Abstract
In this paper, Methacrylic acid (MAA) and 4-vinyl pyridine (4-VP) as functional monomers, Ethylene glycol two methyl acrylate (EGDMA) as crosslinking agent, isopropyl alcohol as the solvent, prepared the Cu(II)- and Pb(II)- imprinted polymers (IIPs) submicron spheres by precipitation polymerization. The presence/absence of the template ion in the preparation of the imprinted polymer was confirmed by EDX spectroscopy, and the structure of the particles was investigated using IR, SEM and BET analysis. From different components of crosslinker/monomer (C/M) ratio analysis, C/M at 1:3 was the optimal ratio for preparing IIPs. Atomic absorption spectroscopy (AAS) was characterized the imprinted polymers absorption behavior. The results show that the maximum adsorption capacity of Cu2+ and Pb2+ -imprinted polymer were 26.9 mg g−1 and 25.3 mg g−1, respectively. They also have good adsorption capacity and superior selectivity property for Cu2+ and Pb2+ in water, respectively. The selectivity factors (α) for Ni2+, Zn2+, Co2+ and Fe2+ were 16.5 (Cu2+) and 12.1 (Pb2+), 13.8 (Cu2+) and 16.2 (Pb2+), 10.8 (Cu2+) and 10.1 (Pb2+), 20.4 (Cu2+) and 20.7 (Pb2+), respectively. The regeneration experiment result demonstrates an excellent re-utilization property of these two type IIPs, after ten uses, the adsorption capacity can maintain above 60%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The industrial wastewater is the serious problems of worldwide pollution. Especially the sewage containing heavy metals is an important factor causing damage to the environment and human body [1]. There are many methods to remove heavy metals from industrial wastewater, such as adsorption on active carbon [2], liquid–liquid extraction [3], membrane [4], chelating resins [5], solid phase extraction [6], and mineral substance [7].
In these methods, Solid phase extraction (SPE) has developed rapidly due to its advantages of simple operation and low cost [8]. However, common separation methods, including SPE, have a fatal disadvantage of poor selectivity. Selective separation is very important in the field of water treatment and metal recovery.
Molecular imprinting is a highly selective separation technique which appeared at the end of the twentieth century [9]. Molecular imprinting works by polymerizing the target molecule (template) with functional monomers using cross-linker. After the removal of template molecules, the functional groups on the binding sites should be arranged in a position suitable for subsequent interaction with template molecules [10], so Molecular imprinting (MIPs) can be recognize and bind the required molecular targets, showing high affinity selectivity [11]. Ion imprinted polymer (IIP) was synthesized with metal ion as template. In most cases, specific ligands (or metal ion complexes with such specific ligands) are used in the polymerization process to form stable complexes with metal ions.
In recent years, metal ion imprinting technology has developed rapidly and a large number of research articles have been published (Zn-IIP [12], Cd-IIP [13], Hg-IIP [14], Cr(VI)-IIP [15], Mn-IIP [16], Co-IIP [17]. Except for the common heavy metals, T. Prasada Rao team prepared Dy-IIP using 5,7-dichloroquinoline 8-ol (DCQ) and 4-vp as the functional monomer [18], the polymer exhibits excellent adsorption capacity and selectivity in the presence of interfering ions. In addition, they also prepared rare earth metal ion-imprinting polymers with templates of Tb [19], Yb [20], Sm [21], La [22] and Ce [23], which were promoted the ion-imprinting technology applications development.
In our previous works, selectively adsorbing Cu-IIP, Ni-IIP, Zn-IIP, Pd-IIP, Pb-IIP and Pt-IIP have been synthesized in particle forms using different polymerization methods [24,25,26,27,28,29]. However, all the previous works have produced particles size area in 200–500 μm, which size are suitable for industrial wastewater treatment ranges. Therefore, in order to expand the application scope of IIPs such as the stationary phase of the liquid chromatography, a new synthesis process was employed on preparing the ion imprinted polymers. This work reports the synthesis of Cu-IIP and Pb-IIP submicron spheres by precipitation polymerization for the enhanced removal of aqueous Cu(II) ions and Pb(II) ions, respectively. Vinyl pyridine (4-VP) and Methacrylic acid (MAA) were used as functional monomers for chelating with the template. The adsorption capacity of the imprinted polymers was measured for several metal ions to demonstrate the selective separation of the template ion, Cu(II) and Pb(II). The reproducibility of the selective adsorption was also tested.
2 Experiment
2.1 Reagents and Instruments
Copper sulfate (CuSO4), Lead nitrate (PbNO3) and isopropanol were from Shanghai combined test chemical reagent co., LTD. (Shanghai, China). Methacrylate (MAA) 99%, 4-vinyl pyridine (4-vp) 95%, Ethylene glycol dimethacrylate (EGDMA) 99% were from Thain chemical technology (Shanghai) co., LTD. (Shanghai, China). Azodiisobutyronitrile (AIBN) was from Tianjin kermio chemical reagent co., LTD. (Tianjin, China). HCl and NaOH were from Huadong Medicine Co., LTD. The monomer and cross-linker were vacuum-distilled and stored at 4 °C before use.
In order to identify the chemical structure of the IIPs, FTIR spectroscopy (Bruker VERTEX70, Bruker, Ettlingen, Germany) was used. Scanning electron microscope (SEM) (S-4800, Hitachi, Tokyo, Japan) and BET analysis (NOVA 4000e, Quantachrome, Boynton beach, Florida, USA) were used to examine the morphology of the prepared polymer particles. The adsorption characteristics of IIPs were investigated using AAS (Z-6100, Hitachi, Japan).
2.2 Preparation of Ion Imprinted Polymer
Cu(II) or Pb(II)-imprinted polymers were prepared as submicron spheres by the precipitation polymerization of self-assembled Cu2+or Pb2+ and MAA, 4-VP complexes. Cu2+ or Pb2+-MAA-4-VP complexes and EGDMA were used at 1:1, 1:2, 1:3 and 1:4 in molar ratio, respectively; AIBN was used at 4 wt% relative to the total monomer system.
2.2.1 Formation of Ion-MAA-4-VP Self-Assembled Complexes
CuSO4 (5 mmol) was dissolved in 60 mL DI water at room temperature, and then MAA (10 mmol) and 4-VP (10 mmol) were added in turn. When the solutions was mixed by stirring for 2 h for sufficient reaction, the obtained precipitates (Cu- MAA-4-VP complex) were filtered and washed three times with DI water, and then dried in 70 °C under vacuum for 24 h. Pb- MAA-4-VP complex was prepared in the same manner.
2.2.2 Preparation of Ion-Imprinted Submicron Particles
Cu(II)-IIPs was synthesized as spherical particles by precipitation polymerization. Firstly, the complex Cu2+-MAA-4-VP (5 mmol) was dispersed in 60 mL of isopropanol, and then the solution was placed in a 250 mL three neck flask. The cross-linker EGDMA (5–20 mmol), 0.12 g of Azobisisobutyronitrile (AIBN) were added into the reactor one by one. The mixture was stirred by mechanical stirrer at room temperature at 250 rpm. The polymerization reaction was conducted at room temperature for 15 min, after which the temperature was increased to 70 °C for 6 h under N2 atmosphere. The product was washed with DI water and acetone to remove any impurities and un-reacted monomer, and then dried under vacuum for 24 h. Pb(II)-IIPs was synthesized in the same way of above. The synthesis process of non-ionic imprinted polymers is the same as that of ionic imprinted polymers, except that template ions.
Template ions within the polymer were removed by extensive washing with 0.5 M HCl under stirring for 2 h. This process was repeated several times and the solution was analyzed by AAS until ions removed completely. The resulting IIP submicron spheres were washed by DI water and then dried in vacuum.
2.3 Adsorption Experiments
2.3.1 Adsorbed Ion
The submicron spheres’ adsorption capacities were investigated for a series of aqueous metal ions. The value of pH value plays an important role at ion adsorption field, so the effects of solution pH on adsorption capacity were studied first. 0.1 g IIP particles was placed in 100 mL aqueous ion (Cu2+or Pb2+) at 30 ppm, pH was adjusted from 2 to 7 by the addition of 0.1 N NaOH and 0.1 N HCl. In order to analyze the maximum adsorption capacity of ion imprinted submicron spheres, 0.1 g of dried IIP was evenly dispersed in 100 mL 5–60 ppm ionic aqueous solutions with different concentrations. After 3 h of magnetic agitation at room temperature, the polymer particles were filtered out using a filtration membrane. Atomic absorption spectrometer (AAS) was used to determine the ion concentration of the original solution and residual solution after adsorption, which were recorded as C0 and Ce respectively. The adsorption capacity of ion imprinted polymer was calculated according to the formula:
where, Q represents the adsorption capacity of the polymer (mg•g−1); C0 and Ce as the concentration of ions before and after adsorption (mg• l−1), respectively; V is the volume (L) of an aqueous solution; W, the mass of the polymer sample (g).
2.3.2 Selective Adsorption Experiment
The selectivity of IIPs to target ions was studied by competitive adsorption of four typical ions: Ni2+, Zn2+, Fe2+ and Cd2+. A 100 ml mixed aqueous solution containing template ions and competing ions was prepared, and the concentration of each ion was 30 ppm. The pH value of the solution was adjusted to 6.5. After adsorption equilibrium was reached; the concentration of the other metal ions was also measured using AAS.
The distribution ratio (D) is given by Eq. (2):
where, v is the volume of the solution (mL), m is the mass of the polymer (g), Co and Ce are the initial and final concentrations of metal ions (mg L−1), respectively.
The selectivity factor for template ions (Cu2+or Pb2+) in competition with other ions was calculated according to Eq. (3):
where, DT and DM represent the distribution ratios of template ions and other metal ions, respectively.
2.3.3 Recycling Experiment
The recycling experiment was performed at a pH of 6.5. A certain amount of dried IIPs was evenly dispersed in 100 mL 30 ppm target ionic aqueous solutions. After 3 h of magnetic agitation at room temperature, the IIPs were filtered out using a filtration membrane. The adsorption capacity of IIPs was determined by Atomic absorption spectrometry (AAS). After that, the IIPs adsorbing target ions were dispersed in the 0.5 M HCl and washed for 2 h to remove the target ions. Then this batch of IIPs to carry out the adsorption target ion experiment and test its adsorption capacity. This process is repeated more than 10 times to test IIPs repeatability.
2.3.4 Structural Characterization of Ion Imprinted Polymers
The molecular structure of ion imprinted polymer before and after adsorption was analyzed by FT-IR and EDX. The morphology of polymer submicron spheres was observed by SEM. The specific surface area of synthetic polymer was determined by BET method.
3 Results and Discussion
3.1 Polymerization Mechanism
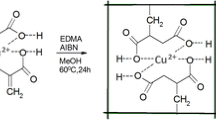
The two type’s Ion imprinted polymers (IIPs) were synthesis with Methacrylate (MAA) and 4-vinyl pyridine (4-VP) (functional monomers). Both functional monomers can chelate with template ions to form ligands, which gives strong imprinting sites towards the template ions. Although both MAA and 4-vp can form complexes with ions, respectively, the formation time is too long (about over 24 h). When these two monomers both to be used, the synergistic effect will play an important role, and this lead to the complex formed in a short time, it greatly improves the reaction efficiency. Because the carboxylic acid group from MAA was dissociated in aqueous solution, and gave off the hydrogen ions, this is the source of protonation of 4-VP, which will be attracted by N. In this way, the positively charged of template ion will be attracted by the carboxylic acid group as negatively charged, which will rapidly form the complex. Following, the complex was cross-linked with EGDMA to synthesize the IIPs spheres (Fig. 1).
3.2 IR Analysis
Figure 2 shown the IR spectra of ion-containing polymers, ion-removed (imprinted) polymers, and non-imprinted polymers, all curves show similar IR spectra, indicating similarity in the backbone structures, except for the binding groups to the template ions(Cu2+, Pb2+). The C=O, C=C, and C–O stretching vibration peaks from MAA at 1679, 1649, and 1201 cm−1, respectively [30]. The characteristic band of pyridine ring was observed at 1638 cm−1 [31]. The characteristic of the structure of the characteristic peak of Cu–O and Pb–O was occurred in ion-containing polymers while not in ion-removed (imprinted) polymers. It is proved that the template ion removed from polymer completely, and the eluting process has not caused damage to the polymer backbone, that’s the implication of the recognition site was perfectly preserved.
3.3 EDX Analysis
Figure 3 show the EDX result on Cu(II), Pb(II) containing and removed (imprinted) from polymers. The absence of Cu(II) ions from the imprinted polymer is confirmed by the energy dispersive x-ray analysis (EDX). The clear presence of Cu(II) ions before washing of template (Fig. 3a) is undetected after its washing process (Fig. 3b), indicating its complete removal. The same phenomenon is observed on Pb (II) (Fig. 3c, d).
3.4 SEM Analysis
The surface morphology of the Cu2+ and Pb2+ -IIPs was characterized using SEM. Figure 4 shows the shape of the imprinted particles. The 0.5–2 μm diameter submicron spheres evident in the figure revealed that the precipitation polymerization was appropriate to prepare well defined, spherical IIP particles. One phenomenon worth noting is that the diameter of Pb2+ -IIPs was smaller than Cu2+ -IIPs under the same preparation conditions, but the agglomeration was also accompanied it. This phenomenon may be caused by the different dispersibility of the two template ion-monomers complex in the solvent.
For primary synthetic variables affecting the particle size and morphology, the minimum amount of cross-linker (EGDMA) to obtain spherical and uniformly shaped particles in a desired size range was determined. Figure 5 shows the size of Cu2+ -IIPs particles produced at different components of cross-linker/monomer(C/M) ratio range of 1:1 to 1:4. The cross-linker plays an important role in particle formation and aggregation. With insufficient EGDMA amount (ratio1:1, 1:2) (Fig. 5a, b), the resulting particles were not spherical and were aggregated with one another. When the ratio was increased from 1:1 to 1:3, the perfect sphere particles were appeared at this condition. When the ratio continues to rise to 1:4, the spheres were going to be smaller. As the particle size became uniform from ratio1:3, the lowest amount of EGDMA was chosen as the optimal component to produce Cu2+ -IIPs particles.
3.5 BET Method for Determination of Specific Surface
As Table 1 Shown, the specific surface area of the copper ion imprinted polymer gradually increased with increasing cross-linker amount. It can explain that the amount of crosslinking agent was underused on C/M 1:1 and C/M 1:2, only a small fraction of the microspheres exist, most of which were aggregation. Therefore, the specific surface area was smaller than other two groups.
However, excessive cross-linker also has an adverse effect on polymerization. The amount of crosslinking agent on C/M 1:4 was more than C/M 1:3, much more cross-linker make its surface area and pore volume going to be smaller, which is not conducive to improving the adsorption efficiency of IIPs. It is indicated that the increased crosslinking agent in a moderate range was better for improving the specific surface area, the total pore volume and the average pore radius, but adverse effect on excessive amount, which led to adsorption capacity and efficiency decreased.
3.6 Adsorption Properties of Ion-Imprinted Polymers
In order to determine the optimum pH for Cu2+ and Pb2+ recognition, adsorption isotherms were obtained over a pH range of 2 to 7. As shown in Fig. 6, the adsorption capacity for target ions increased with increasing pH value. The cause is the proton dissociation of the carboxyl groups in the host molecules plays an important role in adsorbing metals. The sorption capacity was very low owing to the protonation of MIIP in the acidic condition. As the imprinted polymers sorption capacity was near its maximum at pH 6.5, this pH was used in the further adsorption studies.
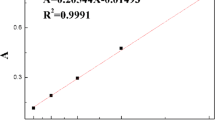
The concentration of target ions adsorbed by imprinted particles was studied at pH 6.5, the test concentration area from 5–60 ppm. As shown in Fig. 7, the amount of Cu2+ and Pb2+ adsorbed per unit mass of IIPs, increased with increasing initial metal ion concentration up to a concentration of 26.9 mg g−1 and 25.3 mg g−1, respectively, which is the maximum adsorption capacity of the prepared IIPs spheres. The binding constant is a parameter that describes the ion binding between metal ions and polymers. The slope of two curves was fitted by the formula: y = a + b*x, that’s the binding constant. The binding constants of Cu(II)- and Pb(II)-IIPs were 0.29717 and 0.28297, respectively.
In order to test the adsorption kinetics and special selectivity of Cu2+ and Pb2+ -imprinted polymer, Atomic absorption spectrometer (AAS) was used to detect target ion. From Fig. 8, Cu(II)- or Pb(II)-IIPs had a high linear adsorption efficiency for Cu2+ or Pb2+ respectively before 60 min. Because there were a large number of active sites in the IIPs spheres at the beginning of adsorption, target ions could bind to the recognition sites easily on the surface of the imprinted polymer. After 60 min, the recognition site on the surface of imprinted polymer was almost occupied by the ions, it lead to the absorption stop increasing. It is implied the ion imprinted polymer can reach the adsorption equilibrium in only 60 min. The binding constants were calculated as 0.1257 and 0.12031 respectively by fitting the two curves.
An anti-interference experiment result was shown in Fig. 9. When the ion imprinted polymer is in a solution containing template ion and other ions of similar value and size, the target ions absorbed by imprinted polymer has the highest adsorption capacity. But using non-imprinted polymers, the amount of target ions adsorption decreases greatly under the same conditions. It is indicated that ion imprinting technology has excellent specific selectivity to template ions. Tables 2, 3 shows the distribution ratio (D) and selectivity factor (α) of Cu(II)-, Pd(II)-IIPs. The selectivity coefficients of the IIPs for Cu2+(Pb2+)/Ni2+, Cu2+(Pb2+)/Zn2+, Cu2+(Pb2+)/Co2+ and Cu2+(Pb2+)/Fe2+, respectively, which are much higher than those of non-imprinted polymers at pH 6.5.
IIPs spheres adsorbent can be used repeatedly after generation using HCl. Figure 10 shows the reproduction of the adsorption capacity after repeated adsorption cycles. As the adsorption capacity for target ions decreased slightly during 10 repetitions, the adsorption capacity of regenerated particles was still maintained at a high degree. The regeneration times of ion-imprinted polymers synthesized in this work was compared with those in literatures, and the results are shown in Table 4. According to the results, this work illustrates higher regenerability for target ions than other ones synthesized using different functional monomers.
4 Conclusion
In this paper, Cu(II)- and Pb(II)-ion-imprinted polymer submicron spheres were synthesized by precipitation polymerization. The functional monomers methacrylate and 4-vinyl pyridine were self-assembled with template ions and then co-polymerized with ethylene glycol dimethacrylate in the presence of template ions for the preparation of a tight network structure. From BET analyzed, the crosslinking agent amount increases appropriately, its specific surface area, pore volume and average pore radius of IIPs can be significantly increased, which is conducive to improving the adsorption efficiency and adsorption capacity on template ions. From different components of cross-linker/monomer(C/M) ratio analysis, C/M at 1:3 was the optimal ratio for preparing IIPs. The adsorption kinetics and adsorption selectivity experiments of ion imprinted polymer on template ions show that IIPs has high adsorption efficiency and adsorption capacity. The regeneration experiment result demonstrates an excellent re-utilization property of the tow type IIPs.
References
N.N. Dil, M. Sadeghi, J. Hazard. Mater. 351, 38 (2018)
L. Elci, M. Dogan, Fresen. J. Anal. Chem. 330, 610 (1988)
E. Carasek, Talanta 51, 173 (2000)
M.L. Tummino, R. Nistico, C. Riedo, D. Fabbri, G. Magnacca, Chem-Eur. J. 27, 660 (2020)
D. Wang, B. Zhang, L.F. Xu, L.N. Huang, Bull. Chem. Soc. Jpn. 93, 92 (2020)
O. Almeida, R.M. Menezes, L.S. Nunes, V.A. Lemos, F.G. Velasco, Environ. Technol. Innov. 21, 101336 (2020)
B. Unnikrishnan, C.W. Lien, H.W. Chu, H.W. Chu, H.W. Chu, J. Hazard. Mater. 401, 123397 (2020)
P.G. Krishna, J.M. Gladis, T.P. Rao, G.R. Naidu, J. Mol. Recog. 18, 109 (2005)
G. Wulff, A. Sarhan, Angew. Chem. Int. Ed. 11, 341 (1972)
H.H. Yang, S.Q. Zhang, W. Yang, X.L. Chen, Z.X. Zhang, J.G. Xu, X.R. Wang, J. Am. Chem. Soc. 126, 4054 (2004)
A. Ma, A. Ms, B. Bs, J. Electroanal. Chem. 879, 114788 (2020)
M. Saraji, H. Yousefi, J. Hazard. Mater. 167, 1152 (2009)
D.K. Singh, S. Mishra, J. Hazard. Mater. 164, 1547 (2009)
D.K. Singh, S. Mishra, Desalination 257, 177 (2010)
G. Bayramoglu, M.Y. Arica, J. Hazard. Mater. 187, 213 (2011)
G.M. Murray, K.A. Van Houten, G.L. Southard 2007 WO Patent 2007/055767A1.
A. Bhaskarapillai, N.V. Sevilimedu, B. Sellergren, Ind. Eng. Chem. Res. 48, 3730 (2009)
V.M. Biju, J.M. Gladis, T.P. Rao, Anal. Chim. Acta 478, 43 (2003)
M.R. Ganjali, A. Ghesmi, M. Hosseini, M.R. Pourjavid, M. Rezapour, M. Shamsipur, M.S. Niasari, Sens. Actuators B 105, 334 (2005)
M.R. Ganjali, L. Naji, T. Poursaberi, M. Shamsipur, S. Haghgoo, Anal. Chim. Acta 475, 59 (2003)
M.R. Ganjali, M.R. Pourjavid, M. Rezapour, S. Haghgoo, Sens. Actuators B 89, 21 (2003)
M.R. Ganjali, A. Daftari, M. Rezapour, T. Puorsaberi, S. Haghgoo, Talanta 59, 613 (2003)
M. Shamsipur, M. Yousefi, M.R. Ganjali, Anal. Chem. 72, 2391 (2000)
Y. Jiang, D. Kim, Chem. Eng. J. 166, 435 (2011)
Y. Jiang, D. Kim, Polym. Adv. Technol. 24, 747 (2013)
M. Kim, Y. Jiang, D. Kim, React. Funct. Polym. 73, 821 (2013)
Y. Jiang, D. Kim, Chem. Eng. J. 232, 503 (2013)
Y. Jiang, D. Kim, J. Nanosci. Nanotechnol. Revisions 14, 8578 (2014)
Y. Jiang, D. Kim, Ind. Eng. Chem. Res. 53, 13340 (2014)
N.T. Hoai, D. Kim, AIChE J. 55, 3248 (2009)
N.T. Hoai, D.K. Yoo, D. Kim, J. Hazard. Mater. 173, 462 (2010)
R. Huang, N. Shao, L. Hou, X. Zhu, Colloid Surface A 566, 218 (2019)
W. Shen, X. Jiang, Q. An, Z.Y. Xiao, S.R. Zhai, L. Cui, New J. Chem. 43, 5495 (2019)
H. Wang, H. Shang, X. Sun, L. Hou, M. Wen, Y. Qiao, Colloid Surface A 585, 124139 (2020)
A. Bukhari, N.H. Elsayed, M. Monier, Int. J. Biol. Macromol. 155, 795 (2020)
S.D. Masi, A. Pennetta, A. Guerreiro, F. Canfarotta, C. Malitesta, Sensor Actuat B-Chem. 307, 127648 (2019)
B. Ara, M. Muhammad, T.U.Z. Rani, K. Gul, Desalin. Water. Treat. 191, 173 (2020)
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation (No. LQ19E030014). National Natural Science Foundation of China (No. 81901900). National Natural Science Foundation of China (No. 51902135).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Y., Tang, B., Zhao, P. et al. Synthesis of Copper and Lead Ion Imprinted Polymer Submicron Spheres to Remove Cu2+ and Pb2+. J Inorg Organomet Polym 31, 4628–4636 (2021). https://doi.org/10.1007/s10904-021-02065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02065-3