Abstract
The reactions of LiOH·H2O with 1,3,5-benzenetricarboxylic acid (1,3,5-btc) under versatile solvothermal conditions produce a series of lithium coordination polymers, namely {[Li2(1,3,5-btc)(1,3,5-Hbtc)(DMF)](DMF)2}n (1), {[Li3(1,3,5-btc)(DMSO)3](H2O)}n (2), {((CH3)2NH)[Li2(1,3,5-btc)(H2O)]}n (3), {((CH3)2NH)[Li2(1,3,5-btc)(1,3,5-Hbtc)]}n (4), {((CH3)2NH)[Li2(1,3,5-btc)]}n (5) and [Li3(1,3,5-btc)(H2O)]n (6) (DMSO = dimethyl sulphoxide, DMF = N,N-dimethylformamide). Compound 1 and 4 exhibit two-dimensional framework, while compound 2, 3, 5 and 6 show three-dimensional network. The results demonstrate different solvents or different temperatures can generate the structural diversity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It is well known that the structure of compound predetermining its properties is a universal principle in chemistry. So the synthesis of coordination polymers (CPs) with different networks is still a matter of high concern owing to controllable design-synthesis such as different pore size, shape, multifarious topology and tunable host–guest interactions [1,2,3,4,5,6,7,8,9,10]. For this reason, CPs is of great interest as promising application for ion-exchange, gas storage, separation, drug delivery and catalysis [11,12,13,14,15,16,17,18]. The structural diversity of CPs depends on the synthetic method and the choice of metal and organic ligand [19,20,21,22]. The character or shape of multitopic organic linker molecules, typical metal coordination environments or the formation conditions of typical inorganic building blocks can produce an effect on the structure of CPs and can help us to understand and direct the synthesis of CPs. The synthetic method and its conditions under given inorganic building block and organic component are of great importance for realizing modulation of structure. Although there are many novel synthesis techniques [23], such as, microwave (MW) heating, electrochemistry (EC), mechanochemistry (MC), and ultrasonic (US) methods, conventional solvothermal synthesis is still considered to be simple and effective. Generally, the main parameters of solvothermal synthesis can affect the structure of CPs [24,25,26,27,28,29], for example, counterions, the reaction time, the reaction pH values, the reaction temperature, the reaction molar ratio between metal salts and ligands, and the reaction solvents or template agents, and so on.
On the other hand, there were a large amount of reports on using transition metal and its cluster to fabricate intriguing framework. However, how to employ lithium element as inorganic building blocks to construct a variety of CPs with unusual, unprecedented, and inconceivable structures still remains a scientific challenge and wide research interest because of the characteristics of lower valance state, lighter atomic mass and fewer coordination number of lithium element. In the recent few years, many low-density Li-CPs have been successfully synthesized and applied in hydrogen storage materials [30,31,32]. There are two primary strategies for constructing lithium coordination networks. One famous strategy is mixing Li+ and B3+ as the charge-complementary inorganic nodes to control the structural diversity of architectures [33,34,35]. Another strategy is using Li4O4 cubane cluster or Li2O2 half-cubane as building block to realize significantly structural transformation of Li-cluster frameworks [36,37,38,39,40,41]. But these two strategies heavily rely on the change of auxiliary boron-imidazolate ligands and the coordination mode and angle of Li4O4 or Li2O2 cluster, respectively. In order to further enrich synthetic strategy, varying influence factor to synthesize different lithium coordination networks by solvothermal reaction is interesting. Therefore, taking into account the aforementioned topic, we are also interested in our focus on the solvent and temperature-dependent assemblies of lithium coordination networks.
Herein we demonstrate a versatile synthetic method capable of generating six new lithium-based coordination polymers, formulated as {[Li2(1,3,5-btc)(1,3,5-Hbtc)(DMF)](DMF)2}n (1), {[Li3(1,3,5-btc)(DMSO)3](H2O)}n (2), {((CH3)2NH)[Li2(1,3,5-btc)(H2O)]}n (3), {((CH3)2NH)[Li2(1,3,5-btc)(1,3,5-Hbtc)]}n (4), {((CH3)2NH)[Li2(1,3,5-btc)]}n (5) and [Li3(1,3,5-btc)(H2O)]n (6). As might be expected, solvent and reaction temperature dramatically affect structural assembly (Fig. 1). Compound 1 can be constructed only using DMF as solvent under 373.15 K, while compound 2, 3, 5 and 6 can be synthesized by changing component of solvent without altering temperature. Compared to the synthesis condition of 3, compound 4 can be fabricated when temperature rises to 413.15 K from 373.15 K.
2 Experimental
2.1 General Materials and Method
All commercially available reagents were purchased and used without further purification. All syntheses were carried out in 20 mL vial or 23 mL polytetrafluoroethylene lined stainless steel containers under autogenous pressure. PXRD patterns were collected on a Rigaku Dmax2500 diffractometer with Cu Kα radiation (λ = 1.54056 Å) with a step size of 0.05°. Thermal stability studies were carried out on a NETSCHZ STA-449C thermoanalyzer with a heating rate of 15 K min−1 in N2 atmosphere.
2.2 Preparation of 1–6
2.2.1 Synthesis of {[Li2(1,3,5-btc)(1,3,5-Hbtc)(DMF)](DMF)2}n (1)
A mixture of 1,3,5-benzenetricarboxylic acid (1,3,5-btc, 0.331 g 1.58 mmol), LiOH·H2O (0.138 g 3.29 mmol) and N,N-dimethylformamide (DMF, 4 mL) were placed in a vial. The sample was heated at 373.15 K for 3 days and then cooled to room temperature. After washing with DMF, the resulting colorless transparent crystals were obtained in 28.09% yield based on Li.
2.2.2 Synthesis of {[Li3(1,3,5-btc)(DMSO)3](H2O)}n (2)
1,3,5-Benzenetricarboxylic acid (1,3,5-btc, 0.114 g 0.54 mmol), LiOH·H2O (0.084 g 2.00 mmol), 1-propanol (4 mL) and DMSO (4 mL) were mixed in a 20 mL vial. The vial was capped and heated to 373.15 K for 3 day and then cooled to room temperature. After washing with 1-propanol and DMSO, the resulting colorless crystals of compound 2 were collected by filtration and dried in air (yield: 39.78% based on Li).
2.2.3 Synthesis of {((CH3)2NH)[Li2(1,3,5-btc)(H2O)]}n (3)
Solvothermal reaction (using N,N-dimethylformamide (DMF, 3 mL) and 1-propanol (1.5 mL) as solvents) of 1,3,5-benzenetricarboxylic acid (1,3,5-btc, 0.220 g 1.05 mmol) and LiOH·H2O (0.096 g 2.29 mmol) in a 20 mL vial at 373.15 K for 5 days produced colorless crystals and then cooled to room temperature. After washing with 1-propanol and DMF, the resulting compound 3 was obtained in 68.4% yield based on Li.
2.2.4 Synthesis of {((CH3)2NH)[Li2(1,3,5-btc)(1,3,5-Hbtc)]}n (4)
Compound 4 was synthesized following the same synthetic procedure as that for 3: a mixture of 1,3,5-benzenetricarboxylic acid (1,3,5-btc, 0.428 g 2.04 mmol), LiOH·H2O (0.17 g 4.05 mmol), N,N-dimethylformamide (DMF, 4 mL) and 1-propanol (6 mL) were placed in a 23 mL Teflon-lined stainless steel vessel. The sample was heated at 413.15 K for 3 days and then cooled to room temperature. After washing with 1-propanol and DMF, the resulting colorless transparent crystals were obtained in 75.84% yield.
2.2.5 Synthesis of {((CH3)2NH)[Li2(1,3,5-btc)]}n (5)
1,3,5-Benzenetricarboxylic acid (1,3,5-btc, 0.097 g 0.46 mmol), pyrazine (0.044 g 0.55 mmol) and LiOH·H2O (0.069 g 1.64 mmol) were dissolved in N,N-dimethylformamide (DMF, 2 mL) and 1-propanol (4 mL). The mixture was placed in a 20 mL vial. The sample was heated at 373.15 K for 3 days and then cooled to room temperature. After washing with 1-propanol and DMF, the resulting colorless transparent crystals were obtained in 57.84% yield.
2.2.6 Synthesis of [Li3(1,3,5-btc)(H2O)]n (6)
A mixture of 1,3,5-benzenetricarboxylic acid (1,3,5-btc, 0.073 g 0.35 mmol), LiOH·H2O (0.049 g 1.17 mmol), N,N-dimethylformamide (DMF, 2 mL), methanol (1 mL), and H2O (1 mL) were placed in a vial. The sample was heated at 373.15 K for 3 days and then cooled to room temperature. After washing with DMF, the resulting colorless transparent crystals were obtained in 35% yield.
2.3 X-Ray Crystallography
Suitable single crystals of 1–6 were carefully selected under an optical microscope and glued to thin glass fibers. Whereafter, single-crystal X-ray diffraction analyses were performed on a computer-controlled XCalibur E CCD diffractometer with graphite-monochromated Mo Kα radiation (λMo−Kα = 0.71073 Å) at T = 293.2 K. Empirical absorption corrections were made using the SADABS program [42]. The structures were solved using the direct method and refined by full-matrix least-squares methods on F2 by using the SHELX-97 program package. The crystallographic data and details of the structure for six compounds are listed in Table 1.
3 Results and Discussion
3.1 Structure Description
3.1.1 {[Li2(1,3,5-btc)(1,3,5-Hbtc)(DMF)](DMF)2}n (1)
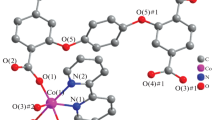
Compound 1 crystallizes in the space group P2(1)/c and exhibits a two-dimensional framework. The asymmetric unit contains two Li(I) centers, two 1,3,5-btc ligands, one coordinated and two isolated DMF solvent molecules (Fig. 2a). The local coordination environment around two Li(I) centers displays same tetrahedron geometric configuration with four oxygen atoms from different organic molecules: Li1 coordinating to four oxygen atoms from three 1,3,5-btc ligands and one DMF molecule, while Li2 coordinating to four oxygen atoms from four 1,3,5-btc ligands. In 1, it is worth noting that the bidentate carboxylate groups of the 1,3,5-btc ligand adopt a µ2-bridging coordination modes and one monodentate coordination mode (Fig. 2b). Two Li2 centers are linked by two µ2-bridging carboxylate groups to bring forth binuclear building block. Each pair of adjacent such binuclear building block is joined by another 1,3,5-btc to result in a chain (Fig. 2c). These chains are further interlinked by the organic 1,3,5-btc ligands and Li1 along a plane to generate the assembly of the two-dimensional framework (Fig. 2d).
3.1.2 {[Li3(1,3,5-btc)(DMSO)3](H2O)}n (2)
A single crystal X-ray diffraction studies revealed that 2 has space group of P2(1)2(1)2(1). As shown in Fig. 3a, each independent crystallographic unit of 2 comprises three Li(I) centers, one 1,3,5-btc ligand, three DMSO molecules (DMSO = dimethyl sulphoxide) and one isolated water molecule. The three metals show two different coordination behaviors: Li1 is five-coordinated with tetragonal pyramid geometric configuration by five oxygen atoms from three carboxylate groups of three 1,3,5-btc ligands and two DMSO molecules, respectively, while both of Li2 and Li3 are four-bounded resulting in tetrahedron coordination geometry by four oxygen atoms from carboxylate groups of 1,3,5-btc ligands and DMSO molecule. In compound 2, three carboxylate groups of one 1,3,5-btc ligand bridge seven Li(I) centers with µ3-bridging coordination mode, µ2-bridging coordination mode and the chelate-bridging coordination mode, respectively. The µ2-bridging carboxylate group of the 1,3,5-btc ligand and chelate-bridging carboxylate group of another 1,3,5-btc ligand link Li1 and Li2, and while Li2 and Li3 are bridged by a bidentate carboxylate group with µ2-bridging coordination mode and an oxygen atom of DMSO. Each pair of adjacent Li1…Li2…Li3 unit in the opposite direction is joined by the carboxylate groups of µ3-bridging fashion and an oxygen atom of another DMSO to result in a chain. Two such chains are further interlinked by the organic 1,3,5-btc ligands along the other two perpendicular directions (Fig. 3b), which will lead to the construction of the three-dimensional framework (Fig. 3c).
3.1.3 {((CH3)2NH)[Li2(1,3,5-btc)(H2O)]}n (3)
Single crystal X-ray analysis revealed that 3 has space group of Pna2(1). As shown in Fig. 4a, the asymmetric unit of 3 consists of two Li(I) centers, one 1,3,5-btc ligand, one water molecule and one isolated dimethylamine (Hdma) cations from the decomposition of DMF solvent. Li1 center is tetrahedrally coordinated by four oxygen atoms from four carboxylate groups of four 1,3,5-btc ligands. Li2 center is trigonal bipyramid coordinated by four oxygen atoms from three carboxylate groups of three 1,3,5-btc ligands and one oxygen atom from the coordinated water molecule. In the structure of 3, three carboxylate groups of the 1,3,5-btc ligand display different coordination modes with the lithium ion. Two bidentate carboxylate groups of the 1,3,5-btc ligand adopt a µ2-bridging coordination mode and the third bidentate carboxylate group also adopts a µ2-bridging coordination mode, but in the meantime, oxygen atoms of this carboxylate group link one Li(I) center with chelate-bridging coordination mode. It is notable that the seven Li(I) centers linked by three carboxylate groups of one 1,3,5-btc ligand are not exactly in the same plane. Two oxygen atoms from one bidentate carboxylate groups with a µ2-bridging mode and two other oxygen atoms from two different carboxylate groups bridge adjacent Li1…Li1 to generate one-dimensional chains and the two 1,3,5-btc ligands linking adjacent Li1…Li1 chains form approximate 60° angle (Fig. 4b), which coordinate to Li2 centers to complete the construction of the three-dimensional framework (Fig. 4c).
3.1.4 {((CH3)2NH)[Li2(1,3,5-btc)(1,3,5-Hbtc)]}n (4)
The structure of 4 with space group of C2/c was characterized by single-crystal X-ray diffraction. In the structure of 4, there are two independent Li centers with same coordination geometries, two 1,3,5-btc ligands with distinct coordination modes and one isolated dimethylamine (Hdma) cations from the decomposition of DMF solvent (Fig. 5a). Two Li centers of compound 4 are tetrahedrally coordinated by four carboxylate oxygen atoms from two 1,3,5-btc ligands which adopt different connection modes, generating an entirely different new crystalline structure. One 1,3,5-btc ligand links five Li centers with two µ2-bridging coordination mode and monodentate coordination mode (Fig. 5b), resulting in one-dimensional chain structure, which are further linked by 1,3,5-btc ligand with one µ2-bridging coordination mode and one monodentate coordination mode to form a layer structure. Furthermore, the parallel stacking of the layers results in a three-dimensional framework (Fig. 5c), and there are no obvious interactions between the layers.
3.1.5 {((CH3)2NH)[Li2(1,3,5-btc)]}n (5)
The three-dimensional structure (space group Pna2(1)) in 5 consists of two Li(I) centers, one 1,3,5-btc ligand, one isolated dimethylamine (Hdma) cations from the decomposition of DMF solvent (Fig. 6a). Similar to 3, three carboxylate groups of the 1,3,5-btc ligand also adopts µ2-bridging coordination mode and µ3-chelate-bridging coordination mode in 5. Although Li1 ions of 3 and 5 are tetrahedrally coordinated by four oxygen atoms from four carboxylate groups of four 1,3,5-btc ligands, Li2 of 5 is also tetrahedrally coordinated by four oxygen atoms (O1, O2, O3, O5) from three carboxylate groups of three 1,3,5-btc ligands, which is different from trigonal bipyramid coordination geometry of Li2 in 3. Due to the very similar bridging-code of 1,3,5-btc ligand and coordination environment of Li (I) center, the construction of frameworks in 3 and 5 is alike. Adjacent Li1…Li1 are bridged by one bidentate carboxylate groups with a µ2-bridging mode and two other oxygen atoms from two different carboxylate groups to generate one-dimensional chain. Two 1,3,5-btc ligands linking three of this one-dimensional chains forms approximate 50° angle (Fig. 6b), which coordinate to Li2 centers to generate new porous structure of 5 (Fig. 6c).
3.1.6 [Li3(1,3,5-btc)(H2O)]n (6)
Different from the above-mentioned five frameworks (1–5), Compound 6 adopts space group of P-1 and features a novel three-dimensional network structure. In the structure of 6, the asymmetric unit consists of three independent Li centers with tetrahedral coordination geometries, one 1,3,5-btc ligand and one coordinated water molecule (Fig. 7a). Li1 ions is coordinated by four oxygen atoms from four carboxylate groups of 1,3,5-btc ligands, while both Li2 and Li3 ions are four-bounded by four oxygen atoms from three 1,3,5-btc ligands and one coordinate water molecule, respectively. It is worth noting that three bidentate carboxylate groups of the 1,3,5-btc ligand not only adopt two µ3-bridging coordination modes, but also show a µ4-bridging coordination mode. These carboxylic groups and µ2-O of water molecule bridge two alternately adjacent Li1…Li3…Li2 units to generate secondary chains, which are further interlinked to result in a one-dimensional chain structure (Fig. 7b). These resulting chains are further interconnected by carboxylic groups to generate a layer, which coordinate to the carboxylic groups from 1,3,5-btc ligands to complete the construction of the three-dimensional framework (Fig. 7c).
3.2 Powder X-Ray Diffraction (PXRD) and Thermogravimetric Analyses
The purities and crystallinities of the bulk samples were checked by powder X-ray diffraction (PXRD). The PXRD patterns of compounds 1, 2, 5 and 6 are illustrated in Fig. S1-S4(ESI). The PXRD patterns of all compounds are in good agreement with the ones simulated from the single crystal structural data. Although TGA curves of 2 and 5 both exhibit four main steps of weight losses, their principles of weight loss from heating are different (Fig. S5-S6). The TGA curve of 2 shows that compound 2 is stable up to 528.15 K. The subsequent three weight losses are 20.88% (from 528.15 to 643.15 K), 3.61% (from 688.15 to 712.15 K), 10.55% (from 788.15 to 873.15 K), respectively, which corresponds to the gradual removal of DMSO. Above 873.15 K, the whole framework begins to collapse due to the decomposition of the organic ligands. The TG trace for 5 reveals that the solvents on the sample surface are gradually removed in proceeding from 303.15 K to 383.15 K with weight loss of 7.77%. For 5, the second weight loss of (16.48%) corresponds to the release of unordered solvent, which starts at 473.15 K and is complete at 550.15 K. The third step of weight loss attributes to the release of Hdma cations. The observed weight loss (12.68%) is lower than the calculated value (16.92%), which starts at 803.15 K and is complete at 885.15 K. Upon further heating, the host framework of 5 begins to decompose.
4 Conclusions
In summary, a series of new Li(I) coordination polymers have successfully synthesized. It is worth noting that only one synthetic method (solvothermal synthesis) and single organic ligand (1,3,5-benzenetricarboxylic acid) were employed to achieve structural diversity. As we expected, various components of solvents can produce different structure of Li(I) coordination polymers without multiple Li-Cluster, while the change of reaction temperature can also lead to new coordination networks, which illustrates that the solvothermal reaction is versatile synthetic method with solvent and temperature as key factors for constructing diversified framework.
5 Supplementary Material
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC reference numbers: 1859125, 1859126, 1859127, 1859128, 1859129, 1859130. These data can be obtained free of charge at: http://www.ccdc.cam.ac. Uk or Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336-033; e-mail: deposit @ ccdc.cam.ac.uk).
References
B.F. Hoskins, R. Robson, J. Am. Chem. Soc. 111, 5962 (1989)
S. Kitagawa, R. Kitaura, S.I. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004)
G. Ferey, Chem. Soc. Rev. 37, 191 (2008)
H.X. Deng, C.J. Doonan, H. Furukawa, R.B. Ferreira, J. Towne, C.B. Knobler, B. Wang, O.M. Yaghi, Science 327, 846 (2010)
H.X. Zhang, F. Wang, H. Yang, Y.X. Tan, J. Zhang, X.H. Bu, J. Am. Chem. Soc. 133, 11884 (2011)
Z.Q. Jiang, G.Y. Jiang, F. Wang, Z. Zhao, J. Zhang, Chem. Commun. 48, 3653 (2012)
H.L. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai, Q. Xu, J. Am. Chem. Soc. 131, 11302 (2009)
F. Wang, Z.S. Liu, H. Yang, Y.X. Tan, J. Zhang, Angew. Chem., Int. Ed. 50, 450 (2011)
M.R. Martinez, C.A. Camur, A.W. Thornton, I. Imaz, D. Maspochbc, M.R. Hill, Chem. Soc. Rev. 46, 3453 (2017)
S.M. Cohen, Chem. Rev. 112, 970 (2012)
J.R. Li, J. Sculley, H.C. Zhou, Chem. Rev. 112, 869 (2012)
M.P. Suh, H.J. Park, T.K. Prasad, D.W. Lim, Chem. Rev. 112, 782 (2012)
H.H. Wu, Q.H. Gong, D.H. Olson, J. Li, Chem. Rev. 112, 836 (2012)
K. Sumida, D.L. Rogow, J.A. Mason, T.M. McDonald, E.D. Bloch, Z.R. Herm, T.H. Bae, J.R. Long, Chem. Rev. 112, 724 (2012)
K. Adil, Y. Belmabkhout, R.S. Pillai, A. Cadiau, P.M. Bhatt, A.H. Assen, G. Maurinb, M. Eddaoudi, Chem. Soc. Rev. 46, 3402 (2017)
P. Horcajada, R. Gref, T. Baati, P.K. Allan, G. Maurin, P. Couvreur, G. Ferey, R.E. Morris, C. Serre, Chem. Rev. 112, 1232 (2012)
M. Yoon, R. Srirambalaji, K. Kim, Chem. Rev. 112, 1196 (2012)
Q. Yang, Q. Xu, H.L. Jiang, Chem. Soc. Rev. 46, 4774 (2017)
P.P. Cui, J.L. Wu, X.L. Zhao, D. Sun, L.L. Zhang, J. Guo, D.F. Sun, Cryst. Growth Des. 11, 5182 (2011)
G.H. Xu, X.Y. He, J.Y. Lv, Z.G. Zhou, Z.Y. Du, Y.R. Xie, Cryst. Growth Des. 12, 3619 (2012)
W.G. Lu, L. Jiang, X.L. Feng, T.B. Lu, Cryst. Growth Des. 8, 986 (2008)
V.R. Pedireddi, S. Varughese, Inorg. Chem. 43, 450 (2004)
N. Stock, S. Biswas, Chem. Rev. 112, 933 (2012)
L.Y. Zhang, J.P. Zhang, Y.Y. Lin, X.M. Chen, Cryst. Growth Des. 6, 1685 (2006)
L. Pan, T. Frydel, M.B. Sander, X.Y. Huang, J. Li, Inorg. Chem. 40, 1271 (2001)
C.M. Liu, S. Gao, D.Q. Zhang, D.B. Zhu, Cryst.Growth Des. 7, 1312 (2007)
R.Q. Fang, X.M. Zhang, Inorg. Chem. 45, 4801 (2006)
Z. Xu, S. Lee, Y.H. Kiang, A.B. Mallik, N. Tsomaia, K.T. Mueller, Adv. Mater. 13, 637 (2001)
C.Y. Su, Y.P. Cai, C.L. Chen, F. Lissner, B.S. Kang, W. Kaim, Angew. Chem., Int. Ed. 41, 3371 (2002)
D. Banerjee, L.A. Borkowski, S.J. Kim, J.B. Parise, Cryst. Growth Des. 9, 4922 (2009)
D. Banerjee, S.J. Kim, W. Li, H.H. Wu, J. Li, L.A. Borkowski, B.L. Philips, J.B. Parise, Cryst. Growth Des. 10, 2801 (2010)
A. Clough, S.T. Zheng, X. Zhao, Q.P. Lin, P.Y. Feng, X.H. Bu, Cryst. Growth Des. 14, 897 (2014)
J. Zhang, T. Wu, C. Zhou, S.M. Chen, P.Y. Feng, X.H. Bu, Angew. Chem. 121, 2580 (2009)
T. Wu, J. Zhang, X.H. Bu, P.Y. Feng, Chem. Mater. 21, 3830 (2009)
T. Wu, J. Zhang, C. Zhou, L. Wang, X.H. Bu, P.Y. Feng, J. Am. Chem. Soc. 131, 6111 (2009)
D.J. MacDougall, J.J. Morris, B.C. Noll, K.W. Henderson, Chem. Commun. 456 (2005)
J.J. Morris, B.C. Noll, K.W. Henderson, Cryst. Growth Des. 6, 1071 (2006)
J.A. Bertke, A.G. Oliver, K.W. Henderson, Inorg. Chem. 51, 1020 (2012)
X. Zhao, T. Wu, X.H. Bu, P.Y. Feng, Dalton Trans. 41, 3902 (2012)
M.S. Shimazu, X. Zhao, D.M. Huynh, X.H. Bu, Cryst. Growth Des. 15, 2550 (2015)
Z.Q. Jiang, Y.L. Tan, S.Y. Wang, B. Li, D. Teng, C. Liao, D.J. Zhou, Y. Kang, J. Inorg. Organomet. Polym. 27, 1583 (2017)
G.M. Sheldrick, SADABS, Program for Area Detector Adsorption Correction; Institute for Inorganic Chemistry (University of Göttingen, Göttingen, 1996)
Acknowledgements
We gratefully acknowledge the support of this work by NSFC (21473202 and 21501176), Science and Technology Planning Project in Sichuan Province (2015RZ0029), Science and Technology Planning Project in Panzhihua City (2014CY-G-24, 2015CY-S-10 and 2016CY-G-4).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, ZQ., Du, Y., Zhu, XJ. et al. Versatile Solvothermal Synthetic Method and Structural Characterization of Lithium Coordination Networks. J Inorg Organomet Polym 29, 1447–1456 (2019). https://doi.org/10.1007/s10904-019-01108-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01108-0