al., Characterization of thin solid films of rare earth oxides formed by the metallo-organic chemical vapour deposition technique, for high temperature corrosion applications. Thin Solid Films 261(1–2), 31–36 (1995)
W. Yang et al., Synthesis of Nd2O3 nanopowders by sol–gel auto-combustion and their catalytic esterification activity. Mater. Chem. Phys. 84(1), 52–57 (2004)
R. Yu et al., Nd2O3 nanoparticles modified with a silane-coupling agent as a liquid laser medium. Adv. Mater. 19(6), 838–842 (2007)
A. Abdelkader et al., Electrochemical synthesis and characterization of a NdCo5 permanent magnet. J. Mater. Chem. 20(29), 6039–6049 (2010)
Z. Xueping et al., Catalytic effect of Gd2O3 and Nd2O3 on hydrogen desorption behavior of NaAlH4. Int. J. Hydrogen Energy 37(10), 8402–8407 (2012)
C. Soliman et al., Neodymium oxide: a new thermoluminescent material for gamma dosimetry. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 251(2), 441–444 (2006)
B. Umesh et al., Nd2O3:Eu3+ nanocrystalline phosphor—a new potential thermoluminescing material for dosimetry. Philos. Mag. Lett. 89(10), 589–597 (2009)
B. Umesh et al., Structural characterization, thermoluminescence and EPR studies of Nd2O3:Co2+ nanophosphors. Mater. Res. Bull. 48(2), 180–187 (2013)
C.W. Nahm et al., Microstructure and varistor properties of ZnO-Pr6O11-CoO-Nd2O3 based ceramics. J. Mater. Sci. Lett. 19(4), 271–274 (2000)
A.A. Dakhel et al., Characterisation of Nd2O3 thick gate dielectric for silicon. Physica Status Solidi (a) 201(4), 745–755 (2004)
K. Byrappa et al., Hydrothermal preparation of neodymium oxide coated titania composite designer particulates and its application in the photocatalytic degradation of procion red dye. J. Mater. Sci. 41(5), 1369–1375 (2006)
A. Ikesue et al., Progress in ceramic lasers. Annu. Rev. Mater. Res. 36(1), 397–429 (2006)
M. Keikhaei et al., Optical properties of neodymium oxide nanoparticle-doped polyvinyl alcohol film. Int. J. Nanosci. 15(04), 1650012 (2016)
H. Liang et al., Radiative properties of Eu (DBM) 3phen-doped poly(methyl methacrylate). J. Mater. Res. 18(8), 1895–1899 (2003)
L. Slooff et al., Rare-earth doped polymers for planar optical amplifiers. J. Appl. Phys. 91(7), 3955–3980 (2002)
S.N. Basahel et al., Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 10(1), 1–13 (2015)
C.H. Nguyen et al., Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: efficiency and degradation pathways. J. Clean. Prod. 202, 413–427 (2018)
B. Rodríguez-Cabo et al., Photocatalytic degradation of methyl orange, methylene blue and rhodamine B with AgCl nanocatalyst synthesised from its bulk material in the ionic liquid [P6 6 6 14] Cl. Water Sci. Technol. 75(1), 128–140 (2017)
S. Kumar et al., Synergetic effect of MoS2-RGO doping to enhance the photocatalytic performance of ZnO nanoparticles. New J. Chem. 40(6), 5185–5197 (2016)
U. Alam et al., Comparative photocatalytic activity of sol–gel derived rare earth metal (La, Nd, Sm and Dy)-doped ZnO photocatalysts for degradation of dyes. RSC Adv. 8(31), 17582–17594 (2018)
W.-C. Lin et al., Electrochemical photocatalytic degradation of dye solution with a TiO2-coated stainless steel electrode prepared by electrophoretic deposition. Appl. Catal. B 140, 32–41 (2013)
B.A. Fil, C. Özmetin, M. Korkmaz, Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull. Korean Chem. Soc. (2012). https://doi.org/10.5012/bkcs.2012.33.10.3184
A. Al-Ahmad et al., Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch. Environ. Contam. Toxicol. 37(2), 158–163 (1999)
W.-T. Jiang et al., Removal of ciprofloxacin from water by birnessite. J. Hazard. Mater. 250, 362–369 (2013)
T.G. Vasconcelos et al., Ciprofloxacin in hospital effluent: degradation by ozone and photoprocesses. J. Hazard. Mater. 169(1–3), 1154–1158 (2009)
P. Huo et al., Enhanced photodegradation of antibiotics solution under visible light with Fe2+/Fe3+ immobilized on TiO2/fly-ash cenospheres by using ions imprinting technology. Chem. Eng. J. 172(2–3), 615–622 (2011)
R. Xiao et al., Sonochemical degradation of ciprofloxacin and ibuprofen in the presence of matrix organic compounds. Ultrason. Sonochem. 21(1), 428–435 (2014)
N.J. Vickers et al., Animal communication: when I’m calling you, will you answer too? Curr. Biol. 27(14), R713–R715 (2017)
S. Jodeh et al., Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. 25(22), 22060–22074 (2018)
H. Wang et al., Preparation of Ag2O/Ag2CO3/MWNTs composite photocatalysts for enhancement of ciprofloxacin degradation. Appl. Surf. Sci. 366, 1–8 (2016)
A. Kadam et al., Morphological evolution of Cu doped ZnO for enhancement of photocatalytic activity. J. Alloys Compd. 710, 102–113 (2017)
M. Lembang, et al., A facile method for green synthesis of Nd2O3 nanoparticles using aqueous extract of Terminalia catappa leaf, in AIP Conference Proceedings (AIP Publishing LLC, 2018)
M.S. Hussien et al., Flexible photocatalytic membrane based on CdS/PMMA polymeric nanocomposite films: multifunctional materials. Environ. Sci. Pollut. Res. 27(36), 45225–45237 (2020)
M.I. Mohammed et al., Optical properties of ZnO nanoparticles dispersed in PMMA/PVDF blend. J. Mol. Struct. 1169, 9–17 (2018)
M.I. Mohammed et al., Synthesis and optical properties of basic fuchsin dye-doped PMMA polymeric films for laser applications: wide scale absorption band. Opt. Quant. Electron. 50(3), 1–12 (2018)
A. El-Mongy et al., Preparation and spectroscopic studies of PbI2-doped poly(methyl methacrylate) nanocomposites films: dielectric and optical limiting approach. Opt. Mater. 100, 109626 (2020)
M.S. Hussien et al., Multifunctional applications of graphene-doped PMMA nanocomposite membranes for environmental photocatalytic. J. Inorg. Organomet. Polym. Mater. 30(7), 2708–2719 (2020)
S. Tabanli, G. Bilir, G. Eryurek, Optical properties and Judd–Ofelt analysis of Nd2O3 nanocrystals embedded in polymethyl methacrylate. J. Rare Earths 36(2), 170–178 (2018)
N. Venkatesan et al., Synthesis and characterization of neodymium based polymer composites and their application in corrosive environment. Rasāyan J. Chem. 8, 321 (2015)
C.K. Sheng et al., Investigation of morphological, structural and electrical properties of Cds/PMMA nanocomposite film prepared by solution casting method. Int. J. Electrochem. Sci. 12, 10023–10031 (2017)
V. Mathur et al., Evaluation of morphological effect on thermal and mechanical performance of PS/PMMA/CdS nanocomposite systems. Adv. Nanopart. 2(03), 205 (2013)
A. Abdelghany et al., Effect of gamma-irradiation on (PEO/PVP)/Au nanocomposite: materials for electrochemical and optical applications. Mater. Des. 97, 532–543 (2016)
E.E. Elemike et al., Phyto-assisted preparation of Ag and Ag–CuO nanoparticles using aqueous extracts of Mimosa pigra and their catalytic activities in the degradation of some common pollutants. J. Inorg. Organomet. Polym. Mater. 29(5), 1798–1806 (2019)
Z. Alrowaili et al., Design of a low-cost laser CUT-OFF filters using carmine dye-doped PVA polymeric composite films. Results Phys. 18, 103203 (2020)
L. Bi et al., Structural, magnetic, and optical properties of BiFeO3 and Bi2FeMnO6 epitaxial thin films: an experimental and first-principles study. Phys. Rev. B 78(10), 104106 (2008)
E.A. Falcao et al., Optical absorption of Nd2O3-doped polyvinylidene fluoride films. Mater. Chem. Phys. 258, 123904 (2021)
L. Aguiar et al., Study of the changes in the polar phase and optical properties of poly(vinylidene fluoride) matrix by neodymium compound addition. Mater. Today Commn. 25, 101274 (2020)
A.S. Hassanien, A.A. Akl, Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50−xSex thin films. J. Alloys Compd. 648, 280–290 (2015)
F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids. Phys. Rev. 92(5), 1324 (1953)
I. Yahia et al., Effects of stabilizer ratio on the optical constants and optical dispersion parameters of ZnO nano-fiber thin films. Superlattices Microstruct. 53, 63–75 (2013)
I. Yahia et al., Multifunction applications of TiO2/poly(vinyl alcohol) nanocomposites for laser attenuation applications. Phys. B: Condens. Matter 556, 48–60 (2019)
O.G. Abdullah et al., Effect of silicon powder on the optical characterization of poly(methyl methacrylate) polymer composites. J. Mater. Sci.: Mater. Electron. 28(5), 4513–4520 (2017)
O.G. Abdullah et al., Optical and structural investigation of synthesized PVA/PbS nanocomposites. J. Mater. Sci.: Mater. Electron. 26(9), 6939–6944 (2015)
H. Elhosiny Ali et al., Optical spectroscopy and electrical analysis of La3+-doped PVA composite films for varistor and optoelectronic applications. J. Mater. Sci.: Mater. Electron. 29(23), 20424–20432 (2018)
J. Tauc et al., Optical properties and electronic structure of amorphous germanium. Physica Status Solidi (b) 15(2), 627–637 (1966)
P. Davis et al., Some optical properties of cadmium telluride. Phys. Rev. 118(4), 1020 (1960)
E. Davis et al., Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 22(179), 0903–0922 (1970)
F. El-Sayed et al., Discussions on the film design and mechanical properties of Y3+/PVA polymeric composite films: enhancement of the electrical conductivity and dielectric properties. J. Mater. Sci.: Mater. Electron. 31(13), 10408–10421 (2020)
M. Morsi et al., UV-irradiation assisted control of the structural, optical and thermal properties of PEO/PVP blended gold nanoparticles. Mater. Chem. Phys. 201, 100–112 (2017)
F. Ali et al., Structural and optical characterization of [(PVA:PVP)-Cu2+] composite films for promising semiconducting polymer devices. J. Mol. Struct. 1189, 352–359 (2019)
S. Yadav et al., Effect of substrate on CuS/PVA nanocomposite thin films deposited on glass and silicon substrate. Soft Nanosci. Lett. 8(2), 9–19 (2018)
M. Mohammed et al., Enhancing the structural, optical, electrical, properties and photocatalytic applications of ZnO/PMMA nanocomposite membranes: towards multifunctional membranes. J. Mater. Sci.: Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-07402-3
S. Hussain et al., Optical and electrical characterization of ZnO/CuO heterojunction solar cells. Optik 130, 372–377 (2017)
M. Mohammed et al., Optical linearity and bandgap analysis of RhB-doped PMMA/FTO polymeric composites films: a new designed optical system for laser power attenuation. Opt. Laser Technol. 121, 105823 (2020)
M.I. Mohammed et al., Dielectric relaxation and thermally activated a.c. conduction in (PVDF)/(rGO) nano-composites: role of rGO over different fillers. J. Mater. Sci.: Mater. Electron. 29(21), 18271–18281 (2018)
A. Ismail et al., A facile method to prepare g-carbon nitride/poly(vinyl alcohol) nanocomposite films with remarkable optoelectrical properties: laser attenuation approach. Opt. Laser Technol. 134, 106600 (2021)
Y. Khairy et al., Optical and electrical properties of SnBr2-doped polyvinyl alcohol (PVA) polymeric solid electrolyte for electronic and optoelectronic applications. Optik 228, 166129 (2021)
M. Mohammed et al., Ammonium iodide salt-doped polyvinyl alcohol polymeric electrolyte for UV-shielding filters: synthesis, optical and dielectric characteristics. J. Mater. Sci.: Mater. Electron. 32(4), 4416–4436 (2021)
S. Duhan et al., Dielectric properties and conductivity enhancement on heat treatment of bismuth silicate glasses containing TiO2. Phys. B: Condens. Matter 404(12–13), 1648–1654 (2009)
M. Costa et al., Dielectric and impedance properties’ studies of the of lead doped (PbO)-Co2Y type hexaferrite (Ba2Co2Fe12O22 (Co2Y)). Mater. Chem. Phys. 123(1), 35–39 (2010)
O. Elkalashy et al., Attempt to tune the dielectric and optical properties in PVA/ZnO composite using tetra ethylene glycol dimethyl ether for light emitting devices. Appl. Phys. A 124(8), 1–12 (2018)
I. Yahia et al., Facile synthesis of graphene oxide/PVA nanocomposites for laser optical limiting: band gap analysis and dielectric constants. J. Mater. Sci.: Mater. Electron. 29(10), 8555–8563 (2018)
G. Appetecchi et al., New concepts in primary and rechargeable solid state lithium polymer batteries. MRS Online Proc. Libr. (1994). https://doi.org/10.1557/PROC-369-495
H.M. Ragab, Spectroscopic investigations and electrical properties of PVA/PVP blend filled with different concentrations of nickel chloride. Phys. B: Condens. Matter 406(20), 3759–3767 (2011)
S. Kaliramna, S.S. Dhayal, N. Kumar, Structural and optical studies of ZnO doped PMMA thin film and its photocatalytic and antibacterial activities. Opt. Mater. 133, 112891 (2022)
M.S. Hussien, I.S. Yahia, Fabrication progress of selective and durable Ni2+-doped Ag3PO4 for visible-light degradation of various textile dyes. J. Photochem. Photobiol. A 368, 210–218 (2019)
N.S. Alkayal et al., Network template-based cross-linked poly(methyl methacrylate)/tin(IV) oxide nanocomposites for the photocatalytic degradation of MB under UV irradiation. J. Mater. Res. Technol. 18, 2721–2734 (2022)
Y. Xie et al., Fabrication of electrospun xylan-g-PMMA/TiO2 nanofibers and photocatalytic degradation of methylene blue. Polymers 14(12), 2489 (2022)
L. Campagnolo et al., Au/ZnO hybrid nanostructures on electrospun polymeric mats for improved photocatalytic degradation of organic pollutants. Water 11(9), 1787 (2019)
N.R. Reddy et al., Photocatalytic hydrogen production from dye contaminated water and electrochemical supercapacitors using carbon nanohorns and TiO2 nanoflower heterogeneous catalysts. J. Environ. Manag. 277, 111433 (2021)
Y. Xie et al., Development of cation exchanger-based nano-CdS hybrid catalyst for visible-light photodegradation of rhodamine B from water. Sci. China Chem. 55(3), 409–415 (2012)
E. Yousif, J. Salimon, N. Salih, Mechanism of photostabilization of poly(methy methacrylate) films by 2-thioacetic acid benzothiazol complexes. Arab. J. Chem. 7(3), 306–311 (2014)
Acknowledgements
The authors would like to thank the financial support by National Authority for Remote Sensing and Space Science under Grant No. (0722/SR/SPA/2022).
Funding
This study was supported by National Authority for Remote Sensing and Space Science (Grant No. 0722/SR/SPA/2022).
Author information
Authors and Affiliations
Contributions
Conceptualization, Software, and Formal Analysis: MIM, ISY. Visualization and Resources: MIM, ISY, DE, NH, ATM, MSAH. Data Curation, Writing—Review & Editing: MIM, ISY, DE, NH, ATM, MSAH. Methodology, Writing—Review & Editing, and Project administration: MIM, ISY, DE, NH, ATM, MSAH. Project Administration and Funding Acquisition: DE, NH. Supervision: MIM, ISY, MSAH.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammed, M.I., Yahia, I.S., Elfiky, D. et al. Structural, Bandgap Analysis, Electrical Properties, and Photocatalytic Applications of PMMA/Nd2O3 Composite Membranes: Towards Multifunctional Materials for Optoelectronic and Environmental Applications. J Inorg Organomet Polym 34, 848–863 (2024). https://doi.org/10.1007/s10904-023-02839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02839-x