Abstract
In this paper, a new La(III) ion-imprinted polymer La(III)-IIP was prepared by imprinting polymerization method, using methacrylic acid as the functional monomer and alkylated mesoporous silica as the carrier. La(III)-IIP was characterized by FTIR and SEM techniques. The material was also used to capture La3+ in low concentration wastewater, and the structural morphology as well as the elemental composition of the material were characterized and analyzed. In addition, based on the characterization and experimental data, the adsorption type of La(III) and the possible adsorption mechanism of the material were further analyzed, and finally, the selective adsorption of lanthanum and the regeneration performance of the material were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The seventeen rare earth elements are composed of lanthanides, yttrium, and scandium [1]. Rare earth elements have excellent magnetic, optical, electrical, and strength properties based on their exceptionally rich electron energy levels and special 4f electron layer structure characteristics [2, 3], making them an indispensable choice for most advanced industrial applications. Owing to their low melting temperatures and good ductility, rare earth elements have been widely used in metallurgy, military applications, electronics, medicine, glass ceramics, agriculture, and new material development [4, 5]. Currently, there are many problems related to resource waste and environmental pollution in the exploitation of rare earth resources; therefore, the efficient enrichment and recovery of rare earth resources has become a key trend. Previously, many methods were developed for the enrichment and recovery of rare earth elements, including extraction chromatography [6], co-precipitation [7], ion exchange [8], membrane separation, and adsorption methods [9, 10]. Among them, the adsorption method is low cost, easy to operate, and has greater potential for the efficient treatment of wastewater. It is also an effective process for the enrichment and recovery of rare earth resources, where the performance of the adsorbent is the most critical influencing factor, and the selection of adsorbent materials is particularly important. The Imprinting Technique is a method applied for the synthesis of adsorbents because of the unique advantages, such as special functional sites for targets, good adsorption and recognition ability.
Depending on the type of target, blotting techniques can be divided into two main categories: molecularly imprinted techniques (MIT) and ion-imprinted techniques (IIT) Molecularly imprinted polymers (MIPs) are prepared by copolymerization of functional monomers and crosslinkers in the presence of a target analyte (imprinted molecule) as a template molecule. In contrast, ion-imprinted polymers (IIPs) are similar to MIPs and retain all the advantages of MIPs, but they recognize ions.Similar to molecularly imprinted polymer MIPs, ion-imprinted polymers (IIPs) have been used for separation, preconcentration, or ion assays [11, 12]. Typically, IIPs are defined as a group of highly selective materials that recognize selected ions from the same matrix in the presence of competing ions [13]. Polymeric adsorbents used for ion imprinting are selective based on the composition, geometry, charge and size of the target ion of the functional monomer. Recent studies have shown that IIPs have promising applications in metal ion recognition, environmental pollution, and metal ion preenrichment and separation [14]. In the present era, IIPs technology as a viable synthetic method for the future of ontogenic polymerization technology through the development of ion- IIPs remains a challenge for various applications, including selective ion recovery from wastewater effluents [15].
Ion-imprinting of silica-based materials has received a lot of attention. Mesoporous silica has demonstrated the ability to adsorb large amounts of metal ions and is widely used in various industries. Mesoporous silica exhibits excellent adsorption–desorption properties, which prompted us to consider it as a very suitable and efficient carrier. In this chapter, a new La(III) ion-imprinted polymer (La-IIP) was prepared by using an imprinting polymerization method, selecting acrylamide as the functional monomer and alkylated mesoporous silica as the carrier. The La(III)-IIP was characterized by FTIR and SEM techniques. The material was also used to capture La3+ in low concentration wastewater, and the structural morphology as well as the elemental composition of the material were characterized and analyzed. In addition, based on the characterization and experimental data, the adsorption type of La(III) and the possible adsorption mechanism of the material were further analyzed, and finally, the selective adsorption of lanthanum and the regeneration performance of the material were investigated.
2 Experimental Section
2.1 Materials and Reagents
Na2SiO3 and polyoxyethylene (20) cetyl ether (PC20) were purchased from Aldrich Chemical Reagent Company, USA) (Sigma-Aldrich), H2SiF6 (35 wt%, Alfa Aesar, UK), lanthanum (III) nitrate hydrate and cerium (III) nitrate hexahydrate were purchased from China Pharmaceutical Chemical Reagent Co. Hexadecyltrimethylammonium bromide, methacrylic acid, ethylene glycol dimethacrylate, arsenate III of azo diisobutyronitrile were purchased from Shanghai Maclean Biochemical Co. Ethanol was purchased from Tianjin Damao Chemical Reagent Factory, and hydrochloric acid, sodium hydroxide and ammonia were purchased from Xilong Science Co. In this experiment, dilute NaOH and HCl solutions were used to adjust the pH values to the desired values. Deionized water was used for all aqueous solutions. All chemical reagents were of analytical grade.
2.2 Preparation of La(III)-IIP
Mesoporous silica (MS-PC20) was prepared according to the route described in the literature [16],The flow chart of La(III)-IIP preparation is shown in Fig. 1. The process has the following main steps: firstly, the prepared MS-PC20 is surface alkylated and methacrylic acid is added as the functional monomer to provide functional groups for the reaction process. Then the template ion La(III) is added for cross-linking polymerization with the cross-linking agent, and finally the template ion is washed away with 2 mol/L HCl to obtain the ion-imprinted polymer La(III)-IIP.
First, to functionalize the MS-PC20 carrier, 5 g of MS-PC20 was stirred in acetonitrile for 1 h. Then 20.76 mmol of CPTES was added and the mixture was soaked in an oil bath at 80 °C for 24 h under neutral atmosphere. The obtained compound was filtered, washed, and dried in a vacuum oven at 110 °C for 4 h. The alkylated MS-PC20-Cl was obtained. Finally, 2 g of MS-PC20-Cl was stirred in acetonitrile for 1 h, followed by the addition of an amount of AM and stirred in an oil bath at 80 °C for 12 h. Then, the obtained solid MS-PC20-AM was filtered, rinsed with ethanol and dried at 50 °C under vacuum. MS-PC20-AM was obtained.
2.2.1 Preparation of Lanthanide Ion-Imprinted Polymers
La(III)-IIP was prepared by surface imprinting method by preparing a mixture of MS-PC20-MAA 50 mg with acetonitrile/methanol 50 mL (60/40 v/v) and dispersed in a circular flask. Then, 0.2 mmol La(NO3)3, 3 mmol EGDMA and 40 mg AIBN were added to the flask. to deoxygenate the solution, the polymerized mixture was purged with N2 for 10 min. The solution was sealed and stirred in an oil bath at 60 °C for 24 h. The surface polymerization reaction was performed by repeatedly washing the solution with methanol and finally with distilled water to remove unreacted material. Then, 2 mol/L HCl was used to leach out the lanthanide ions captured in the polymer until a titration solution free of La(III) ions was obtained. Finally, the white powder with neutral pH was obtained by repeated washing with deionized water. The white powder La(III)-IIP was dried under vacuum at 40 °C for 12 h. As a comparison, a non-imprinted polymer without the addition of La(NO3)3 (La(III)-NIP) was also prepared in parallel.
2.3 Characterization
The functional groups of La(III)-IIP were analyzed by Fourier infrared transform spectroscopy (FT-IR, Nicolet is50) in the range of 3500–400 cm−1 to analyze the functional groups of the material. The morphology and surface structure of the composites were analyzed at different magnifications using a Quanta 200FEG type environmental scanning electron microscope (SEM). The thermal stability of La(III)-IIP was obtained using a thermogravimetric analyzer (TGA 4000) under a nitrogen atmosphere at a heating rate of 10 °C/min from 25 to 900 °C.
2.4 Experimental Methods
2.4.1 Adsorption Experiments
The prepared adsorbent materials were used as adsorbents for La(III) and Ce(III) for the next adsorption experiments to test the adsorption effect of the prepared adsorbent materials on low concentrations of rare earth ions. First, a certain mass of rare earth ions was weighed and dissolved in deionized water using an analytical balance, and 1L of 50 mg/L of lanthanide and cerium ion solution stock solution was prepared. A certain amount of supernatant was transferred to a numbered 25 mL volumetric flask with a pipette, and then 2 mL of pre-prepared HCl solution with a concentration of 0.02 mol/L and 1 mL of azoarsine(III) solution with a concentration of 500 ppm were added to observe the color development phenomenon, and the absorbance was measured by UV for a few moments after shaking well with fixed volume, and the adsorption amount and The adsorption amount and the adsorption rate were calculated according to Eqs. 1 and 2.
In the above equation: Q is the adsorption amount (mg/g), i.e. the ratio of the mass of the adsorbent (mg) to the mass of the adsorbent (g); C0 denotes the initial concentration of La3+ (mg/L); Ce denotes the equilibrium concentration of La3+ (mg/L); V denotes the volume of La3+ solution added to the oscillation (L); m denotes the mass of the adsorbent (g); Ƞ denotes the adsorption rate of the adsorbed rare earth ions (%).
2.4.2 Optimization of Adsorption Experiments
In this paper, in order to further investigate the effect of adsorption conditions on the adsorption capacity of the adsorbent, the optimal adsorption conditions of the adsorbent were investigated and its adsorption mechanism was studied. The effect of each condition on the adsorption capacity of the adsorbent was investigated using the controlled variable method to obtain the optimal adsorption conditions.pH optimization: To investigate the effect of pH on the adsorption of La3+, the pH range of La3+ with C0 = 50 ppm was adjusted to 2–7 using 0.1 mol/L HCl and 0.1 mol/L NaOH, and equal amounts of adsorbent were added and placed into a constant temperature oscillating water bath (set at different temperatures), and the adsorption was oscillated to saturation and measured at different pH The remaining concentration of La3+ in the solution was measured to find the adsorption amount and adsorption rate.
Time optimization: In order to determine the adsorption capacity of the adsorbent on La3+ with time. First, equal volumes of 50 ppm La3+ solutions adjusted to the optimal pH were added to several conical flasks, and then equal amounts of adsorbent were added and shaken at room temperature to measure the remaining La3+ concentrations at different adsorption times, so as to obtain experimental data at different adsorption times.
Optimization of the initial concentration of rare earth ions: a fixed amount of adsorbent was weighed and added to different concentrations of rare earth solutions, and the adsorption was fully shaken at room temperature for a period of time until the equilibrium state, and the remaining ion concentration was measured by filtration, so as to obtain the adsorption amount.
Optimization of temperature: The same mass of adsorbent was added to the same volume of rare earth ion solution with an initial concentration of 50 ppm, and the water bath thermostat was adjusted to different temperatures, and the remaining ion concentration was measured after a specific time of sufficient shaking to obtain the adsorption amount.
To ensure the true accuracy of the experimental data, three parallel experiments were conducted for each studied factor during the optimization of the adsorption test.
2.4.3 Competitive Adsorption Studies
In practical applications, the selective adsorption performance of the adsorbent materials is particularly important. At pH = 6, four coexisting solutions of Ce3+, Gd3+, Y3+, Yb3+, Fe3+ and Al3+ configured with La3+ at 50 ppm were poured into conical flasks, and after the same amount of adsorbent was added to each conical flask for the same time of oscillatory adsorption, the residual ion concentration was detected by filtration with ICP-OES, and the partition coefficients of various ions and the selectivity coefficients for lanthanum(III) The partition coefficients of various ions and the selectivity coefficients for lanthanum(III) were obtained from Eqs. 3 and 4, respectively.
where Kd is the partition coefficient (L/g) and k is the selectivity coefficient of lanthanum(III).
2.4.4 Study of the Elution and Reusability Properties of Adsorbents
The reusability of the adsorbent is an important factor affecting the cost of application. In order to find a suitable eluent and to study the reusability of the adsorbent, five cycles of repeated adsorption/desorption experiments were carried out with the same adsorbent. The specific procedure was to perform adsorption/desorption experiments on a certain concentration of La(III) and Ce(III) under certain adsorption conditions. After the adsorption of the adsorbent material reached saturation, the lanthanide and cerium ions adsorbed by the adsorbent were eluted with an eluent (hydrochloric acid was chosen in this paper), and their elution rates were calculated for several times using the following Eq. 5.
In the above equation: D is the elution rate (%); Qd is the elution capacity (mg/g).
2.5 Analysis of the Adsorption Process
2.5.1 Analysis of Adsorption Kinetics
The kinetic mechanism of La(III) and Ce(III) ion adsorption was further investigated using the proposed primary kinetic model and the proposed secondary kinetic model. The fitted primary kinetic model assumes that the adsorption process is ion diffusion through the boundary layer of the adsorbent surface, and diffusion is the main step in the adsorption process that determines the adsorption rate. In addition, the fitted secondary kinetic model suggests that the adsorption process is mainly controlled by the chemisorption mechanism, i.e., the electron sharing or transfer between the adsorbent and the adsorbate is the main driving force of the adsorption process. Currently, the fitted primary kinetic model (PFO) and the fitted secondary kinetic model (PSO) have been widely used to describe the adsorption rate in liquid–solid interactions.
In many cases, the PFO model is not a good fit for the entire contact adsorption time range and it is usually more applicable to the initial stage of the adsorption process.The PFO equation is expressed as in Eq. 6:
The PSO model fit can predict the equilibrium adsorption amount when the adsorbent reaches adsorption. the PSO equation is expressed as Eq. 7:
where Qe is the equilibrium adsorption amount (mg−/g); Qt is the adsorption amount at time t (mg-g-1); Qm is the theoretical adsorption amount (mg−/g); t is the adsorption time (min); k1 is the PFO rate constant (min−1); k2 is the PSO rate constant (g−/mg/min).
On the other hand, when it comes to solid adsorbents, intraparticle diffusion is a crucial factor. In solid adsorbent particles, the transport process of gas molecules in the pore channel. It is one of the most important factors affecting the adsorption rate and selectivity and is expressed as in Eq. 8:
Kp (mg g/min−0.5) in the IPD expression equation is the rate constant for intraparticle diffusion, C is the intercept constant, and whether the rate-limiting step of the IPD model is unique depends on whether the value of C is zero.
2.5.2 Isothermal Adsorption Model Analysis
Adsorption isotherms are one of the important tools to study the adsorption process and understand the adsorption mechanism. In solid/liquid systems, the commonly used isotherm models include Langmuir model and Freundlich model. In this paper, Langmuir and Freundlich models are used to perform adsorption experiments on lanthanum or cerium ions at different initial concentrations of adsorbent and to analyze the adsorption mechanism.
The Langmuir model equation is shown in Eq. 9, which describes chemisorption based on some assumptions:
-
(1)
The adsorption energy is constant, the adsorbent surface is homogeneous in nature, and the adsorption process occurs on all uniform parts of the surface.
-
(2)
the adsorption layer is localized, a molecule is bound to a binding site (i.e. monolayer adsorption)
-
(3)
In the adsorption process, the adsorption and desorption are dynamically balanced.
$$\frac{{C}_{e}}{{Q}_{e}}=\frac{1}{{K}_{L}{Q}_{m}}+\frac{{C}_{e}}{{Q}_{m}}$$(9)
In addition, the dimensionless separation factor constant (RL) was used to assess the suitability of the adsorbent for the studied adsorbent. an RL between 0 and 1 indicates that the adsorption process can proceed, while an RL greater than 1 does not. the RL expression is Eq. 10.
The Freundlich model equation is shown in Eq. 11. The Freundlich isotherm assumes that the binding sites have inhomogeneous affinity for the adsorbate. It describes well the multilayer adsorption and adsorption on non-homogeneous surfaces of the adsorbent.
In the above equations: Q_m is the theoretical adsorption amount (mg/g); C_m indicates and the initial concentration of adsorbent; K_L and K_F indicate the Langmuir constant and Freundlich constant, respectively; the magnitude of 1⁄n indicates the favorability of adsorption.
2.5.3 Thermodynamic Analysis of Adsorption
The thermodynamic study was carried out to better understand the adsorption mechanism. The adsorption of La(III) or Ce(III) at different temperatures was analyzed by adsorbing materials, and the values of thermodynamic parameters such as reaction enthalpy ∆H, entropy change ∆S and Gibbs free energy ∆G were calculated by fitting Eqs. 12 and 13. In addition to the enthalpy change ∆H, the adsorption process is also affected by the entropy change ∆S. The entropy change ∆S can reflect the entropy change between the adsorbent and the adsorbate molecules and is usually one of the important indicators to determine whether the adsorption process is thermodynamic or physical adsorption. The enthalpy change ∆H and entropy change ∆S can be positive or negative, and finally the Gibbs free energy ΔG is used to determine whether the reaction can proceed spontaneously. If ∆G > 0, the reaction is a non-spontaneous process, and if ∆G < 0, the reaction is spontaneous.
where: Kd denotes the adsorption equilibrium constant; ΔS denotes the entropy change(J/mol/K); ∆H denotes the enthalpy change(kJ/mol); R denotes the gas constant with a value of 8.314 J/mol/K; ΔG denotes the Gibbs free energy change (kJ/mol).
3 Results and Discussion
3.1 Characterization and Analysis
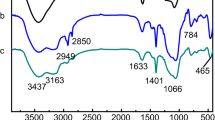
3.1.1 FT-IR Spectroscopy
The molecular structure of the materials was analyzed by FI-IR spectroscopy test. Figure 2 shows the IR comparison between MS-PC20 and lanthanum-imprinted polymer La(III)-IIP, from which it can be seen that both MS-PC20 and La-(III)-IIP have absorption peaks at 3433, 1085, 803, and 468 cm−1. The peaks at 1085 cm−1, 803 cm−1, and 468 cm−1 correspond to the Si–O–Si asymmetric, symmetric stretching vibration peak and bending vibration peak of the MS-PC20 backbone, respectively. In contrast to MS-PC20, La(III)-IIP produced new absorption peaks at 2976 cm−1, 2895 cm−1,1418 cm−1. The peaks at 2976 cm−1 and 2895 cm−1 are the C–H bond stretching vibration peaks, which are probably generated by the grafting of 3-chloropropyltriethoxysilane and acrylamide. 1460 cm−1 corresponds to the C–N bond stretching vibration peak, which can be inferred that acrylamide was successfully grafted to the surface of MS-PC20. In addition, the absorption peak at 1625 cm−1 is the characteristic absorption peak of the C=O bond, which belongs to the cross-linker glycolic acid dimethacrylate, which can indicate that the trace layer was successfully grafted onto the mesoporous silica MS-PC20.
3.1.2 TGA
The thermal stability was investigated by thermogravimetric analysis (TGA) in the range of room temperature ~ 900 °C. The TGA curves of the ion-imprinted polymer La(III)-IIP and the substrate MS-PC20 are shown in Fig. 3. For MS-PC20, the weight loss is about 5.8% when the temperature rises from room temperature to 150 °C. Due to the high specific surface area and porosity of MS-PC20 mesoporous material, there are many incompletely condensed hydroxyl groups in the pore channels, thus showing a certain polarity, which will absorb water molecules that are also polar, so the weight loss here belongs to the physisorbed water on the surface of MS-PC20 and the surface within its pore channels Physisorbed water removal. The weight loss of about 8.3% from 150 to 900 °C is due to the high-temperature decomposition and combustion of the residual surfactant in the prepared MS-PC20 and the high-temperature condensation of the surface silicon hydroxyl groups to form Si–O–Si bonds. the weight loss of La(III)-IIP in the first stage from room temperature to 300 °C is about 8.2%, which is mainly due to the evaporation of physisorbed water. When the temperature was changed from 300 to 900 °C, the weight loss was more pronounced, decreasing by about 66.8%. This may be due to the decomposition and degradation of the organic matter coated on the silica surface. The results indicate that the mesoporous silica surface is covered with a thin layer of polymer therefore the prepared polymer has good thermal stability when the ambient temperature is below 300 °C.
3.1.3 SEM Analysis
As shown by the SEM images of La(III)-NIP and La(III)-IIP in Fig. 4, La(III)-NIP shows a smaller cylindrical structure with relatively obvious size distribution. It can be seen that the polymerization process of MS-PC20 without La(III) ion as the target ion is an incomplete polymerization process. As can be seen in Figs. 4a and b, the leached La(III)-IIP and the unleached La(III)-IIP become rough and stick together. We also see that their ordered morphology is strongly disrupted during the ion-imprinted polymerization process.EDS plots to study the elemental composition of La(III)-IIP clearly show that four elements (C, N, O and Si) are present in La(III)-IIP and are more uniformly distributed in the sample.
3.2 Effect of pH
Solution pH affects the ionization and surface charge of the adsorbent. The adsorption of La3+ on the adsorbent is a surface reaction, so pH plays an important role in the adsorption of La3+ by La(III)-NIP和 La(III)-IIP. In this study, the pH test range was 2–7 because La3+ would precipitate in an alkaline environment so it was not considered.
As shown in Fig. 5, at pH 2, the adsorption amount and adsorption rate of La(III)-NIP和La(III)-IIP were extremely small or even absent, and in the more acidic environment of pH < 4, the adsorption amount and adsorption rate of La(III)-NIP和 La(III)-IIP increased sharply with increasing pH. This significant increase can be attributed to the fact that when the solution pH was low, a higher amount of H+ occupied the adsorbent surface adsorption sites with adsorption effect, and La3+ formed a competitive adsorption with H+. In addition, at lower pH environments, the number of positively charged adsorbent surface sites increases, and the direct electrostatic repulsion between the positively charged adsorbent mass and the positively charged molecules on the adsorbent surface increases, so that both the adsorption amount and adsorption rate decrease with decreasing pH. At pH 4–6, the adsorption and adsorption rate of the three adsorption materials gradually increased with decreasing acidity intensity. This can be further explained as the increase in pH caused the number of H+ and H3O+ ions in the solution to decrease, the degree of ionization of the agent is further enhanced, which leads to the enhancement of electrostatic attachment between the adsorption agent and the La3+. However, the high pH of the solution also has a negative effect on the adsorption of lanthanide ions. Due to the presence of a large number of anions in water, the positively charged lanthanide ions will be surrounded by anions and form negatively charged atomic groups, which negatively affects the adsorption effect of lanthanide ions, so the adsorption amount and rate increase more slowly. At pH 7, a small amount of lanthanide ions may form hydroxide precipitation, and a portion of the lanthanide ions in the solution will be adsorbed and part will be precipitated, so the adsorption amount and adsorption rate will suddenly increase. In summary, to ensure that the adsorption process is not heavily influenced by ion precipitation, a pH value of 6 was chosen for subsequent experiments.
3.3 Effect of Contact Time on La3+ Adsorption and Kinetic Study
A good quality adsorbent needs to have not only high adsorption capacity and adsorption rate, but also fast adsorption rate, so the examination of contact time is crucial, and kinetic studies are the basis for evaluating the interaction mechanism between the target metal ions and the adsorbent. The effect of adsorption time on the adsorption of La(III)-IIP and La(III)-NIP on La(III) ions was investigated (Fig. 6). The adsorption rates were faster at the initial stage, then gradually slowed down and gradually reached the adsorption equilibrium at 20–30 min. We speculate that: (1) in the initial stage, there are enough binding sites on the polymer surface to make La(III) easily bound to the adsorbent; (2) at the end of the fast adsorption stage, the external binding sites are gradually occupied by La(III), the number of available binding sites decreases, and La(III) needs to diffuse to the interior of the adsorbent for further adsorption, resulting in a slow adsorption rate until the adsorption equilibrium is reached.
The adsorption rate of La(III)-IIP was fast and the adsorption of La3+ was basically equilibrated within 30 min, and the adsorption capacity of La(III)-IIP reached 219.89 mg/g at 30 min, while the adsorption capacity of the control La(III)-NIP was 149.21 mg/g at 30 min when the adsorption reached equilibrium, which was much lower than that of La(III)-IIP (219.89 mg/g), which indicates the stronger adsorption performance and faster adsorption rate of the imprinted material. In summary, the contact adsorption time of 30 min was subsequently chosen to explore La(III)-IIP, respectively.
The kinetic mechanism of La(III) ion adsorption was further investigated using a pseudo-first-order kinetic model and a pseudo-second-order kinetic model. The pseudo-first-order kinetic model assumes that the adsorption process is ion diffusion through the boundary layer of the adsorbent surface and the adsorption process is controlled by the diffusion step. In the proposed second-order kinetic model, the adsorption process is controlled by a chemisorption mechanism, and the adsorption process involves electron sharing or electron transfer between the adsorbent and the adsorbate. The proposed first-order kinetic model and the proposed second-order kinetic model expressed as Eqs. (6) and (7), respectively, were fitted to the experimental data of lutetium ion adsorption by La(III)-IIP with time, and the adsorption process and mechanism of La3+ on this adsorbent were further analyzed, and the fitted curves are shown in Fig. 7.
There is a strong interaction between the adsorbate and adsorbent, and to further investigate the adsorption mechanism, the pseudo-first-order kinetic model (Eq. (6) and pseudo-second-order kinetic model (Eq. (7) were used to analyze the adsorption kinetics of La3+ on the adsorbents [17,18,19,20]. The fitted curves are shown in Fig. 8b–d), and the kinetic parameters and correlation coefficients were calculated from the fitted equations as shown in Table 1.
From Table 1, the values of the correlation coefficients (R2 = 0.9813–0.9922) obtained by the PSO model for the Imprinted material La(III)-IIP were higher than those of the PFO model. The quantified values (Qe cal) calculated with the PSO model were closer to the experimentally obtained ones (Qe exp). The results indicate that the adsorption rate on La3+ is controlled by the interaction of La3+ with the active site. The initial fast adsorption is mainly attributed to the rapid uptake of lanthanide ions by the Imprinted material La(III)-IIP. The subsequent slow adsorption was attributed to the slow diffusion of lanthanide ions through the pores [21].
3.4 Effect of Initial Concentration and Isothermal Adsorption Model
The isotherm adsorption line reflects the interaction pattern between the adsorbent and the adsorbent, and the equilibrium adsorption isotherm is usually used to determine the adsorption capacity. Controlling the rest of the conditions as constant, Fig. 9 shows the variation of the adsorption rate for different initial concentrations of La3+, which can be seen to be 100% for low concentrations. Figure 9 shows the adsorption isotherm plots of the La(III)-IIP to investigate the variation of the adsorption performance of the adsorbent materials at each temperature (308 K, 323 K, 338 K) with the variation of the initial concentration of La3+, and the results show that at the same temperature, the adsorption performance of the La(III)-IIP for La3+ adsorption properties of both composites La(III)-IIP gradually increased with increasing initial concentration of La3+ (Table 2).
To analyze the experimental data, the Langmuir and Freundlich models were used to fit the experimental data. The Langmuir model assumes that the adsorption process takes place homogeneously across the surface and that the adsorbent adsorbs in a single molecular layer without any interaction between the surfaces; the Freundlich model is used for adsorption on non-homogeneous surfaces with different adsorption energies. The two model equations are shown in Eqs. (9) and (11) [22,23,24,25].
In addition, the dimensionless separation factor constant (RL) was used to assess the suitability of the adsorbent for the studied adsorbent [26]. The RL expression is given by Eq. (10).
The fitted figure for this La(III)-IIP is shown in 8. The fit parameters obtained from the fit plots are presented in Tables 3 and 4, respectively, and the applicability of the model is analyzed by comparing the correlation coefficients R2 of the two models. From the table, it can be seen that the correlation coefficient R2 is greater for the Langmuir model of the three adsorbent materials, La(III)-IIP, indicating that the experimental data of La3+ adsorption on the three adsorbent materials are more suitable for the Langmuir model, and the monomolecular layer adsorption of La3+ occurs between the homogeneous site on the surface of the three adsorbents and its active site without other effects, and the fitted The calculated values of RL are all between 0 and 1, indicating that the adsorption is reasonably effective.
3.5 Thermodynamic Study of Adsorption
To investigate the spontaneity of the adsorption process of the adsorption materials, La(III)-IIP, the thermodynamic investigation was carried out. The adsorption performance of La3+ by the three adsorbent materials at 298 K, 308 K, 318 K, 328 K and 338 K is shown in Fig. 10, from which it can be seen that the adsorption capacities are all lowest at T = 298 K and highest at T = 338 K. This indicates that the adsorption processes are all heat-absorbing reactions and high temperature is favorable for their adsorption. This may be due to the fact that with the increase of temperature, La3+ moves faster and more toward the adsorbent surface, and also possesses more energy for binding to the active sites on the adsorbent surface.
The values of thermodynamic parameters such as reaction enthalpy ΔH, entropy change ΔS, and Gibbs free energy ΔG were calculated by fitting Eqs. (12) and (13) to the three adsorbents at different temperatures. Gibbs free energy ΔG was used to determine whether the reaction proceeded spontaneously [27, 28]
As shown in Table 3, the positive values of ΔH for the three adsorbent materials confirm the heat-absorbing nature of the adsorption process, the positive values of ΔS reveal the good affinity of the adsorbent materials for the adsorption of La(III) ions, and ΔG decreases with increasing temperature, indicating that the spontaneity of the reaction increases with increasing temperature. The absorption efficiency of La(III) was highest at T = 338 K.
3.6 Competitive adsorption studies
To investigate the selectivity of La(III)-IIP, selective adsorption experiments were conducted at pH 7 in a mixture of La3+, Ce3+, Gd3+, Yb3+, Y3+, Al3+ and Fe3+, which have the same valence and similar ionic radii and may coexist in low concentration wastewater discharged from mines. The specific steps were as follows: Ce3+, Gd3+, Yb3+, Al3+ and Fe3+ were configured with La3+ at pH 7 into four groups of coexisting solutions of 100 ppm, respectively, and poured into conical flasks, and after adding 10 mg of adsorbent to each conical flask for the same time of oscillatory adsorption, the remaining concentration of the ions was measured by ICP-OES through 0.22 um filter membrane filtration. The partition coefficients of each ion calculated using Eqs. (3) and (4) as well as the lanthanide ion selection coefficients are shown in Table 4.
Relative to the non-imprinted material La(III)NIP, La(III)-IIP shows good selectivity for La(III). the La(III)-IIP material has a high selectivity coefficient, on the other hand, La3+/Y3+, La3+/Ce3+, La3+/Gd3+, La3+/Yb3+, La3+/Al3+ and La3+/ The relative selectivity coefficients (k) of Fe3+ were 4.02, 2.23, 4.07, 2.89, 12.62 and 18.13, respectively, which were much higher than 1. It is noteworthy that the imprinted silica adsorbent had the ability to selectively adsorb La3+ ions in the presence of other interfering ions. In this study, the selectivity of the non-ionic imprinted polymer was much lower than that of the ionically imprinted polymer due to the fact that the cavities in the imprinted polymer act as specific cavities that allow selective ion adsorption during ion adsorption. Thus, the cavities formed in the structure of the host imprinted polymer have a specific shape and size, which would prevent them from readily absorbing ions larger or smaller than the imprinted template, as the fixed recognition cavities complement the target ions in shape, size and properties, suggesting that the cavities left by elution of La3+ not only result in an improved adsorption rate, but the La(III)-IIP material has a higher Kd, template ions bind significantly differently from competing ions on the blotted polymer, suggesting that La(III)-IIP can be used as a selective adsorbent for the separation of template ions in the presence of other ions.
3.7 Recyclability Experiments
The reproducibility and stability of an adsorbent are important factors for commercial use. In this study, 0.1 mol/L HCl was used as the eluent, and the adsorbent was usually added to 30 mL of La3+ solution with a concentration of 50 mg/L to saturate the adsorption. The ions were washed out with the eluent and deionized water to recover the adsorbent, and finally dried and used again for the next adsorption; this process was repeated five times. As shown in Fig. 11, the adsorption effect of La(III)-IIP decreased after ten times but still maintained greater than 74% of the initial adsorption effect, indicating that the adsorbent not only has high adsorption capacity and adsorption rate but also high repeatability and stability.
4 Conclusions
In this study, the lanthanide ion-imprinted polymer La(III)-IIP was prepared under N2 atmosphere using MS-PC20 as the carrier, acrylamide (AM) as the functional monomer, ethylene glycol dimethacrylate as the cross-linker and diisobutyronitrile as the initiator.The results are summarized as follows. The results are as follows.
-
(1)
La-IIP has a strong adsorption affinity, specific recognition ability and excellent selectivity with a maximum adsorption capacity of 219.89 mg/g.
-
(2)
The quasi-first-order and quasi-second-order kinetic fitting of the experimental data led to the conclusion that La-IIP is more consistent with the quasi-second-order kinetic model, indicating that the adsorption process of La-IIP is mainly chemisorption.
-
(3)
According to the isothermal adsorption model, the results of the Langmuir and Freundlich adsorption model fitting showed that La-IIP was more consistent with the Langmuir adsorption model.
-
(4)
The results of the thermodynamic fitting of La-IIP indicate that the adsorption process is a stable process heat absorption spontaneous reaction.
-
(5)
In addition, the ion-imprinted polymer material La(III)-IIP has better stability and regeneration performance. Surface ion imprinting technology continues to develop and become more mature, providing more reference methods for the treatment and adsorption of metal ions in waste ores
References
A. Tk, C. Akb, C. Alb et al., Adsorption of rare earth metals from wastewater by nanomaterials: a review. J. Hazard. Mater. 386, 121632 (2020)
F.H. Robert, C. Thibault, E.C. Bren et al., Magnetic Field Directed Rare-Earth Separations. Angew. Chem. Int. Ed. 132(5), 1867–1872 (2019)
M.J. Simon, T.W. Timothy, W. Zhehan et al., Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 13, 1–7 (2018)
A. Ioannis, B. Amit, C.L. Eder, Adsorption of rare earth metals: a review of recent literature. J. Mol. Liq. 221, 954–962 (2016)
T. Yue, S. Lu, F. Chong et al., Distribution of rare earth elements (REEs) and their roles in plants growth: a review. Environ. Pollut. 298, 118540 (2021)
H. Minowa, M. Ebihara, Separation of rare earth elements from scandium by extraction chromatography. Anal. Chim. Acta 498(1–2), 25–37 (2003)
F. Nicolas, B. Germain, B. Dominique et al., Determination of rare earth elements and other trace elements (Y, Mn Co, Cr) in seawater using Tm addition and Mg(OH)2 co-precipitation. Talanta 85(1), 582–587 (2011)
A. Tsuyoshi, W. Yuezhou, K. Mikio et al., Separation of rare earths in nitric acid medium by a novel silica-based pyridinium anion exchange resin. J. Alloys Compd. 408, 1008–1012 (2006)
D. İlayda, E. Duygu, R.K. Ali, Graphene oxides for removal of heavy and precious metals from wastewater. J. Mater. Sci. 51, 6097–6116 (2016)
Z. Yongfeng, Z. Yian, W. Aiqin, A simple approach to fabricate granular adsorbent for adsorption of rare elements. Int. J. Biol. Macromol. 72, 410–420 (2014)
M. Shamsipur, J. Fasihi, A. Khanchi et al., A stoichiometric imprinted chelating resin for selective recognition of copper (II) ions in aqueous media. Anal. Chim. Acta 599(2), 294–301 (2007)
A. Koohpaei, S. Shahtaheri, M. Ganjali et al., Application of multivariate analysis to the screening of molecularly imprinted polymers (MIPs) for ametryn. Talanta 75(4), 978–986 (2008)
B. Ooa, Macroporous copolymer networks. Prog. Polym. Sci. 25(6), 711–779 (2000)
J. Fu, L. Chen, J. Li et al., Current status and challenges of ion imprinting. J. Mater. Chem. A 2015(3), 13598–13627 (2015)
Y. Huang, R. Wang et al., Review on Fundamentals, preparations and applications of imprinted polymers. Curr. Org. Chem. 22(16), 1600–1618 (2018)
O.Y. Kim, Preparation of mesoporous silica by the rapid gelation of Na2SiO3 and H2SiF6 in aqueous surfactant solution. Microporous Mesoporous Mater. 285, 137–141 (2019)
M.H. Mostafa, S.E. Rizk, A.A. Nayl, Adsorption kinetics and modeling of gadolinium and cobalt ions sorption by an ion-exchange resin. Part. Sci. Technol. 34(6), 716–724 (2015)
H. Azhar-Abdul, A. Hamidi-Abdul, M.J. Megat-Azmi et al., Comparison study of ammonia and COD adsorption on zeolite, activated carbon and composite materials in landfill leachate treatment. Desalination 262(1–3), 31–35 (2010)
L. Sheng-Yu, G. Jin, Q. Bin et al., Kinetic models for the adsorption of lead ions by steel slag. Waste Manage. Res. 262(1–3), 31–35 (2009)
S. Qianqian, F. Yi, L. Zhenya et al., The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution. Chem. Eng. J. 325, 71–79 (2017)
L. Xing, C. Ya-Shuo, Z. Qian et al., Removal of iodine from aqueous solution by PVDF/ZIF-8 nanocomposite membranes. Sep. Purif. Technol. 238, 116488 (2019)
J.S. Piccin, T. Cadaval, L. Pinto et al., Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations (Springer International Publishing, New York, 2017)
M.A. Al-Anber, Adsorption of ferric ions onto natural feldspar: kinetic modeling and adsorption isotherm. Int. J. Environ. Sci. Technol. 12, 139–150 (2013)
S. Gaurav, N. Mu, Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling. J. Mol. Liq. 310, 113025 (2020)
P.S. Neetu, Synthesis, characterization and sorption behavior of zirconium(IV) antimonotungstate: an inorganic ion exchanger. Desalination 267(2–3), 277–285 (2011)
F. Mojtaba, A. Asghar, L. Milad Jamal et al., Adsorption of lead(II) and chromium(VI) from aqueous environment onto metal-organic framework MIL-100(Fe): synthesis, kinetics, equilibrium and thermodynamics. J. Solid State Chem. 291, 121636 (2020)
G. Ahmet, Y. Mehmet, S. Mustafa et al., The investigation of adsorption thermodynamics and mechanism of a cationic surfactant, CTAB, onto powdered active carbon. Fuel Process. Technol. 81(1), 57–66 (2003)
S. Fabiano Bisinella, M. Aparecido Nivaldo, B. Carlos Eduardo et al., Monolayer–multilayer adsorption phenomenological model: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 284, 1328–1341 (2015)
Acknowledgements
The work was supported by the Nature Science Foundation of China (51664042).
Funding
Project supported by the Nature Science Foundation of China (51664042).
Author information
Authors and Affiliations
Contributions
Author Contribution Statement: YH, First author: Designed the study, conducted the study, analyzed most of the data, and wrote the paper. The remaining authors contributed to refining the idea, performing additional analysis, and finalizing the paper.
Corresponding authors
Ethics declarations
Competing interest
All authors disclosed no relevant relationships.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Li, X., Qin, Y. et al. Preparation of Lanthanide Ion Surface Imprinted Polymers Based on Mesoporous Silica and Their Adsorption Properties. J Inorg Organomet Polym 33, 3638–3650 (2023). https://doi.org/10.1007/s10904-023-02769-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-023-02769-8