Abstract
In this paper, we reported on obtaining and study of polymer nanocomposites based on isotactic polypropylene and lead sulfide nanoparticles. SEM analysis showed that the average size of nanoparticles of lead sulphide based on PP + 1%PbS 7–13 nm; PP + 3% PbS is 13–18 nm, for PP + 5%PbS 11–20 nm and for nanocomposites PP + 10%PbS 13–24 nm. UV–Vis spectroscopy determined that the band gap for nanocomposites based on PP + 1%PbS is 2.2 eV; for PP + 3%PbS 2.1 eV; for PP + 5%PbS 2.3 eV, and for PP + 10% PbS 1.7 eV. The photoluminescence properties of nanocomposites based on PP + PbS are investigated. It is established that the intensity of photoluminescence increases with increasing concentration of nanoparticles of PbS to 3% of content, and then decreases. The effect of temperature–time crystallization on the structure and photoluminescent properties of nanocomposites based on PP + PbS was also studied. It is established that in the case of cooling of PP + PbS films in liquid nitrogen, the intensity of the photoluminescence increases sharply compared with cooling in water and with slow spontaneous cooling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor materials in the form of clusters distributed in an organic polymer matrix have recently attracted an increasing interest of researchers working in the area of physics and chemistry of low-dimensional systems. Such materials exhibit unusual electronic and optical properties, in comparison with bulky materials. For the production of nanoscale semiconductor materials, a number of methods are used, such as sol–gel technology, Langmuir–Blodgett method, molecular beam epitaxy and the method of multicyclic processing. One of the main obstacles to the possible use of nanocomposites on the base semiconductor nanoparticles in optoelectronics is the low luminescence efficiency of nanoclusters due to the high density of surface states. The density of surface states depends both on the method of preparation and on the subsequent processing of nanoclusters and the matrix into which the nanoparticles (NPs) are incorporated. It is possible to regulate the structure of the formed material by studying the structure of composites, revealing of the link between the nature of supramolecular structure and the properties of polymers, controlling the methods of introducing nanoparticles into polymer matrixes, and also by varying the various technological factors, in order to obtain the required properties. Studying the structure of such materials allows us to predict their properties, and by changing the properties one can judge the structure of the nanocomposite.
Polypropylene (PP) at the present time is one of the most prevalent synthetic polymer that is widely applied in many fields. Introduction of nanosized inorganic particles in polymer allows to design the structure of material by means of transfer of plain volume filled system to the system with given supramolecular architecture and spatial distribution of the filler, that promotes the creation the polymer with new technological and operating properties. Polypropylene is a polycrystalline polymer having attractive mechanical properties such as strength and elasticity at ambient temperature. PP with fillers is considered to be one of best thermoplastic materials. The thermoplastic polymer such as polypropylene that is fundamentally distinguished by its structure and functional designation is a matter of interest in term of preparation on basis of polypropylene new nanocomposite polymer structures. The nanocomposite on the basis of above mentioned polymer and PbS nanoparticles is a matter of indubitable interest for targeting the design of new nanocomposite materials. So, the study of the dependence of properties of nanocomposite on the characteristics of its individual components can lead to the preparation of nanocomposite with set of practically important properties [1,2,3,4,].
Lead sulphide is widely used in an infrared technique as well as in micro-and optoelectronics. The electrical properties of the lead sulphide significantly change upon transition from bulk crystalline to nanocrystalline state as in the case with other semiconductors. This fact promises new opportunities for the use of its optical features in the visible and infrared regions. As a narrow band semiconductor the lead sulphide is widely used for obtaining the temperature sensitive transducers, detectors operating in the infrared region (850–3100 nm) of spectrum, photoresistors and selective sensors. Properties of lead sulphide are significantly changed with a decrease in nanoparticles’ sizes up to nanometer range. Therefore, an obtaining smaller lead sulphide nanoparticles and the polymer nanocomposite materials on their basis is of great scientific and practical interest.
Nanostructured lead sulfide, predominantly in the form of thin films, can be used to expand the spectral range of IR photodetectors and detectors designed to detect heat sources, in photomultipliers and night vision devices, in solar batteries and optical devices. In this regard, it should be expected that the creation of composite materials, on the basis of polypropylene containing lead sulfide nanoparticles, will create technological film materials that will find wide application for the creation of new generation optical devices. These materials must have new properties, one of which is the manifestation of a size effect in the optical range [1,2,3,4,5].
In this work lead sulphide NPs synthesized using chemical coo precipitation method proposed by Ramazanov et al. [2] in which CTAB and sodium sulphide were used as capping and precipitated agent respectively for lead acetate. PP + PbS based nanocomposites prepared using the ex-situ and hot pressing method proposed by Maharramov et al. [1]. The hot pressing method is one of the defect-free methods for introducing nanoparticles into a polymer matrix, where it is possible to introduce nanoparticles to large volume contents. The limiting concentration of the dispersed filler is determined by the degree of change in the properties of the composite, the rheological properties, the reprocessing, and the viscosity limit of the composites. The degree of high filling cannot be determined by injection molding and extrusion. Literary analysis shows that it is impossible to achieve filling in the polymer above 20–25% using these methods. It should also be noted that using these methods, even at low concentrations, it is impossible to obtain defect-free composite structures.
In this paper, polymer nanocomposites based on PP/PbS were obtained by ex-situ and hot pressing methods, the structure, optical and photoluminescence properties of polymer nanocomposites PP/PbS were studied.
2 Experimental Section
2.1 Materials
All chemicals were used as received: polypropylene [(PP grade Moplen HF500 N, homopolymer); density 0.92 g/cm3 at 25 °C, Mw = 250000, Mn = 67000, melt mass-flow rate—MFR = 11.5 g/10 min (2300 °C, 2.16 kg), melting T = 162 °C], lead acetate trihydrate (316512 Aldrich Pb(CH3COO)2 × 3H2O), sodium sulfide (Na2Sx9H2O, PLC 141687), CTAB (cetyltrimethylammonium bromide, C19H42BrN, AB 117004), toluene (PLC 141745).
2.2 Synthesis of Polymer Nanocomposites
PbS nanoparticles were synthesized in work [1, 2]. PP + PbS basednanocomposites were synthesized by ex-situ method in solution of the polypropylene polymer. PbS nanoparticles were added to the polymer solution at various weight contents of PbS and mixed for an hour until a homogeneous mixture was obtained. The mixture was transferred to a Petri dish and dried in a vacuum oven during a day. Then, thin films of nanocomposites were obtained from these samples by hot pressing at a melting point of polypropylene and a pressure of 10 MPa. The cooling of the films after hot pressing was carried out in water (cooling rate was 200 deg/min), in liquid nitrogen (cooling rate was 2000 deg/min) and air (cooling rate was 20 deg/min).
2.3 Characterization
X-ray diffraction analysis was performed with Rigaku Mini Flex 600 XRD diffractometer at ambient temperature. In all the experiments, Cu K α radiation operating at 15 mA and 30 kV was used. The samples were scanned in the 20–70° range of Bragg angle 2θ. The images of the nanocomposite samples have been obtained by a scanning electron microscopy (SEM, Jeol JSM-7600 F). Scanning was performed in SEI mode at an accelerating voltage of 15 kV and a working distance of 4.5 mm. Energy dispersive micro-X-ray analysis was performed using the device X-Max 50 (Oxford Instruments). The UV–vis spectra have been recorded on Spectrophotometer Specord 250 Plus at 200–700 nm and ambient temperature. Photoluminescent properties of nanocomposite films were examined using a spectrofluorimeter Varian Cary Eclipse at a wavelength range of 200–900 nm. The morphology of nanocomposites has been studied by the AFM method on Integra-Prima (NT-MDT, Zelenograd). For scanning, were used a special silicon cantilevers, prepared by plasma chemical method of etching, with 20 nm radius of curvature and 1–5 Hz resonance frequency. The measurements have been carried out at the regime of semi-contact × of scanning area were 1 microscopy in the air, were fixed the changes in amplitude of oscillation of cantilever’s needle that defines the topography of surface. The rate of scanning and number of scanned lines on image were 1969 Hz and 256, respectively.
3 Results and Discussion
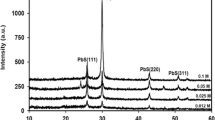
In Fig. 1 shows the diffraction pattern of lead sulfide nanoparticles. As can be seen, the main peaks on the diffractogram at 2θ correspond to 26.780 (111); 30.11 (200); 43.37 0 (220); 51.1° (311); 53.97° (222); 62.54° (400); 68.63° (331); 71.0° (420); 79.38° (422) and 85.19° (511) belong to lead sulfide nanoparticles. PbS crystallizes in a cubic lattice of type B1 (NaCl) with a Fm3m space group with lattice parameters of 0.594, 0.5936, or 0.5929 nm, in accordance with the ICCD PDF database (00-210-4270). Other peaks in the diffractogram belongs to the surfactant CTAB, which coats and stabilizes the surface of lead sulphide nanoparticles.
Figure 2 shows the diffraction patterns of nanocomposites based on PP and PbS, depending on the content of PbS nanoparticles. As can be seen from the diffraction patterns with increasing content of PbS nanoparticles in the polymer matrix, the crystallinity of polymer nanocomposites increases.
Figure 3 shows SEM images of nanocomposites based on PP/PbS as a function of the concentration of PbS nanoparticles. As can be seen from Fig. 3, the average size of nanoparticles based on PP + 1%PbS 7–13 nm; PP + 3%PbS is 13–18 nm, for PP + 5%PbS 11–20 nm and nanocomposites PP + 10%PbS 13–24 nm.
Figures 4 and 5 show the EDS spectrum and the mapping of the PP + PbS based nanocomposites by elements over the entire area of the site. As can be seen from the mapping spectrum, the maps of the element of lead and sulphur coincide with each other and it is therefore established that the nanocomposite consists mainly of nanoparticles of lead sulphide and polymeric polypropylene matrix. EDS spectrum also shows that nanocomposite consist mainly of polypropylene and lead sulfide nanoparticles.
In Fig. 6 are shown the UV absorption spectra (a) and plot of (αhν)2 versus the energy for the PP/PbS nanocomposite as a function of the concentration of PbS nanoparticles. It is clearly seen from the Fig. 6 that, compared with the bulk lead sulfide (band gap for bulk lead sulphide 0.93), the band gap of nanocomposites based on PP + PbS increases. The increase in the band gap correlates well with the inherent quantum-size effect for nanoparticles. As also can be seen, from Fig. 6, as the concentration of PbS nanoparticles increases, the width of the band gap of nanocomposites decreases. The decrease in the width of the forbidden band as a function of the concentration of PbS nanoparticles is explained by the increase and agglomeration of nanoparticles depending on the content of PbS nanoparticles [6,7,8,9,10,11].
Figure 7 shows the photoluminescence spectra of nanocomposites PP + PbS as a function of the concentration of PbS nanoparticles. It is established that the intensity of photoluminescence increases with increasing concentration of nanoparticles to 3% of content, and then decreases. Photoluminescence spectra were obtained by excitation wavelength of 365 nm. Figure 8 shows the excitation and emission spectra in the visible regions for a nanocomposite PP + PbS. It can be seen that the excitation and emission spectra are mirror images of each other, which is a proof that the peak at a wavelength of 765 nm is a photoluminescent peak belonging to PbS nanoparticles [12,13,14,15,16,17,18,19].
Have also been studied the effect of various technological conditions on the optical properties of polymer nanocomposites based on PP + PbS. Figure 9 shows the absorption spectra and plot of (αhν)2 versus the energy for the nanocomposite samples based on PP + PbS for various temperature–time modes of crystallization of polymer nanocomposites.
As can be seen, from Fig. 9b with a change in the temperature–time crystallization regime, i.e. conditions of crystallization of nanocomposites based on PP + PbS in water, liquid nitrogen and slow cooling, the band gap varies differently. The decrease in the band gap in the case of quenching nanocomposites in liquid nitrogen is explained by the fact that smaller crystallites are formed in the mode of rapid cooling in liquid nitrogen compared with slow cooling and cooling in the mode of quenching in water.
Figure 10 shows AFM images of the surface of polymer nanocompositions based on PP + PbS, depending on the temperature–time crystallization regime. As can be seen, with a change in the temperature–time mode of crystallization of nanocomposite films, the supramolecular structure of nanocomposites changes dramatically.
Figure 11 shows a histogram of the surface roughness ofnanocompositions based on PP + PbS, depending on the temperature–time crystallization regime. Thus, the average surface roughness for nanocomposites based on PP + PbS obtained in regime of cooling in the water is 20–120 nm, in the slow cooling regime is 50–150 nm, in the cooling in liquid nitrogen regime is 10–50 nm.
Figure 12 shows the photoluminescence spectra of nanocomposites based on PP + PbS obtained in the regime of slow cooling (1), in water (2) and in liquid nitrogen (3). The photoluminescence spectra were obtained upon excitation of nanocomposite films at light length of 365 nm. It can be seen that with a change in the temperature–time mode of crystallization of the films of nanocomposites, the intensity of photoluminescence varies greatly. So in the case of cooling of PP + PbS films in liquid nitrogen, the intensity of the photoluminescence increases sharply compared with cooling in water and with slow spontaneous cooling.
This can be explained by the fact that in the mode of slow cooling Tcryst. = 20 deg/min, larger molecular structures are formed, and this leads to a weakening of interactions with nanoparticles. In the rapid cooling mode, smaller crystallites form in liquid nitrogen and nanoparticles play the role of the center of the nucleus of crystallization. As a result, interfacial interactions between the polymer and nanoparticles increase and the photoluminescence intensity increases. Consequently, it has been established that the PP matrix is not just passive matrices for nanoparticles, but also strong interfacial interactions occur between polymer matrices and nanoparticles of lead sulfide, and this, in its turn affects the luminescent properties of PP + PbS nanocomposites.
4 Conclusion
In this paper, we reported on obtaining and study of polymer nanocomposites based on isotactic polypropylene and lead sulfide nanoparticles. SEM analysis showed that the average size of nanoparticles based on PP + 1%PbS 7–13 nm; PP + 3% PbS is 13–18 nm, for PP + 5%PbS 11–20 nm and nanocomposites PP + 10%PbS 13–24 nm. UV–Vis spectroscopy determined that the band gap for nanocomposites based on PP + 1% PbS is 2.2 eV; for PP + 3%PbS 2.1 eV; for PP + 5%PbS 2.3 eV, and for PP + 10% PbS 1.7 eV. The photoluminescence properties of nanocomposites based on PP + PbS are investigated. It is established that the intensity of photoluminescence increases with increasing concentration of nanoparticles of PbS to 3% of content, and then decreases. The effect of temperature–time crystallization on the structure and photoluminescent properties of nanocomposites based on PP + PbS was also studied. It is established that in the case of cooling of PP + PbS films in liquid nitrogen, the intensity of the photoluminescence increases sharply compared with cooling in water and with slow spontaneous cooling. Therefore, for such systems, it was proved that the spectral region is expanded and it makes possible to apply such nanocomposites in various fields of science and technology.
References
A.M. Maharramov, M.A. Ramazanov, F.V. Hajiyeva, S.S. Amirov, Investigation the structure and dielectric properties of PP + PbS nanocomposites synthesized on the basis of polypropylene polymer irradiated by accelerated heavy ions. J. Optoelectron. Biomed. Mater. 8(1), 15–20 (2016)
A.M. Magerramov, M.A. Ramazanov, F.V. Hajiyeva, Structure and dielectric properties of nanocomposites on the basis of high-density polyethylene and lead sulfide. J. Chalcogenide Lett. 11(4), 175–180 (2014)
L. Bakueva, I. Gorelikov, S. Musikhin, X.S. Zhao, E.H. Sargent, E. Kumacheva, PbS quantum dots with stable efficient luminescence in the near-IR spectral range. Adv. Mater. 16(11), 926–929 (2004)
A.A. Novruzova, M.A. Ramazanov, A. Chianese, F.V. Hajiyeva, A.M. Maharramov, U.A. Hasanova, Synthesis, structure and optical Properties of PP + PbS/CdS hybrid nanocomposites. Chem. Eng. Trans. 60, 61–66 (2017)
M.A. Ramazanov, A.M. Maharramov, A. Chianese, A.A. Novruzova, G.Y. Maharramova, The effect of electric discharge treatment on the optical properties of hybrid PP/PbS/CdS nanocomposites. J. Ovonic Res. 14(2), 113–117 (2018)
P. Lutsyk, L. Dzura, A. Kutsenko, Ya. Vertsimakha, J. Sworakowski, Photovoltaic and optical properties of a polymer-PbS nanocomposite. Semicond. Phys. Quantum Electron. Optoelectron. 8(3), 54–59 (2005)
P. Chandana, L. Sosa-Vargas, J.J. Ojeda, A.K. Sharma, A.N. Cammidge, M.J. Cook, A.K. Ray, Charge transport in lead sulfide quantum dots/phthalocyanines hybrid Nanocomposites. Org. Electron. 44, 132–143 (2017)
A.H. Khan, S. Pal, A. Dalui, J. Pradhan, D.D. Sarma, S. Acharya, Solution-processed free-standing ultrathin two-dimensional PbS nanocrystals with efficient and highly stable dielectric properties. Chem. Mater. 29(3), 1175–1182 (2017)
E.G. Durmusoglu, M.M. Yildizhan, M.A. Gulgun, H.Y. Acar, Production of small, stable PbS/CdS quantum dots via room temperature cation exchange followed by a low temperature annealing processes. J. Phys. Chem. C 121(45), 25520–25530 (2017)
K. Stadelmann, A. Elizabeth, N.M. Sabanés, K.F. Domke, The SERS signature of PbS quantum dot oxidation. Vib. Spectrosc. 91, 157–162 (2017)
M.H. Patel, T.K. Chaudhuri, V.K. Patel, T. Shripathi, U. Deshpande, Optical properties of PbS/PVP nanocomposites films. AIP Conf. Proc. 1728(1), 020106 (2016)
S. Jing, S. Xing, C. Zhao, Direct synthesis of PbS/polypyrrole core-shell nanocomposites based on octahedral PbS nanocrystals colloid. Mater. Lett. 62(1), 41–43 (2008)
T. Tohidi, K.J. Ghaleh, R.M. Rezaei, Characterization of PbS/PVA/GQDs nanocom-posite prepared by chemical bath deposition method. Eur. Phys. J. Appl. Phys. 68(1), 10403 (2014)
C. Lü, C. Guan, Y. Liu, Y. Cheng, B. Yang, PbS/Polymer nanocomposite optical materials with high refractive index. Chem. Mater. 17(9), 2448–2454 (2005)
H.M. Patel, T.K. Chaudhuri, V.K. Patel, T. Shripathi, U. Deshpande, N.P. Lalla, Dip-coated PbS/PVP nanocomposite films with tunable band gap. RSC Adv. 7, 4422–4429 (2017)
S.P. Mondal. Growth and characteristics of PbS-polymer nanocomposites for flexible opto-electronic device applications, in International Conference on Fiber Optics and Photonics (2014). https://doi.org/10.1364/PHOTONICS.2014.T3A.72
T.K. Chaudhuri, A.J. Kothari, D. Tiwari, A. Ray, Photoconducting nanocomposite films of PbS nanocrystals in insulating polystyrene. Phys. Status Solidi 210(2), 356–360 (2013)
S. Khan, Z. Jiang, S.M. Premathilka, J. Hu, A. Voevodin, P.J. Roland, R.J. Ellingson, L. Sun, Ultrathin colloidal PbS/CdS core/shell nanosheets. Nanomaterials 2(60), 3685–3690 (2017)
T. Serrano, J.L. Cavazos, Y. Pena, I. Gomez, Synthesis and characterization of PbS/ZnScore/shell nanoparticles by microwave method. Chalcogenide Lett. 11(1), 21–28 (2014)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramazanov, M.A., Hajiyeva, F.V. Influence of Crystallization Temperature–Time Technological Factors on Structure and Photoluminescent Properties of Polymer Nanocomposites on the Base of Isotactic Polypropylene (PP) and Lead Sulphide (PbS) Nanoparticles. J Inorg Organomet Polym 29, 2168–2175 (2019). https://doi.org/10.1007/s10904-019-01176-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01176-2