Abstract
The present investigation showed the green synthesis of silver nanoparticles (AgNPs) using Ficus benghalensis (F. benghalensis) leaf extract. UV–Vis spectra of the biofabricated AgNPs displayed its maximum peak of absorption at 461 nm. High resolution-transmission electron microscopy images displayed the shape of AgNPs as spherical with an average diameter of 35 nm size. The analysis of X-ray diffraction confirmed the presence of crystalline AgNPs. The analysis of Fourier-transform infrared spectroscopy confirmed the existence of bioconstituents such as terpenoids, phenolics and flavonoids, which functions as bio-reducing agents. When compared with F. benghalensis extract, AgNPs displayed the considerably greater bioactivities. The exceptional antimicrobial functionalities of AgNPs against both the gram positive and gram negative bacteria makes them appropriate candidates for the production of antibiotics against the species that are resistant to traditional antibiotics. The assay of HDFa cell scratch confirmed that the AgNPs have greater ability of wound healing than the leaf extract of F. benghalensis. Altogether, the obtained results showed the application of synthesized AgNPs in the production of novel drugs that are used for wound healing in nursing care after rectal surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several researchers have reported the fabrication of silver nanoparticles (AgNPs) by reducing Ag ions utilizing Allium cepa (onion) extract [1]. P. vulgaris, S. marcescens and K. pneumoniae were resistant towards onion extract. Although, AgNPs fabricated using the same extracts inhibited bacterial growth, B. subtilis and P. aeruginosa showed a large diameter of inhibition in presence of AgNPs [1]. AgNPs were fabricated from the leaves of Lantana camara in the study reported by Patil and his co-workers [2]. They displayed significant antimicrobial effect over P. aeruginosa, S. aureus and E. coli in comparison with the standard ciprofloxacin. These findings were related with antimicrobial effect of petroleum ether extraction and essential oil separated from the L. camara leaves. In comparison to the antimicrobial effect of petroleum ether extraction and essential oil separated from L. camara leaves the antimicrobial effect of AgNPs was greater showing that the membrane permeability of AgNPs was dependent on its dose. Moreover, these comparative studies revealed that the morphology and size of AgNPs affects the antimicrobial activity [2]. On the other hand, the high aqueous dispersibility of green fabricated AgNPs may increase their scope for preparation of various polymer nanocomposites, which have reported widely in sensors [3], electrolysis [4], antibacterial activity [4] and wound healing activity [5].
The major objectives of the present study are the wound healing activities of green fabrication of AgNPs with poly-phenolic F. benghalensis leaf extract using a technique established previously [6, 7], which determines the effect of various factors on green fabrication approaches (pH, time and metallic salt). The current work mainly focused on application of F. benghalensis leaf extract mediated AgNPs towards wound healing activity. Also, the antibacterial activity of prepared AgNPs have shown in present work. The characterization of green fabricated AgNPs was performed using specific analytical techniques like, transmission electron microscopy (TEM), Fourier transforms infrared spectroscopy (FT-IR) and Ultraviolet–Visible (UV–Vis) Spectrometry. We also evaluated the antimicrobial property and wound healing activity of green fabricated AgNPs in the current study.
2 Materials and Methods
2.1 Preparation and Characterization of F. benghalensis
10 g of Spruce bark was added to distilled water of 100 mL in an Erlenmeyer flask and placed on ultrasonic water bath for about half an hour at 70 °C to obtain the F. benghalensis extract (SBE). The solution obtained was filtered followed by centrifugation, and then stored at 4 °C for further usage.
Total polyphenol content (TPC) was determined by performing spectrophotometric analysis using Folin–Denis reagent [8]. TPC of the clarified SBE was calculated depending upon the calibration plot of gallic acid, the results were represented as mg GAE/g biomass.
2.2 Fabrication of AgNPs
In one of the earlier study, the green fabrication approach was optimized by estimating the ratio of saline and extract solution or the influence of silver nitrate (AgNO3) concentration [5]. Silver acetate (AgC2H3O2) and AgNO3 were used to prepare the silver solutions. In brief, spruce bark of 10 mL was mixed with 1mM AgNO3/AgC2H3O2 of 90mL.The extract solutions were brought to pH 9 utilizing NaOH and pH 4 utilizing HNO3. The color change of the solution placed on an ultrasonic water bath for 3 h at 60 °C, indicated the complete synthesis of AgNPs. The obtained AgNPs were washed several times to remove impurities and dried in oven to obtain pure AgNPs, which later was dispersed in double distilled water for TEM and DLS analysis.
2.3 Characterization
In UV–Vis spectroscopic data analysis, AgNPs had been measured at a wavelength ranging from 350 nm to 700 nm and at 1nm resolution. The samples of AgNPs were examined initially and after time intervals of 1 h, 2 and 3 h. For FTIR analysis, the AgNPs powder was mixed with KBr in a mortar (1:100 ratio) and made pellet using pellet maker. FTIR Thermo Nicolet 380 Spectrophotometer was used to observe the spectrum of the tested and extract solutions at a wavenumber ranging from 4000 to 400 cm−1. TEM analysis was utilized to characterize the particle size and morphology of fabricated AgNPs utilizing JEOL 100 U TEM microscope at 100 kV of operating voltage.
2.4 Testing for Antimicrobial Activity
2.4.1 Antimicrobial Activity
Antimicrobial activity over B. cereus, a gram-positive bacteria and E. coli, a gram-negative bacterium was examined individually by Kirby-Bauer disk diffusion method [9]. Muller-Hinton (MH) nutrient culture medium was used to perform all the microbial experiments. Agar plates and broth (nutrient agar medium) were prepared. The microbial cultures were grown in MH nutrient medium during the night on an orbital shaker at 28 °C and 250 rpm. UV–Vis spectrophotometer was used to measure the microbial growth at an absorbance wavelength of 600 nm over non-inoculated nutrient agar medium as a control. For both of the bacterial strains, individual plates were prepared containing 20 µL microbial cell culture of concentration 1 × 106 CFU/mL which was evenly distributed and these culturing plates had been incubated at a temperature of 37 °C overnight. Later, the standard disks with 6 mm diameter were soaked separately with 20 µL of AgNO3, F. benghalensis extract, AgNPs, Rifampicin (positive-control) and water (negative-control) at a concentration of 1000 µg/mL. These standard disks had been placed aseptically on the two microbial culture plates and later incubated at a temperature of 37 °C for a day. The zone of inhibition (ZOI) of microbial cell growth was assessed and considered as an indicator for antimicrobial activity.
2.5 Antimicrobial Mechanism of Action
Determination of bacterial morphological changes under Scanning Electron Microscopy (SEM) was used to study the Antimicrobial Mechanistic Action. Briefly, 100 µL bacterial culture cells at a concentration of 1 × 106 CFU/mL were cultured individually along with 100 µL volume of AgNO3, Rifampicin (positive-control), water (negative-control), 1000 µg/mL of AgNPs and F. benghalensis extract for 6 h. The reaction solution was centrifuged for about 30 min at 3000×g. The pellet was washed three times using phosphate buffered saline (PBS) prior to prefixing with 2.5% of glutaraldehyde for about 30 min. The pellet was washed again thrice using PBS. The sample dehydration was facilitated by the increasing of ethanol concentration from 30 to 100% for about 15 min each. In the final step, amyl acetate was added and dried using critical point drying method. The morphology of these dried samplings (after covering the samples with gold using a sputter coater) was observed using SEM analysis (operated at 10 kV of accelerating voltage).
2.6 Assessment of Wound Healing Ability
The study of in-vitro wound healing activity was carried out in accordance with the standard method with minor changes [10]. In a 6 welled microtiter plate containing DMEM medium, human dermal fibroblast cells (HDFa) were grown at a seeding density of 5.0 × 105 cells/well. These culturing plates had been incubated in a humidified environment consisting of 5% CO2 at 37 °C for a day. The culture medium was discarded and cells were cleaned thrice using PBS. Test sample used was the medium consisting of either AgNO3, Rifampicin (positive control), water (negative control), 1000 µg/mL of AgNPs or F. benghalensis extract. 1 mL of sample mentioned previously was individually added into 6-welled microtitre plate consisting of cells. Sterile yellow tip was used to scratch the cells and migration of cells was seen under microscope (Leica BM IRB) with 10x magnification followed by time intervals at 28 and 48 h.
3 Results and Discussions
3.1 Characterization of AgNPs
The spherical shape of bio-fabricated AgNPs having an average diameter of 28 nm was confirmed by the HR-TEM analysis (Fig. 1A–C). Additionally, no aggregation was observed as they were parted from one another by inter-particle distance. Moreover, the poly crystallite nature of the bio-fabricated AgNPs was shown by the selected area electron diffraction (SAED) pattern (Fig. 1D). Therefore, it was concluded that AgNPs fabricated using aqueous extract of F. benghalensis leaf were predominantly spherical, crystalline and mono-dispersed in nature. The presence of elemental Ag in sample and AgNPs formation was confirmed by an intense absorption peak of silver was recorded at 3 keV in the EDS micrograph (Fig. 1E).
The elemental composition of AgNPs displayed maximum amount of Ag only. The crystallite morphology of bio-fabricated AgNPs was verified by the analysis of XRD (Fig. 2A). Four characteristic diffraction peaks were noticed at 2θ angles of 76.56°, 64.12°, 44.16° and 38.02° related to (311), (220), (200) and (111) crystal indexing planes, which attributed to four faced FCC structure of Ag. Our findings were noticed to be similar with the earlier reports [11,12,13]. In addition, few smaller peaks were shown by the XRD spectra because of bioorganic phase crystal impurities which obtained from plant extract [14]. The crystalline size was calculated by Scherrer formula utilizing full width at half maximum of highest peak (111) and the average crystalline size was found to be 28 nm, which is in agreement with TEM results.
The electro kinetic potential and mean grain size of fabricated AgNPs was confirmed by performing the DLS analysis. As depicted in Fig. 2B, average particle size was found to be 35 nm. However, the findings of DLS study displayed that the mean particle size was 28 nm with narrow particle size distribution because of their minor difference between the smallest and largest size of NPs. Additionally, the observed value of surface zeta potential was − 27.6 mV (Fig. 2C). The zeta potential greater than − 20 mV displayed that the fabricated NPs have adeq uate electrostatic repulsion among each other in order to remain stable in the aqueous leaf extract solution [15]. The negative electro kinetic potential of AgNPs was attained because of the stabilizing effect of bio-constituents in the extract solution. Additionally, the biomolecules present on the surface of AgNPs increase the stability of NPs by avoiding their agglomeration, increasing their scope of applications in different fields of science. On the other hand, the surface bioconstituents existed also improved the aqueous dispersibility of NPs. Hence, it was concluded that the bio-fabricated AgNPs are of high importance in different biological applications.
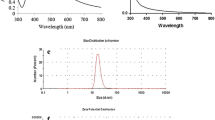
The time of bio-fabrication determined from UV–Vis spectroscopic analysis was found to be 2 h (Fig. 3). It is well reported that silver colloids have maximum absorbance at a wavelength ranging from 400 to 500 nm because of its Surface plasmon resonance (SPR) [16]. However, these values may increase (bathochromic displacement) as the duration of biosynthesis increases. After placing the solution for about 1 h in an ultrasonic water bath, the AgNPs were visible under UV–Vis spectrophotometer and the maximal absorbance was noted at 461nm (Fig. 3). After, 2 h of synthesis time, there is no further change in SPR absorption band was observed which indicated the completion of AgNPs synthesis.
3.2 FTIR Analysis of Plant Extract and AgNPs
Moreover, FTIR analysis was carried out to confirm the possible functionalities present in the aqueous extract of P. longum leaf and its resultant AgNPs, which plays an important role in capping, reducing and stabilizing the AgNPs. In Fig. 4, FT-IR spectrum of SBE prior to AgNPs synthesis and after its fabrication was plotted. The SBE spectrum has numerous oscillation bands which indicated the complexity of SBE. Strong bands observed at 1605 cm−1 and 3394 cm−1 were attributed to carbonyl group and –O–H bond of phenolic group. AgNPs have very intense, clear and well defined bands at 1070, 1516, 1605, 2931 and 3385 cm−1 for aromatic ethers, –OH carboxylic, > C = O, –C–H from aldehydes and –O–H phenolic bonds respectively. In both the spectrum, an intense band observed at a wavenumber 1384 cm−1 attributed to stretching vibrations of asymmetrical carboxylate ion (COO−), a newly developed functionality followed by the reduction of silver (Fig. 4). The synergistic activity between phenol groups and the other constituents of SBE might have added to the NPs stability [17].
3.3 Antimicrobial Activity
3.3.1 Zone of Inhibition (ZOI)
Zone of inhibition (ZOI) was the criteria used for the determination of antimicrobial effect of AgNPs and plant extract over E. coli and B. cereus (Fig. 5). Monitoring of the plates for microbial growth was carried out at an interval of 12 h. ZOI was negligible in AgNO3 and it was not observed in untreated sample (negative control) in both of the microbial cultures. ZOI for AgNPs was greater when compared to plant extract and the positive control used was Rifampicin in both the microbial cultures (data not shown). The effect of inhibition was elevated up to 24 h with ZOI values for E. coli and B. cereus as Rifampicin (38mm, 33mm), AgNO3 (10 mm, 8 mm), AgNPs (37 mm, 34 mm) and plant extract (27 mm, 26 mm) correspondingly. Therefore, the ZOI values were almost similar for AgNPs and no bacterial regrowth was observed (Fig. 5). The outcomes showed that the inhibition of gram-positive bacteria (B. cereus) was less in comparison with the gram-negative (E. coli) bacteria.
Antimicrobial activity was evaluated further by investigating cellular viability of produced microbial cell colonies in nutrient broth. The microbial cell colonies had been selected from margings of ZOI and grown in MH nutrient medium overnight. The cell viability was determined by UV–Vis spectroscopic analysis at a wavelength of 600nm. After a day, no growth of bacterial cells was observed in AgNPs and minimum growth was observed in F. benghalensis extract for gram positive bacteria. The bacterial growth was minimum in both AgNPs and F. benghalensis extract for gram negative bacteria. The growth of bacterial cells was significant in Rifampicin.
3.4 Mechanism of Action of Antimicrobials
The changes in the morphology of bacteria were determined by studying the mechanism of action of antimicrobials. AgNPs can penetrate easily into bacterial cells because of their smaller size and exhibit bactericidal activity as observed from the SEM photographs (Fig. 6 represents B. cereus and Fig. 7 represents E. coli). In untreated cells, uniform cellular morphology was noticed with an average particle size of 1 μm width and 3 μm length (i). Most of bacterial cells were ruptured when treated with rifampicin (iii) whereas treatment with AgNO3 showed slight distortion of bacterial cell structure (ii). The bacteria treated with F. benghalensis extract showed a slight distortion of cell structure (iv) whereas most of the bacterial cells were damaged significantly when treated with AgNPs (v).
3.5 Wound Healing Potential
Cell scratch assay was performed on HDFa cell lines to determine the in vitro wound healing activity. After a day, the groups treated with AgNPs (e), F. benghalensis extract (d) and Rifampicin (c) showed greater cell migration at the wound site in comparison to AgNO3 (b) and control (a) (Fig. 8). Moreover, in groups treated with AgNPs, large number of cells appeared after 2 days in comparison with the other treatment groups (Fig. 9). The wound closure area was measured using given formula:
The wound closure percentage was least in control (53%) followed by AgNO3 (55%), F. benghalensis extract (62%), AgNPs (70.2%) and Rifampicin (84%). These results suggested the wound healing ability of AgNPs.
4 Conclusions
In conclusion, we showed the green synthesis of AgNPs using F. benghalensis leaf extract. The XRD results have confirmed the presence of crystalline AgNPs. The analysis of Fourier-transform infrared spectroscopy (FTIR) confirmed the existence of compounds like terpenoids, phenolics and flavonoids, which functions as bio-reducing agents. The exceptional antimicrobial functionalities of AgNPs against both the gram positive and gram negative bacteria makes them appropriate candidates for the production of antibiotics against the species that are resistant to traditional antibiotics. The assay of HDFa cell scratch test confirmed the greater wound healing ability of AgNPs than the leaf extract of F. benghalensis. Altogether, the obtained results showed the application of synthesized AgNPs in the production of novel drugs that are used for wound healing after the rectal surgery.
Data Availability
The data generated or analyzed during the present work was available on reasonable request from the corresponding author.
References
E.Z. Gomaa, Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: a green approach. J. Genet. Eng. Biotechnol. 15, 49–57 (2017)
P. Shriniwas, K. Subhash, Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 10, 76–81 (2017)
M.A. Deshmukh, B.C. Kang, and T.J. Ha, Non-enzymatic electrochemical glucose sensors based on polyaniline/reduced-graphene-oxide nanocomposites functionalized with silver nanoparticles. J. Mater. Chem. C 8, 5112–5123 (2020)
M. Ginting et al., A simple one-pot fabrication of silver loaded semiinterpenetrating polymer network (IPN) hydrogels with self-healing and bactericidal abilities. RSC Adv. 9(67), 39515–39522 (2019)
F. Liu, Y. Yuan, L. Li, S. Shang, X. Yu, Q. Zhang, S. Jiang, Y. Wu, Synthesis of polypyrrole nanocomposites decorated with silver nanoparticles with electrocatalysis and antibacterial property. Compos. B: Eng. 69, 232–236 (2015)
Talmaciu AI (2017) Contributions concerning the elucidation of the mechanisms involved in the processes of extraction and characterization of polyphenols with biological activity using non-conventional techniques, Ph.D. Thesis, “Gheorghe Asachi” Technical University of Iasi, Iasi, Romania
C. Tanase, L. Berta, N.A. Coman, I. Ros, A. Ca, F. Man, A. Toma, L. Mocan, D. Jakab Farkas, A. Biró, Mare, Investigation of in vitro antioxidant and antibacterial potential of silver nanoparticles obtained by biosynthesis using beech bark extract. Antioxidants 8, 459 (2019)
C. Tanase, E. Domokos, S. Cosarcă, A. Miklos, S. Imre, J. Domokos, C.A. Dehelean, Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. Bioresources 13, 2247–2267 (2018)
A.W. Bauer, W.M. Kirby, J.C. Sherris, M. Turck, Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45(4), 493–496 (1966)
E.Y. Ahn, H. Jin, Y. Park, Assessing the antioxidant, cytotoxic, apoptotic and wound healing properties of silver nanoparticles green synthesized by plant extracts. Mater. Sci. Eng. C. Mater. Biol. Appl. 101, 204–216 (2019)
D. Kumar, G. Kumar, V. Agrawal, Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) wall bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol. Res. 117, 1–13 (2017)
M. Govindarajan, M. Rajeswary, R. Sivakumar, Larvicidal and ovicidal efficacy of Pithecellobium dulce (Roxb.) Benth. (Fabaceae) against Anopheles stephensi Liston and Aedes aegypti Linn. (Diptera: Culicidae). Indian J. Med. Res. 138, 129–134 (2014)
M. Govindarajan, G. Benelli, One-pot green synthesis of silver nanocrystals using Hymenodictyon orixense: a cheap and effective tool against malaria, chikungunya and Japanese encephalitis mosquito vectors? RSC Adv. 6, 59021–59029 (2016)
A.A. Abdel Hamid, M.A. Al-Ghobashy, M. Fawzy, M.B. Mohamed, M.S.A. Mohamed, A. Mottaleb, Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed (Potamogeton pectinatus L.). ACS Sustain. Chem. Eng. 1, 1520–1529 (2013)
C.D. Patil, S.V. Patil, H.P. Borase, B.K. Salunke, R.B. Salunkhe, Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 110, 1815–1822 (2012)
A. Demirbas, B.A. Welt, I. Ocsoy, Biosynthesis of red cabbage extract directed AgNPs and their effect on the loss of antioxidant activity. Mater. Lett. 179, 20–23 (2016)
E.C.B.A. Alegria, A.P.C. Ribeiro, M. Mendes, A.M. Ferraria, A.M.B. Do Rego, A.J.L. Pombeiro, Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 8, 320 (2018)
Funding
The authors declare that no funds, grants, or other support were received related to this work.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the design and study conception. Analysis, data collection and material preparation were performed by HL. The first draft of the manuscript was written and revised by HL, SL, YZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that no relevant financial or non-financial interests related to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, H., Liu, S. & Zhang, Y. Development of Novel Green Synthesized Silver Nanoparticles with Superior Antibacterial and Wound Healing Properties in Nursing Care After Rectal Surgery. J Inorg Organomet Polym 32, 773–780 (2022). https://doi.org/10.1007/s10904-022-02223-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02223-1