Abstract
Novel SnO2/SiC photocatalysts with nanosheet morphology were successfully fabricated by mechanical alloying and subsequent aging of SnCl2·2H2O, NH4HCO3, SiC, and 2-methylimidazole powders. The photocatalysts were mainly composed of SiC and SnO2. When the SiC content of the raw materials was higher, small amounts of Sn6O4(OH)4 also existed in the product. TEM results show that SiC nanocrystallines were well combined with SnO2 nanosheets. These SnO2/SiC photocatalysts showed good photocatalytic degradation efficiency to methyl orange (MO) under visible-light irradiation. The MO was rapidly reduced by 99% within 45 min by SiC/SnO2 composite. The excellent photocatalytic performance was mainly attributed to the formation of SnO2/SiC heterojunction, which reduced the recombination of photogenerated electrons and holes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dye wastewater pollution has attracted increasing attention due to the rapid development of printing and dyeing industry. At present, the main methods for treatment of dye wastewater are adsorption, chemical oxidation, ultrasonic degradation, biological degradation, and photocatalytic oxidation. Photocatalytic oxidation [1] has the unique advantages of low energy consumption and high degradation efficiency; this method has gained increasing attention in the academia and production enterprises. Research and development of semiconductor catalysts, such as TiO2 [2,3,4,5], is the core of photocatalytic oxidation for dye wastewater treatment. SnO2 is a wide band gap semiconductor (Eg = 3.6 eV) and has excellent photoelectric performance and chemical stability; as such, SnO2 is a common photocatalytic material [6, 7]. However, some disadvantages of SnO2 that seriously limit its application in the field of photocatalysis include fast recombination of carrier and hole pairs, low light conversion efficiency, and low visible-light response. Different semiconductors with band structure have been matched to build heterostructures with improved photocatalytic efficiency [8, 9]. At present, SnO2 is combined with TiO2 [10], ZnO [11], Fe2O3 [12], C3N4 [13], graphene [14], and other materials to obtain significantly improved visible photocatalytic performance.
SiC, as a traditional non-metallic semiconductor material, is rich in carbon and silicon and found in the Earth’s crust. SiC has high carrier transport rate and chemical stability and is thus a potential photocatalyst [15, 16]. SiC and SnO2 have matching conduction band and valence band potentials. SiC/SnO2 composites can be used in photocatalytic water splitting [17, 18] and electrochemical oxidation of wastewater [19]. However, no report is available about heterojunction materials constructed by SnO2 and SiC for photocatalytic degradation.
In our previous study [20], Sn6O4(OH)4 powders were prepared by mechanical alloying with SnCl2·2H2O and NH4HCO3 as raw materials; SnO2 nanoparticles were obtained by aging Sn6O4(OH)4 powders at a certain time in air. However, the as-synthesized SnO2 material did not have visible-light catalytic degradation performance. In the present study, we prepared SnO2/SiC composites through mechanical alloying and subsequent aging. The visible-light photocatalytic degradation of methyl orange (MO) was also studied.
2 Experimental Procedure
Reagent-grade SnCl2·2H2O, 2-methylimidazole, and NH4HCO3 were obtained from Tianjin Beichen Fangzheng Reagent Factory. SiC powders (average size of 40 nm, 99.9% purity) were purchased from Beijing Deke Island Gold Technology Co., Ltd. The raw material formula is listed in Table 1. These raw material powders were weighted and mechanically ground on a small grinder for 25 min. The ground powders were allowed to stand in air for 30 min before immersing in deionized water for 10 days. Finally, the powders were washed several times and dried. The obtained powders were named SnO2, 1% SiC/SnO2, 3% SiC/SnO2, 5% SiC/SnO2, 7% SiC/SnO2, 9% SiC/SnO2, and 11% SiC/SnO2. The detailed test equipment and conditions were reported in previous research [16]. In the present work, the concentrations of the photocatalyst and MO are 1 g/L and 15 mg/L, respectively.
3 Results and Discussion
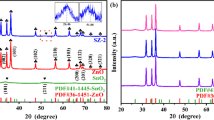
Figure 1 shows that the XRD pattern of different photocatalytic materials. The synthesized products were mainly composed of SnO2 and SiC. MA-synthesized Sn6O4(OH)4 precursor was basically transformed to SnO2 by aging. The reaction equation for the formation of SnO2 was as follows.
Compared with other fabrication technologies [10,11,12,13,14], this aging technology had the advantages of mild conditions, cost-effectiveness, and simplicity.
When the SiC content was low (≦ 2%), the SiC peaks were difficult to find. The SiC peaks began to appear when the SiC content was 4%. With increasing SiC content, the intensity of the diffraction peaks of SiC increased. Weak Sn6O4(OH)4 peak began to appear when the SiC content was increased to 7%. The intensity of the Sn6O4(OH)4 peak obviously increased when the SiC content was increased to 11%. Hence, adding excess SiC may inhibit the transformation from Sn6O4(OH)4 into SnO2 and could degrade the photocatalytic properties of the composites.
The diffraction peaks of SnO2 widened, indicating the fine grains of SnO2. Average grain size was estimated from the highest intensity peaks of SnO2 (2θ = 26.56°) by using the Scherrer’s equation. The average grain sizes were 6–7 nm for SnO2 and SnO2/SiC composites. Hence, we obtained very fine SnO2 nanocrystallines by using the proposed method.
Figure 2 shows the SEM images of (a) SnO2 and (b) 7%SnO2/SiC composite and present similar serious aggregates. These aggregates were composed of nanoparticles with size of several to dozens of nanometer.
Figure 3a shows that the as-synthesized SnO2 mainly consisted of severely agglomerated nanocrystallines with size of less than 10 nm; this finding is consistent with the XRD results. Nanosheets with thickness of about 3 nm and length of about 30 nm were also detected. The TEM image (Fig. 3b) of 7%SnO2/SiC composite was identical to that of the SnO2 sample. Figure 3c shows the HRTEM image of 7%SnO2/SiC composite. The tight interface of SiC and SnO2 was observed, indicating the formation of a good heterostructure. This SnO2/SiC heterostructure may enhance the separation of photoelectron pairs, thereby enhancing the photocatalytic performance.
The compositional information and elemental chemical states of 7% SiC/SnO2 were probed with X-ray photoelectron spectroscopy (XPS). Spectrum decomposition was performed using the casaXPS program with Gaussian functions after the subtraction of a Shirley background.
Figure 4a shows the survey spectra, which confirmed the existence of Si, C, Sn, and O. As shown in Fig. 4b, the peaks centered at 486.98 and 495.38 eV are assigned to the Sn3d5/2 and Sn3d3/2 of Sn4+ in SnO2, respectively. The O peaks (Fig. 4c) at 530.74, 531.81, and 534.29 eV are assigned to O–Sn–O, surface hydroxyl oxygen (–OH), and adsorption oxygen groups, respectively. Figure 4d and e show the high-resolution spectra of Si 2p and C 1s, respectively. The peaks centered at 101.8 and 284.6 eV are attributed to Si 2p and C 1s of SiC, respectively. The C=O bond mainly rose from oxygen absorbed on the surfaces of the SiC nanostructures.
The photoluminescence spectrum of the sample was recorded to investigate the separation effect of photogenerated electrons and holes in the composites (Fig. 5). The intensity of all emission peaks of SnO2/SiC composites was lower than that of SnO2, suggesting the low recombination probability of electron holes in the composites. The peak intensity of 7% SnO2/SiC composite was the lowest, indicating that the electron–hole recombination probability of the catalyst was the lowest. This composite was considered the most conducive to the separation of electrons and holes in the photocatalytic system. Hence, the introduction of SiC effectively improved the luminescent properties of SnO2 and rendered them conducive to photocatalytic reaction.
The typical UV–Vis diffuse reflection spectra of SiC, SnO2, and composites are shown in Fig. 6a. SnO2 showed weak absorption capacity, indicating that it possessed a wide band gap, whereas pure SiC had an obvious absorption band from 200 to 800 nm. SiC/SnO2 composites had obviously stronger absorption capacity than SnO2 in the visible region. These results indicated that the SiC/SnO2 composites exhibited efficient visible-light utilization, which can result in good photocatalytic performance.
Figure 6b shows that the band-gap energy (Eg) values of SiC/SnO2 composites were about 2.65 eV. Single-phase SnO2 and SiC had a high Eg value of 2.78 and 2.99 eV, respectively. Thus, their combination significantly reduced their band gap, thereby improving the utilization efficiency of visible light.
Figure 7 compares the photocatalytic degradation of MO by SnO2 and SnO2/SiC composites under visible light. SnO2 exhibited weak ability to photocatalytic degradation of MO. Moreover, SnO2/SiC composites had good degradation ability. The photocatalytic degradation abilities of 1% SiC/SnO2, 3% SiC/SnO2, 5% SiC/SnO2, and 7% SiC/SnO2 composites were better, and they degraded 99% MO within 45 min. The enhancement can be explained by the synergetic effect on the efficient electron–hole separation at the SnO2/SiC photocatalyst interface. Figure 1 shows that the 9%SiC/SnO2, and 11% SiC/SnO2 composites contained some Sn6O4(OH)4 impurity. Obviously, adding excess SiC inhibited the transformation of Sn6O4(OH)4 to SnO2, which decreased the content of SnO2 photocatalysts in the composites.
Table 2 shows the visible photocatalysis results of the fabricated SnO2/SiC composite and SnO2-based catalysts in other studies. The SnO2/SiC composite showed higher photocatalytic degradation rate than the other SnO2-based photocatalysts. Hence, SnO2/SiC photocatalyst could be a new highly efficient catalyst.
To evaluate the photocatatyic cyclic performance of the 7% SnO2/SiC composite, four cycling experiments were performed for MO degradation. Figure 8a shows that after several cycles, no obvious change occurred in the photocatalytic-degradation activity. Additionally, the XRD (Fig. 8b) pattern and XPS (Fig. 8c–g) data remained stable. These findings indicated the reusability and high stability of the 7% SnO2/SiC composite.
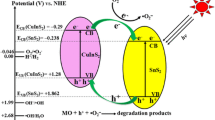
The proposed mechanism of photocatalytic degradation over SnO2/SiC nanosheets is shown in Fig. 9. The locations of the conduction and valence bands of SiC and SnO2 were confirmed by the Mott–Schottky plot. The valence-band maximum values of SiC and SnO2 were estimated at 1.6 and 3.78 eV, and the corresponding conduction-band minimum values of SiC and SnO2 were around − 1.4 and 0 eV, respectively. The conduction band of SiC was more negative than that of SnO2, resulting in the transfer of electrons generated in the conduction band from SiC to SnO2 nanoparticles. The valence band of SnO2 was more positive than that of Si; as such, the hole transferred from the valence band of SnO2 to the valence band of SiC. This migration of electrons and holes between SnO2 and SiC effectively reduced the band gap of SnO2. This phenomenon further improved the mobility of electrons, inhibited the recombination of electrons and holes, and prolonged the lifetime of electrons and holes, which are conducive to photocatalytic degradation. The holes in the valence band oxidized MO into CO2 and H2O and interacted with OH− to form ·OH radicals, which also oxidized MO into CO2 and H2O. In addition, the electrons on the conduction band reduced the adsorbed O2 into O2− and oxidized MO into CO2 and H2O.
4 Conclusions
In this work, SiC/SnO2 composites were synthesized by mechanical alloying combined with aging. The TEM, XPS, UV–Vis DRS, and PL studies showed the formation of SiC/SnO2 heterojunction, which inhibited the recombination of photogenerated electron–hole pairs. Hence, the fabricated SiC/SnO2 composites exhibited excellent visible-light photodegradation of MO. In particular, 7%SiC/SnO2 composite possessed the highest photodegradation rate, that is, it degraded 99% MO within 45 min.
References
A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972)
R. Raliya, C. Avery et al., Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl Nanosci 7, 253–259 (2017)
M. Ritu, K. Vijay, J. Nirav et al., Au–TiO2-loaded Cubic g-C3N4 nanohybrids for photocatalytic and volatile organic amine sensing applications. ACS Appl Mater Int 10, 34087–34097 (2018)
P.A. Aprilya, W. Sri, P.P. Yuniar et al., Optimisation of methyl orange photodegradation using TiO2-zeolite photocatalyst and H2O2 in acid condition. IOP Conf Ser: Mater Sci Eng 546, 042047–042055 (2019)
N. R.Zha, X.Guo Reddeppa et al., Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J Mater Chem A 3, 6565–6574 (2015)
D. Venkatesh, S. Pavalamalar, K. Anbalagan, Selective photodegradation on dual dye system by recoverable nano SnO2 photocatalyst. J Inorg Organomet Polym 29, 939–953 (2019)
M.U. Khalid, S.R. Khan, S. Jamil, Morphologically controlled synthesis of cubes like tin oxide nanoparticles and study of its application as photocatalyst for congo red degradation and as fuel additive. J Inorg Organomet Polym 28, 168–176 (2018)
L. Mohan, N. Sisupalan, K. Ponnusamy et al., One step synthesis and characterization of ZnO–ZnSe heterostructures by chemical precipitation and its solar photocatalytic activity. J Inorg Organomet Polym 30, 2626–2632 (2020)
A. Phuruangrat, P. Keereesaensuk, K. Karthik et al., Synthesis and characterization Ag nanoparticles supported on Bi2WO6 nanoplates for enhanced visible-light-driven photocatalytic degradation of rhodamine B. J Inorg Organomet Polym 30, 1033–1040 (2020)
S. Weerachai, The self-cleaning and photocatalytic properties of TiO2 doped with SnO2 thin films preparation by sol-gel method. Energy Proc 89, 170–176 (2016)
P. Petronela, A. Anton, O. Niculae et al., Photocatalytic degradation of rhodamine B dye using ZnO–SnO2 electrospun ceramic nanofibers. Ceram Int 42, 6775–6781 (2016)
H.L. Xia, H.S. Zhuang, T. Zhang et al., Visible-light-activated nanocomposite photocatalyst of Fe2O3/SnO2. Mater Lett 62, 1126–1128 (2008)
Y.P. Zang, L.P. Li, X.G. Li et al., Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem Eng J 246, 277–286 (2014)
B. Quan, W. Liu, Y.S. Liu et al., Quasi-noble-metal graphene quantum dots deposited stannic oxide with oxygen vacancies: synthesis and enhanced photocatalytic properties. J Colloid Interf Sci 481, 13–19 (2016)
J. Weng, P. Lee, Y. Chen et al., Degradation of abiotic orange II dye and biotic E. coli by highly porous SiC-AgCl/Ag0 photocatalyst. J Inorg Organomet Polym 30, 1760–1768 (2020)
J.Y. Hao, Y.Y. Wang, X.L. Tong et al., Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int J Hydrog Energy 37, 15038–15044 (2012)
C.Y. Li, H.B. Ouyang, J.F. Huang et al., Synthesis and visible-light photocatalytic activity of SiC/SiO2 nanochain heterojunctions. Mater Lett 122, 125–128 (2014)
J.J. X.Liao, M.M. Chen, Wang et al., Enhanced photocatalytic and photoelectrochemical activities of SnO2/SiC nanowire heterostructure photocatalysts. J. Alloys Compd 658, 642–648 (2016)
X.L. Zhang, D. Shao, W. Lyu et al., Utilizing discarded SiC heating rod to fabricate SiC/Sb-SnO2 anode for electrochemical oxidation of wastewater. Chem Eng J 361, 862–873 (2019)
B.Y. Liang, Mechanical-induced spontaneous formation of SnO2/Sn6O4(OH)4 photocatalysts at room temperature. Mater Lett 252, 155–157 (2019)
Acknowledgements
This project was sponsored by the National Natural Science Foundation of China (Grant No. 51864028), the Natural Science Foundation of Henan (18A430035), the Natural Science Foundation of Henan (Grant No. 18A430035), International S&T Cooperation Program of China (Grant No. 2015DFR50620).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Dai, Z., Liang, B. et al. Facile Synthesis of SnO2/SiC Nanosheets for Photocatalytic Degradation of MO. J Inorg Organomet Polym 31, 303–310 (2021). https://doi.org/10.1007/s10904-020-01702-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-020-01702-7