Abstract
The present work addresses a facile synthesis of Indium doped zinc oxide (IZO) cellulose acetate nanocomposite membrane. The membrane was prepared by casting method. Various weights of In:ZnO nanoparticles were added to solution formed by dissolution of cellulose acetate granules in acetone. The harvested membrane, after acetone evaporation, was characterized by various techniques including X ray diffraction, scanning electron microscopy, energy dispersive X ray and elemental mapping, Fourier transform infrared spectroscopy. The results show that the In:ZnO nanoparticles are well embedded in the cellulose acetate host matrix. The elemental mapping reveals that the nanoparticles are uniformly distributed. The optical characterization reveals the reduction of the transmittance in the UV (A and B range) of the CA/IZO composite with increasing the weight of the added IZO powder. This reduction was attributed to ZnO UV absorption. No noticeable peak assigned to ZnO bond are observed. However, IR peaks are shifted towards the higher wavenumber due to the change of the bonds environment with including IZO in the CA matrix. The antibacterial action of the synthetized nanocomposite membranes was tested against Escherichia coli (E. coli). Staphylococcus aureus (S. aureus). The assay results have shown that the membrane has no activity against (E. coli). In contrary, the synthetized membrane exhibits an interesting antibacterial activity against S. aureus. The inhibition region varies from 6 to 15 mm with increasing the weight ratio of filler. A zone of inhibition (ZOI) of 18 mm was observed for the membrane prepared with 30% wt. of In:ZnO. We noticed that the ZOI radius increases with the added weight of IZO. Due the settling down of the nanoparticles only one face of the membrane exhibits an antibacterial activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hybrid polymeric nanocomposite films have gained increasing attention during the last decade, due to their interesting and promising physical, chemical, and optical properties [1,2,3,4]. Merging metal or metallic oxide nanoparticles with polymers leads to the improvement of the physicochemical properties and behavior of the polymer [5]. Hence, the produced composites represent a new class of materials suitable for applications in several fields [6,7,8,9,10,11,12,13,14]. A large variety of polymers have been used for the production of various composite materials with interesting mechanical properties, thermal stability, biocompatibility, and high chemical resistance. These unique characteristics of nanostructured composites were a reasonable stimulus for the researcher and industrial activity in the polymer based nanocomposite. The most commonly used composite are produced by the combination of polymer matrices including poly(methyl methacrylate), polycarbonate, polystyrene and thermosets (e.g., epoxy, silicone) with various inorganic fillers in particular oxides namely: SiO2, TiO2, CeO2, ZnO, ferric oxide, semiconductors: CdS, PbS, CdTe, CdSe, minerals: clays, CaCO3 and metal: Au, Ag, Cu, Ge, Fe) [10]. Among these polymers, the cellulose acetate (CA) based materials have attracted an increasing interest. Cellulose with a chemical formula (C6H10O5)n is a commercially available, naturally obtained polymer [15,16,17,18,19]. Cellulose acetate biopolymer is characterized by its high mechanical strength, good biocompatibility, biodegradability, hydrophilicity properties [20, 21], thus making it highly desirable in numerous biomedical, antibacterial applications and wound healing [22,23,24,25,26,27,28]. Recently, wound-healing properties of numerous metal nanoparticles have been demonstrated [29]. Substantial research attention has been paid to metal and metal oxide nanoparticles (NPs) due to their exceptional physical properties, this have enabled their broad use in diverse applications [30,31,32,33]. These nanoparticles particles embedding into a polymer matrix yields to original composites with new properties owing the advantages of both components. Beisl et al. [34] have prepared cellulose acetate with silver nanoparticles to improve their antibacterial properties. Amin et al. [35] have added TiO2 to the mechanical properties of bioplastic. Al Saeed et al. [36] prepared scaffold base on CA loaded with copper oxide nanoparticles. They noticed and improvement in the plastic hydrophilicity beside its ability to kill infectious microbes abundant in wounds. Jatoia et al. [37] have synthetized a composite formed by carbon nanotubes and silver nanoparticles incorporated into polymer matrix with the goal to minimize the direct contact of silver nanoparticles with human cells to reduce silver leaching. Matosa et al. [38] have produced composite membranes by combining magnetic nanoparticles with cellulose acetate membranes for magnetic hyperthermia application. Ahmed et al. [39] have introduced ZnO, Ag and CuO nanoparticles into of cellulose acetate and ε-olycaprolactone matrix blend. They investigated the synthetized composite viability and antibacterial activity against Staphylococcus aureus (S. aureus) and E. coli. Alay et al. [40] have prepared a nanocomposite via combination of nanostructured zinc oxide (ZnO) and graphene oxide with cellulose acetate (CA). They reported that this combination yields to some good bio-composite materials for wound healing application. Fahmi et al. [41] have synthetized nanocomposite membrane composed of cellulose acetate and collagen matrix blend embedded with MnFe2O4 nanoparticles and apply it in drug delivery. Kalaycıoğlua et al. [42] have prepared a wound dressing composite materials composed of cerium oxide nanoparticles introduced into chitosan and cellulose acetate polymer matrix. Baday et al. [43] have decorated cellulose acetate nanocrystals during the synthesis with Ag and ZnO nanoparticles. The obtained nanocomposites, have been experienced as some effective adsorbents for lead ion Pb(II). Haider et al. [44] have synthetized cellulose acetate nanofibers decorated with CuO nanoparticles and apply them as a potential wound dressing material, they exhibited excellent antibacterial efficacy against both E. coli and S. aureus bacteria’s. Zinc oxide nanoparticles have been studied extensively in the past years. Their unique properties enable their multidisciplinary applications in several industrial fields [45,46,47,48,49]. Therefore, developments of nanoparticles with antimicrobial properties are of considerable interest. In the last few years, multi-functional metals and metal oxides have attracted interest for their antimicrobial activities. Metal oxide nanoparticles find many applications in physical, chemical and biological fields. Metal oxides nanoparticles, such as TiO2, ZnO, CuO, SiO2, SnO2 and MgO have proven an interesting antibacterial properties. ZnO has attracted a special interest due to its application in health care products, UV radiation blocking. The antibacterial activity of ZnO NPs was reported earlier [50, 51]. It has been demonstrated that ZnO nanostructures can be successfully used against both Gram-positive and Gram-Negative bacteria [52, 53]. Due to their interesting properties, ZnO nanoparticles can be introduced into polymers matrix in order to tune polymers properties that may covers a wide area of applications. To the best of our knowledge, the incorporation of Indium doped ZnO in CA matrix is not studied.
The preset work deals with the synthesis of nanocomposites by incorporating IZO nanoparticles into CA matrix. The antibacterial activity of the synthetized nanocomposite is investigated. The structural, compositional properties of the synthetized composite is firstly investigated and the antibacterial activity against E. coli and S. aureus bacteria’s is also addressed. During the IZO/CA composite preparation, the ratio of the nanoparticle mass, regarding the biopolymer, was varied in order to investigate the effect of nanoparticles concentration.
2 Experimental Details
2.1 Nanoparticles Preparation
In-doped ZnO nanoparticles were prepared via sol gel technique. The starting solution was prepared by dissolving zinc acetate dehydrate Zn(CH3COO)2·2H2O (Sigma-Aldrich, > 99.5% purity) in methanol with a 0.1 M molarity. The weight of the added dopant source indium chloride InCl3 (Sigma-Aldrich, > 99.5% purity) is calculated to achieve the desired In/Zn ratio of 3 at%. After stirring for 10 min at room temperature, the solution was placed in an autoclave heated at supercritical condition at 250 °C for 10 h. The resultant material was washed several times with mixture of ethanol and water for purification and then dried at 60 °C for 1 h in an oven. The harvested nanopowder was then calcinated at 400 °C for 2 h in an open oven.
2.2 Nanocomposite Fabrication
Cellulose acetate was purchased from BDH, (Germany, 99%) and was used as received without any additional treatment. 2 g of CA granules were dissolved in 20 ml pure acetone. IZO nanopowder was added to the dissolved CA solution. A set of IZO/CA bioplastic films were prepared by varying the weight percentage of the added IZO nanoparticles regarding the CA mass, the used weigh ratios were 5, 10, 15 and 25%. The solution was mixed in a beaker and ultrasonically treated to obtain a well-dispersed and homogeneous solution. A glass petri dish was then filled with the obtained solution for casting. The dish was covered with aluminum foil to avoid any environmental particles contamination. The solution was dried during 24 h at room temperature, the bioplastic film is formed after acetone evaporation.
2.3 Nanoparticles and Biopolymer Characterizations
The structure of the prepared In doped ZnO nanoparticles and IZO/CA composite were characterized by X-ray diffraction (XRD) using X’pert Pro diffractometer using the Cu-Kα radiation in the 2θ range from 20° to 80°. The nanoparticles and IZO/CA films morphology was analyzed by mean of scanning electron microscopy (SEM) observations using Zeiss Cross Beam 540 instrument, equipped by an energy dispersive X ray (EDX) spectrometer for elemental analysis and element mapping. The transmission optical properties, of the IZO/CA polymer films were acquired via the UV–Vis spectrophotometer (Bio Aquarius CE 7250, UK). The Fourier transform infrared (FTIR) absorption coefficients of the bioplastic films were measured using a Brucker spectrophotometer in the wavenumber range 400–4000 cm−1.
2.4 Antibacterial Activity
The antibacterial activity assay of the obtained bioplastic films was evaluated by disk diffusion method regarding Gram positive (Staphylococcus aureus (ATCC 43300)), and Gram negative species (Escherichia coli ATCC 25922)).
The prepared IZO/CA films were cut into disk specimens (diameter of 6 mm). The cut discs were washed with distilled water and sterilized at 120 °C/20 min. Thereafter, 100 µl of bacterial inoculums with a concentration of 108 CFU/ml, have been served on plates containing Mueller Hinton agar. The testing discs are then placed on the surface of the media. Thereafter, the plates have been incubated for 24 h to 48 h at 37 °C. The diameters of inhibition zones were measured to assess the antibacterial activity.
3 Results and Discussion
Figure 1 represents XRD diffraction pattern recorded for In-doped ZnO synthetized nanopowder. As can be seen, the obtained nanopowder is polycrystalline, its XDR pattern is composed of different peaks assigned to the diffraction plan (100), (002), (102), (110), (103) and (112) of the ZnO Wurtzite hexagonal structure (according to JPCD card No. 36–1451). No other secondary peaks originating from In related phases such In2O3 or In metallic was observed. This indicating the pure quality of the synthetized nanopowder and also that In is incorporated and diluted in the ZnO lattice and do not form any secondary phases, confirming thereafter the In doping success of ZnO nanoparticles. The crystallite size is calculated using Hall–Williamson method. The estimated crystallite sizes were in the order of 34.2 nm.
The nanopowder morphology was studied using SEM observation. Figure 2a shows the SEM images of In-doped ZnO nanopowder. The nanopowder is composed of agglomeration of spherical nanoparticles. To confirm the In incorporation, we have reported in Fig. 2b the recorded EDX spectra in IZO nanopowder, as can be deduced the synthetized nanoparticles are composed of the element Zn, O and In necessary for IZO formation.
Figure 3 represents the XRD pattern of bioplastic IZO/CA films composite prepared with different IZO weigh ratios. The peaks related to ZnO phase appears with IZO filler adding. The ZnO peaks intensities increase with increasing the mass of the added ZnO nanoparticles. An interesting feature is the emergence of the same peaks assigned to the diffraction plan (100), (002), (102), (110), (103) and (112) of the ZnO Wurtzite hexagonal structure as observed in the IZO nanoparticles (Fig. 1). For the pure CA XRD diffraction pattern is composed with a broad peak confirming its amorphous structural nature.
The SEM images of the synthetized bioplastic composite are shown in Fig. 4a–d. At 5% of added filler mass ZnO nanoparticles image starts to be visible. At higher ratio 15 and 25% the SEM images reveals clearly the presence of the added ZnO nanoparticles. The aspect of the nanoparticles is comparable to that observed in the starting IZO nanopowder (Fig. 2a). This suggests that the added IZO nanoparticles are imbedded in the CA matrix.
SEM images of bioplastic nanocomposite sheets prepared with incorporation of different In:ZnO nanoparticles weights into the cellulose acetate a pure cellulose acetate polymer, b nanocomposite prepared with 5 wt% of IZO nanoparticles, c nanocomposite prepared with 15 wt% of IZO nanoparticles and d nanocomposite prepared with 25 wt% of IZO nanoparticles
To assess the uniform distribution of the IZO nanoparticles in the host matrix, we have proceeded with elements mapping (Fig. 5a–d) and EDX composition in three different region (Fig. 5e, f) in the IZO/CA film prepared with a mass ratio of 15% taken as example. Figure 5a–d show the different element forming the IZO nanoparticles. The elements In, Zn and O, as can be deduced from these figures, are well uniformly distributed in the whole film regions.
The films transmittance spectra in the UV–Vis range are reported in Fig. 6. The film transmittance variation in the visible range 400 to 800 nm can be explained in term of the film thickness. The absorption threshold is red shifted with increasing the IZO weigh concentration. Indicating the band gap reduction from 3.26 to 3 eV of the IZO/CA composite with increasing the weight ratio of ZnO introduced in the polymer matrix as shown in the insert Fig. 6. Culicia et al. [54] have investigated the effect of CeO2 nanoparticles on the optical properties of the CA polymer and their UV filtration, they reported a reduction of the band gap from 3.36 to 3.28 eV with increasing the concentration of CeO2 filler in CA matrix. The CA based nanocomposites can be used for photocatalysis for waste water treatment, this reduction in the composite band gap can be benefit for this technique. This may lead to energy consumption reduction by avoiding the UV lamp use.
An interesting feature that can be deduced from the transmittance spectra is the reduction of the transmittance in the UV (A and B range) i.e. 280–400 nm. The same behavior has been reported also by Culicia et al. [46] in the Ce2O/CA composite. This suggests the increase of the composite blue radiation absorption with increasing ZnO nanoparticles in CA matrix. This may suggest that the CA filling with ZnO can be a method that improves the UV visible filtering of the CA polymer. The observed increase in the absorption in the UV range may be attributed to the ZnO nanoparticles. Owing a band gap of 3. 17 eV, ZnO absorbs the UV radiations.
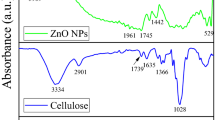
Figure 7 shows the FTIR spectra recorded in the frequency range between 400 and 4000 cm−1 of different IZO/CA nanocomposite prepared with different weight filler ratio. The whole spectra exhibit peaks located at 1058 cm−1 assigned to the C–O vibrational mode, 1234 cm−1 assigned to the C–O–C anti symmetric stretching, 1377 cm−1 due to C–OH vibration, 1768 cm−1 due to C = O carbonyl vibrational stretching vibration and 2380 cm−1 due to the C–H group vibration. All these peaks are characteristic of cellulose acetate and assigned respectively to the following bonds vibration. Peaks related to ZnO are not present, only a very small weak peak located at 450 cm−1 assigned to ZnO is observed only in the composite prepared with the larger weight filler ratio of 25 wt%. However as shown in Fig. 8, IR peaks are shifted towards the higher wavenumber. This due to change of the bonds environment with including IZO in the CA matrix.
The antibacterial performance of IZO/CA composite against gram-negative E. coli and gram-positive S. aureus bacterial has been evaluated using the disk diffusion method. Figure 9 show the zone of inhibition (ZOI) obtained in the antimicrobial assays. As can be seen (Fig. 9a), pure CA do not show any antibacterial activity, while IZO/CA composite exhibits significant antibacterial activity against S. aureus (Fig. 9a). In Table I is collected the measured ZOI radius in various membranes. The inhibition region varies from 6 to 15 mm with increasing the weight ratio of filler. The same conclusion has been reported by Fahmi et al. [41]. They inferred that the antibacterial activity of the Chitosan/CelAc films increased considerably by adding nano cerium oxide in the films.
The IZO/CA do not exhibit any antibacterial activity against E. coli, no halo around the sample was observed (Fig. 9a). The same conclusion has been outlined by Jatoia et al. [37] reported the inactivity of CA/carbon nanotube/Ag against E. coli and a significant activity against S. aureus. This result agrees with various studies that demonstrated the high sensitivity of gram positive bacterium (S. aureus) compared to gram negative bacterium (E. coli) vis-à-vis different antibacterial agents [55, 56].
The suggested mechanism of antibacterial activities composite has been based on generation of reactive oxygen species (ROS), such as hydroxyl, oxide and oxygen radicals which are negatively charged. It was reported that E. coli (Gram negative bacteria) cell membranes were more negative and rigid than S. aureus [48]. Moreover, E. coli enjoys a double lipopolysaccharide membrane that presents a barrier to many substances. Thus owing E. coli greater resistance by comparison to S. aureus bacteria.
An interesting feature is that the antibacterial performance depends on the film face. Indeed, the synthetized films faces have different color one face is more clear (face A) than the second one (face B) as shown in Fig. 10. The face A do not exhibit any bacterial activity in contrary to the face B (Fig. 10). This may suggest that during the antibacterial assay using the face B, the bacteria is in contact with the antibacterial agent i.e. ZnO nanoparticles, while in the assay with the face A there is no contact with the antibacterial agent. Thereafter, one can conclude that the face B is ZnO rich than the face A. This may be due to the fact that during film casting the nanoparticles, due to the gravity, settle down and condensate at the bottom as shown in the cross section SEM image of the bioplastic sheet (Fig. 11). Thus making the face B ZnO rich and then may explain the different behavior of the films faces regarding the antibacterial activity.
4 Conclusion
In the present we have succeeded in the preparation of bioplastic membrane containing In doped ZnO/cellulose acetate nanocomposite. The influence of weigh ratio of embedded IZO nanoparticles incorporated in the cellulose acetate host matrix. The XRD analysis and the SEM observations reveal that the IZO nanoparticles are well introduced in the acetate network and well uniformly dispersed in the whole polymer matrix according to the EDX element mapping observation. The synthetized membrane was tested against the gam positive (S. aureus) and gam negative (E. coli) bacteria. The optical characterization reveals the reduction of the transmittance in the UV (A and B range) of the CA/IZO composite with increasing the weight of the added IZO powder. This reduction was attributed to ZnO UV absorption. No noticeable peak assigned to ZnO bond are observed. However, IR peaks are shifted towards the higher wavenumber due to the change of the bonds environment with including IZO in the CA matrix. From the assay tests we concluded that the IZO/CA nanocomposite has a good antibacterial activity against gam S. aureus, the inhibition region varies from 6 to 15 mm with increasing the weight ratio of IZO filler weight. While, no antibacterial activity against E. coli bacteria was detected. Moreover, due the nanoparticles settlement at the bottom of the preparation Petri dish during polymer casting, the synthetized polymer has an active face (containing ZnO nanoparticles) and inactive face (free from ZnO nanoparticles).
Change history
12 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10904-022-02477-9
References
L.E. Aguilar, A. Ghavami Nejad, C.H. Park, C.S. Kim, Nanomedicine 13(2), 527 (2017)
G. El-Barbary, M.K. Ahmed, M.M. El-Desoky, A.M. Al-Enizi, A.A. Alothman, A.M. Alotaibi, A. Nafady, Synth. Met. 278, 116824 (2021)
S. Beis, S. Monteiro, R. Santos, A.S. Figueiredo, M. Guadalupe, M. Sanchez-Loredo, M.A. Lemos, F. Lemos, M. Minhalma, M. Norberta de Pinho, Water Res. 149, 225 (2019)
Y. Wu, G. Xu, W. Zhang, C. Song, L. Wang, X. Fang, L. Xu, S. Han, J. Cui, L. Gan, Carbohydr. Polym. 267, 118166 (2021)
J. Lostea, J.-M. Lopez-Cuesta, L. Billona, H. Garay, M. Save, Progress Polym. Sci. 89, 133 (2019)
M. Abbas, M. Buntinx, W. Deferme, R. Peeters, Nanomaterials 9, 1494 (2019)
M. Choi, G. Murillo, S. Hwang, J.W. Kim, J.H. Jung, C.Y. Chen, M. Lee, Nano Energy 33, 462 (2017)
E. Tang, S. Dong, Colloids Polym. Sci. 287, 1025 (2009)
S. Tachikawa, A. Noguchi, T. Tsuge, M. Hara, O. Odawara, H. Wada, Materials 4, 1132 (2011)
S. Li, M.M. Lin, M.S. Toprak, D.K. Kim, M. Muhammed, Nano Rev. 1, 1 (2010)
S. Zinatloo-Ajabshir, S.A. Heidari-Asil, M. Salavati-Niasari, Sep. Purif. Technol. 280(1), 119841 (2022)
H. Etemadi, S. Afsharkia, S. Zinatloo-Ajabshir, E. Shokri, Polym. Eng. Sci. 61, 2364–2375 (2021)
S. Zinatloo-Ajabshir, M. Mousavi-Kamazani, Ceram. Int. 47(17), 23702–23724 (2021)
H. Safajou, M. Ghanbari, O. Amiri, H. Khojasteh, F. Namvar, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Int. J. Hydrogen Energy 46(39), 20534–20546 (2021)
S. Sharaf, M.E. El-Naggar, Cellulose 25(9), 5195–5204 (2018)
M.S. Abdelrahman, T.A. Khattab, A. Aldalbahi, M.R. Hatshan, M.E. El-Naggar, J. Environ. Chem. Eng. (2020). https://doi.org/10.1016/j.jece.2020.104573
A. Aldalbahi, M.E. El-Naggar, M.K. Ahmed, G. Periyasami, M. Rahaman, A.A. Menazea, J. Mater. Res. Technol. 9, 15045 (2020)
A. Aldalbahi, M. El-Naggar, T. Khattab, M. Abdelrahman, M. Rahaman, A. Alrehaili, M. El-Newehy, Polymers 12, 2501 (2020)
A. Abdel-Karim, M.E. El-Naggar, E.K. Radwan, I.M. Mohamed, M. Azaam, E.R. Kenawy, Chem. Eng. J. 405, 126964 (2021)
A. Zucchelli, M.L. Focarete, C. Gualandi, S. Ramakrishna, Polym. Adv. Technol. 22, 339–349 (2011)
A.R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y.S. Lee, C.S. Kim, Carbohydr. Polym. 102, 884 (2014)
T.J. Sill, H.A. von Recum, Biomaterials 29, 1989 (2008)
S. Tungprapa, I. Jangchud, P. Supaphol, Polymer 48, 5030 (2007)
T.P. Armedya, M.F. Dzikri, S.C.W. Sakti, A. Abdulloh, Y. Raharjo, S. Wafiroh, M.Z. Fahmi, BioNanoScience 9, 274 (2019)
A.W. Jatoi, Z. Khatri, F. Ahmed, M.H. Memon, J. Surf. Deterg. 18, 205 (2015)
A. W., Jatoi, I. S. Kim, and Q.Q. Ni, Appl. Nanoscience, 1 (2018)10.
H. Lee, A.W. Jatoi, Y. Kyohei, K.-O. Kim, K.H. Song, J. Lee, Text. Res. J. 88, 630 (2018)
A.R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y.S. Lee, C.S. Kim, Carbohyd. Polym. 102, 884 (2014)
S.K. Nethi, S. Das, C.R. Patra, S. Mukherjee, Biomater. Sci. 7, 2652 (2019)
S. Subhadarshini, R. Singh, D.K. Goswami, A.K. Das, N.C. Das, Langmuir 35(52), 17166–17176 (2019)
S. Subhadarshini, R. Singh, A. Mandal, S. Roy, S. Mandal, S. Mallik, D.K. Goswami, A.K. Das, N.C. Das, Langmuir 37(31), 9356–9370 (2021)
S. Subhadarshini, E. Pavitra, G.S.R. Raju, N.R. Chodankar, D.K. Goswami, Y.-K. Han, Y.S. Huh, NCh. Das, A.C.S. Appl, Mater. Interfaces 12(26), 29302–29315 (2020)
S. Subhadarshini, E. Pavitra, G.S.R. Ma Raju, N.R. Chodankar, A. Mandal, S. Roy, S. Mandal, M.V. Basaveswara Rao, D.K. Goswami, Y. Suk Huh, N.C. Das, Ceram. Int. 47(11), 15293–15306 (2021)
S. Beisl, S. Monteiro, R. Santos, A.S. Figueiredo, M.G. Sanchez-Loredo, M.A. Lemos, F. Lemos, M. Minhalma, M. Norberta de Pinho, Synth. Met. 278, 116824 (2021)
M.R. Amin, M.A. Chowdhury, M.A. Kowser, Heliyon 5, 02009 (2019)
S.I. Al-Saeedi, N.S. Al-Kadhi, G.M. Al-Senani, O.A. Almaghrabi, A. Nafady, Int. J. Biol. Macromol. 182, 464 (2021)
A.W. Jatoi, H. Ogasawara, I.S. Kim, Q.Q. Ni, Mater. Sci. Eng. C 110, 110679 (2020)
J.R. Ricardo, I.P. Matos, C. Chaparro, J.C. Silva, M.A. Valente, J.P. Borges, P.I. Soares, Carbohydr. Polym. 198, 9 (2018)
M.K. Ahmed, A.A. Menazea, A.M. Abdelghany, Int. J. Biol. Macromol. 155, 636 (2020)
A.A. Aly, M.K. Ahmed, Int. J. Pharm. 598, 120325 (2021)
M.Z. Fahmi, R.A. Prasetya, M.F. Dzikri, S. Candra, W. Sakti, B. Yuliarto, I. Ferdiansjah, Mater. Chem. Phys. 250, 123055 (2020)
Z. Kalaycıoğlu, N. Kahya, V. Adımcılar, H. Kaygusuz, E. Torlak, G. Akın-Evingür, F.B. Erim, Eur. Polym. J. 133, 109777 (2020)
A.A. Badawy, A.F. Ghanem, M.A. Yassin, A.M. Youssef, M.H. Abdel Rehim, Environ. Nanotechnol. Monit. Manage. 16, 0501 (2021)
M.K. Haider, A. Ullah, M. Nauman Sarwar, Y. Saito, L. Suna, S. Park, I.S. Kim, Int J. Biol. Macromol. 173, 315–326 (2021)
Z. Lu, J. Gao, Q. He, J. Wu, D. Liang, H. Yang, Carbohydr. Polym. 156, 460 (2017)
P.K. Dutta, S. Tripathi, G.K. Mehrotra, J. Dutta, Food Chem. 114, 1173 (2009)
I.S. Elashmawi, E.M. Abdelrazek, A.M. Hezma, A. Rajeh, Phys. B Condens. Matter 434, 57 (2014)
E.M. Abdelrazek, I.S. Elashmawi, A. Hezma, A. Rajeh, Int. J. Modern Appl. Phys. 1, 83 (2012)
S.M. Hassan, A.I. Ahmed, M.A. Mannaa, Ceram. Int. 44, 6201 (2018)
R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M.F. Benedetti, F. Fievet, Nano Lett. 6, 866 (2006)
G.X. Tong, F.F. Du, Y. Liang, Q. Hu, R.N. Wu, J.G. Guan, X. Hu, J. Mater. Chem. B 1, 454 (2013)
R.R. Krishna, T.K. Ranjit, C.M. Adhar, Langmuir 27, 4020 (2011)
J. Nicole, R. Binata, T.R. Koodali, C.M. Adhar, Microbiol. Lett. 279, 71 (2008)
M.E. Culica, A.L. Chibac-Scutaru, V. Melinte, S. Coseri, Materials 13, 2955 (2020). https://doi.org/10.3390/ma13132955
K. Ravichandrana, R. Rathia, M. Banetoc, K. Karthikaa, P.V. Rajkumara, B. Sakthivela, R. Damodaran, Ceram. Int. 41, 3390 (2015)
S. Anithaa, S. Muthukumaran, Mater. Sci. Eng. C 108, 110387 (2020)
Acknowledgements
This research work was funded by institutional Fund Projects under Grant number (IFPRC-197-130-2020). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, Jeddah Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original version of this article unfortunately contained two mistakes. The co-author N.H. Alonizan is affiliated only to the following institution:
Department of Physics, College of Science, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam, 31441, Saudi Arabia.
The grant number in the Acknowledgments section should be IFPRC-197-130-2020. The Acknowledgments section should read as follows:
Acknowledgments
This research work was funded by institutional Fund Projects under Grant number (IFPRC-197-130-2020). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, Jeddah Saudi Arabia.
The original article was corrected.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aida, M.S., Alonizan, N.H., Hussein, M.A. et al. Facile Synthesis and Antibacterial Activity of Bioplastic Membrane Containing In Doped ZnO/Cellulose Acetate Nanocomposite. J Inorg Organomet Polym 32, 1223–1233 (2022). https://doi.org/10.1007/s10904-021-02171-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02171-2